Abstract
The decline in malaria across Africa has been largely attributed to vector control using long-lasting insecticidal nets (LLINs). However, this intervention has prompted widespread insecticide resistance (IR) and been associated with changes in mosquito behaviour that reduce their contact with LLINs. The relative importance and rate at which IR and behavioural adaptations emerge are poorly understood. We conducted surveillance of mosquito behaviour and IR at 12 sites in Burkina Faso to assess the magnitude and temporal dynamics of insecticide, biting and resting behaviours in vectors in the 2-year period following mass LLIN distribution. Insecticide resistance was present in all vector populations and increased rapidly over the study period. In contrast, no longitudinal shifts in LLIN-avoidance behaviours (earlier or outdoor biting and resting) were detected. There was a moderate but statistically significant shift in vector species composition from Anopheles coluzzii to Anopheles gambiae which coincided with a reduction in the proportion of bites preventable by LLINs; possibly driven by between-species variation in behaviour. These findings indicate that adaptations based on insecticide resistance arise and intensify more rapidly than behavioural shifts within mosquito vectors. However, longitudinal shifts in mosquito vector species composition were evident within 2 years following a mass LLIN distribution. This ecological shift was characterized by a significant increase in the exophagic species (An. gambiae) and coincided with a predicted decline in the degree of protection expected from LLINs. Although human exposure fell through the study period due to reducing vector densities and infection rates, such ecological shifts in vector species along with insecticide resistance were likely to have eroded the efficacy of LLINs. While both adaptations impact malaria control, the rapid increase of the former indicates this strategy develops more quickly in response to selection from LLINS. However, interventions targeting both resistance strategies will be needed.
Subject terms: Ecology, Evolution
Introduction
Long-lasting insecticidal nets (LLINs) and Indoor Residual Spraying (IRS) are the main malaria vector controls tools1,2 recommended by the World Health Organisation (WHO)3. Both interventions have had a significant impact on malaria control in Africa; with LLINs being primarily responsible for the > 50% reduction in prevalence since 20004. The success of these interventions rely on their ability to exploit innate aspects of malaria vector behaviour including a preference for feeding on humans (anthropophagy), inside houses (endophagy), during sleeping hours, and to rest inside houses (endophily) after feeding5,6. Consequently, LLINs and IRS are expected to work best against anthropophilic, endophilic and endophagic vectors that have high susceptibility to insecticides7,8. Historically, most of the major malaria vectors in Africa (members of the Anopheles gambiae complex) were described as having highly anthropophilic9,10 and endophilic resting behaviour11, characterized by late night-biting11,12. This combination of traits is thought to account for the early success of current vector control approaches in Africa13.
However global progress on malaria control has stalled since 201514, with a slowing of decline in cases and deaths, and even an increase in some settings. This stagnation has been most pronounced within a small group of high-burden countries including ten in Africa plus India. This slowdown is hypothesized to be driven by the widespread emergence of insecticide resistance (IR) in malaria vectors that has now been documented in almost all African countries15. Resistance has been facilitated by the reliance of almost all LLIN products on a single class of insecticides (pyrethroids). Vectors have developed a range of resistance mechanisms against pyrethroids including. target site mutations16, cuticular resistance17 and metabolic resistance18. Additionally, there are reports of vectors shifting key behaviours that predispose them to LLINs including their host choice19, biting time7,20 and location (indoor versus outdoors21) and resting22. Such shifts can arise either through ecological changes in vector species composition (with more zoophilic, exophagic and exophilic species being favoured23,24) or evolutionary adaptations reflected by behavioural changes within species. Examples of within species behavioural changes include the apparent switch in biting time from late night to morning in An funestus in Benin25 and from indoor to outdoor resting in An. arabiensis in Tanzania19. Such ecological and adaptive changes have been documented in several settings26,27. Regardless of how changes in vector behaviour arise, they are expected to erode LLIN effectiveness; with outdoor biting alone estimated as being responsible for over 10 million malaria cases in Africa28.
Ecological shifts and evolutionary adaptations in mosquito behaviour could both reduce malaria control by enabling mosquitoes to avoid contact with LLINs. In contrast, IR allows mosquitoes to tolerate contact with LLINs by avoiding immediate mortality. Understanding the relative capacity of mosquito vectors to mount IR and behavioural avoidance in response to LLINs is necessary to estimate the risk posed by each process to malaria control and highlight where mitigation efforts should be prioritized. For instance, rapid emergence of behavioural avoidance mandates incorporation of interventions that target mosquitoes outside homes (e.g. spatial repellents, endectocides, larvicides etc.29). In contrast, the rapid emergence of insecticide resistance in vectors who continue to feed and rest indoors indicates that switching to alternative insecticide classes in indoor-based interventions (e.g. next generation nets) or using non-insecticidal methods inside the home (house screening30,31) may have greater impact.
Burkina Faso is amongst the 10 high burden malaria countries in Africa where prevalence appears to be rising despite several rounds of mass LLIN distribution32. The Anopheles gambiae complex (An. arabiensis, An. coluzzii and An. gambiae) and An. funestus species group are the primary vectors in this country33,34. Resistance to insecticides (DDT and Dieldrin) was first detected in Burkina Faso in the late 1960s35,36. Over the past two decades IR has intensified in vector populations in Burkina Faso15,37 with levels now being amongst the highest in Africa38. This high rate of IR is hypothesized to be responsible for the limited impact of LLINs in Burkina Faso39, but less is known about the potential additional impacts of mosquito behavioural adaptations and changes in vector species composition. In the current study we estimated the potential epidemiological impact of temporal shifts in vector abundance, insecticide resistance and behaviour in two ways. First, we estimated expected human exposure to infected mosquitoes in term of the “Entomological Inoculation Rate “(“EIR”) between study years. The EIR is considered to be one of the most direct estimates of human exposure to malaria and has a relatively good correlation with human epidemiological outcomes such as malaria incidence40,41. Additionally, we estimated the degree of protection expected to be obtained from use of an effective LLIN during typical sleeping hours, as a function of the time and location of mosquito biting42. In combination this information will enable assessment of malaria transmission and the expected impact of current control tools as vector behavioural and physiological resistance rises.
In Burkina Faso, LLIN mass-distribution campaigns started in 2010. Approximately, ~ 8.4 and 10.5 million nets were distributed during campaigns held in 2010 and 2013 respectively. Much of the knowledge on malaria vector behaviours comes from before the period of mass LLIN distribution43–45 and showed that > 50% of An. gambiae s.l. bite indoors and biting activity was concentrated late at night (01 h–06 h), with only few studies thereafter46,47. We conducted an observational study to measure the intensity and rate of change in insecticide and behavioural resistance traits within malaria vector populations over 2 years in the Cascades Region. The study was undertaken after a mass LLIN distribution held in July 2016 where ~ 10.6 million pyrethroid-only net were distributed. Aims were to (1) update information on vector ecology and behaviour in a high burden African country, (2) quantify and compare the magnitude and rate of change in insecticide and behavioural resistance traits, and malaria vector species composition and (3) estimate the potential impact of changes in these ecological and physiological traits on malaria transmission and LLIN effectiveness.
Results
A total of 49,280 female mosquitoes were caught in Human Landing Catches (HLCs), of which most were female An. gambiae s.l. (N = 40,220; Table 1). A total of 927 mosquitoes were collected from the Resting Box Traps (RBTs) of which 584 were female An. gambiae s.l. (Table 2). Within the subset (~ 20% of total) of An. gambiae s.l. collected in HLCs that were identified to species level by polymerase chain reactions (PCR), 53.2% were An. coluzzii, 45.9% were An. gambiae and 0.28% were An. arabiensis. Within the 449 resting female An. gambiae s.l. identified by PCR, An. coluzzii was dominant (61.25%) followed by An. gambiae (38.08%), An. arabiensis (n = 2, 0.45%) and one hybrid between An. coluzzii and An. gambiae (0.22). Due to the low number of An. arabiensis, this species was not included in statistical analysis of species-specific differences.
Table 1.
Total number of female Anophelines caught using Human Landing Catches in 12 villages of southwestern Burkina Faso, from October 2016 to December 2018.
| Village | An. coustani | An. funestus | An. gambiae s.l | An. nili | An. obscurus | An. pharoensis | An. rufipes | An. ziemanni | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | ||
| Dangouindougou | 0 | 4 | 3 | 3 | 788 | 786 | 0 | 1 | 0 | 0 | 11 | 14 | 1 | 0 | 0 | 0 | 1611 |
| Gouera | 0 | 0 | 0 | 5 | 762 | 867 | 0 | 3 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 1642 |
| Nianiagara | 0 | 0 | 1 | 0 | 451 | 522 | 4 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 984 |
| Nofesso | 0 | 0 | 1 | 0 | 340 | 540 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 881 |
| Ouangolodougou | 0 | 0 | 0 | 0 | 265 | 409 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 675 |
| Sitiena | 31 | 12 | 1 | 1 | 2549 | 2589 | 160 | 306 | 5 | 8 | 25 | 34 | 0 | 0 | 1 | 0 | 5722 |
| Tengrela | 49 | 88 | 4 | 3 | 4579 | 4598 | 25 | 47 | 1 | 0 | 63 | 152 | 2 | 5 | 2 | 3 | 9621 |
| Tiefora | 1 | 2 | 4 | 2 | 3917 | 4754 | 14 | 24 | 0 | 0 | 52 | 119 | 0 | 0 | 0 | 0 | 8889 |
| Timperba | 0 | 1 | 0 | 1 | 445 | 413 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 861 |
| Tondoura | 0 | 0 | 1 | 0 | 1121 | 1096 | 2 | 5 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2227 |
| Toumousseni | 0 | 2 | 2 | 2 | 2670 | 3056 | 8 | 17 | 0 | 0 | 14 | 29 | 0 | 0 | 0 | 0 | 5800 |
| Yendere | 2 | 1 | 0 | 4 | 1272 | 1431 | 5 | 9 | 0 | 0 | 9 | 9 | 0 | 0 | 0 | 0 | 2742 |
| Total | 83 | 110 | 17 | 21 | 19,159 | 21,061 | 218 | 413 | 6 | 8 | 179 | 366 | 3 | 5 | 3 | 3 | 41,655 |
Results are displayed by trapping location (IN = indoor and OUT = outdoor) and village.
Table 2.
Total number of female Anophelines caught using Resting Bucket Traps in 12 villages of southwestern Burkina Faso, from October 2016 to December 2018.
| An. coustani | An. funestus | An. gambiae s.l | An. nili | An. pharoensis | An. rufipes | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | IN | OUT | ||
| Dangouindougou | 0 | 0 | 0 | 1 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 8 |
| Gouera | 0 | 0 | 0 | 4 | 11 | 25 | 0 | 0 | 0 | 0 | 0 | 11 | 51 |
| Nianiagara | 0 | 0 | 0 | 0 | 8 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 14 |
| Nofesso | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Ouangolodougou | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Sitiena | 0 | 0 | 0 | 0 | 22 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 41 |
| Tengrela | 0 | 2 | 2 | 1 | 142 | 101 | 0 | 2 | 1 | 1 | 5 | 0 | 256 |
| Tiefora | 0 | 0 | 0 | 2 | 58 | 73 | 0 | 1 | 0 | 0 | 0 | 0 | 134 |
| Timperba | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Tondoura | 0 | 0 | 0 | 0 | 12 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| Toumousseni | 0 | 0 | 0 | 0 | 32 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 72 |
| Yendere | 0 | 0 | 0 | 0 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Total | 0 | 2 | 2 | 8 | 300 | 284 | 0 | 3 | 1 | 1 | 5 | 12 | 618 |
Results are displayed by trapping location (IN = indoor and OUT = outdoor) and village.
Insecticide resistance
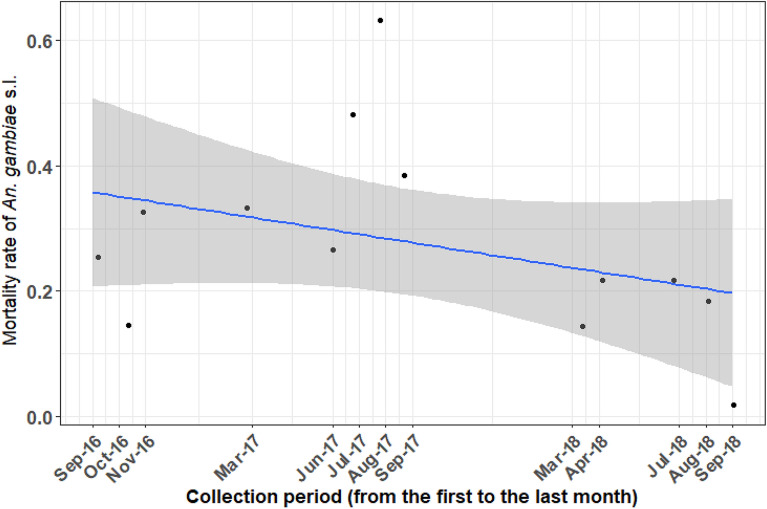
The overall mortality of An. gambiae s.l. in the 24 h following exposure to the diagnostic dose (DD) of deltamethrin was 23.33% [95% CI 14.63–32.05%], indicating a resistant population according to WHO criteria. The mortality of An. gambiae s.l. following exposure to the DD of deltamethrin declined significantly (df = 1, χ2 = 20.91, p = 0.001; Supplementary Table 1) over the study period, falling from ~ 38% at the beginning (October 2016) to ~ 17% toward the end (September 2018, Fig. 1). As expected, mortality 24-h post-exposure generally rose with increased concentration of deltamethrin (0.25%: 64.14% [95% CI 55.27–73.0]; 0.5%: 86.03 [95% CI 81.87–90.2%]; 0.75%: 87.48% [95% CI 81.52–92.43%]). The predicted reduction in post-exposure mortality over time as observed with the DD was also detected at 15X the DD (df = 1, χ2 = 11.25, p = 0.001; Supplementary Table 1). Long-term increases in IR were evident after controlling for statistically significant variation between villages (all concentrations) and season (only detected in bioassays using the DD; Supplementary Table 1).
Figure 1.
Mean predicted mortality of Anopheles gambiae s.l. 24 h following exposure to a diagnostic dose of deltamethrin across the study period. Black dots indicate mean predicted mortality at each study month between September 2016 to September 2018 across 9 villages in southwestern Burkina Faso. The blue line indicates the predicted linear change in An. gambiae s.l. across the study period based on the final model, with the grey-shaded area indicating the 95% confidence intervals.
The human biting rate and Anopheles gambiae s.l. species composition
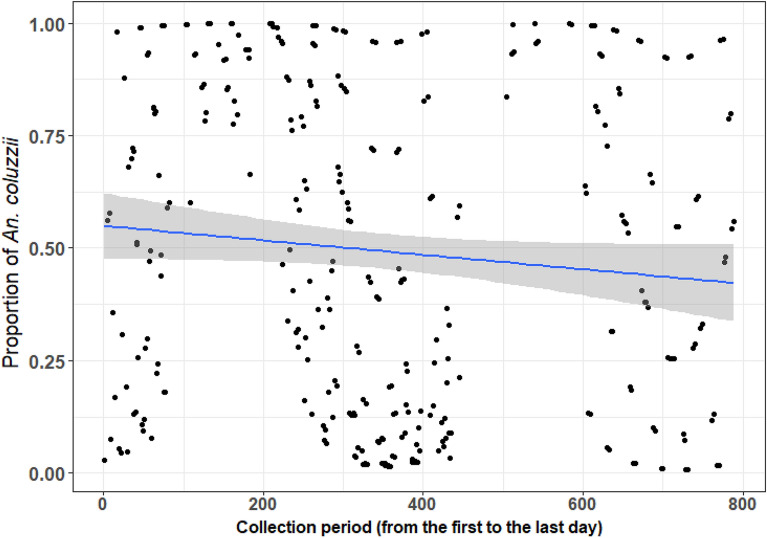
Across the whole study period and pooling across sites, the mean Human Biting rate (HBR) of An. gambiae s.l. was ~ 17 bites per person per night (bppn). After controlling for seasonal and spatial variations, there was evidence of a 39% longitudinal reduction in HBR (Supplementary Table 2) from the beginning to end of the study period. Similarly, after controlling for variations due to season and village there was also evidence of 23% decline in the proportion of An. coluzzii relative to An. gambiae over the study period (Supplementary Table 2, Fig. 2).
Figure 2.
Mean predicted long-term trends in Anopheles coluzzii proportion within the An. gambiae complex from 12 villages in southwestern Burkina Faso. The blue line represents the predicted regression line from a model accounting for additional variation due to village and trapping location (inside and outdoors houses), with the grey-shaded area indicating the 95% confidence intervals.
Anopheles gambiae s.l. behaviours (biting and resting) and host choice
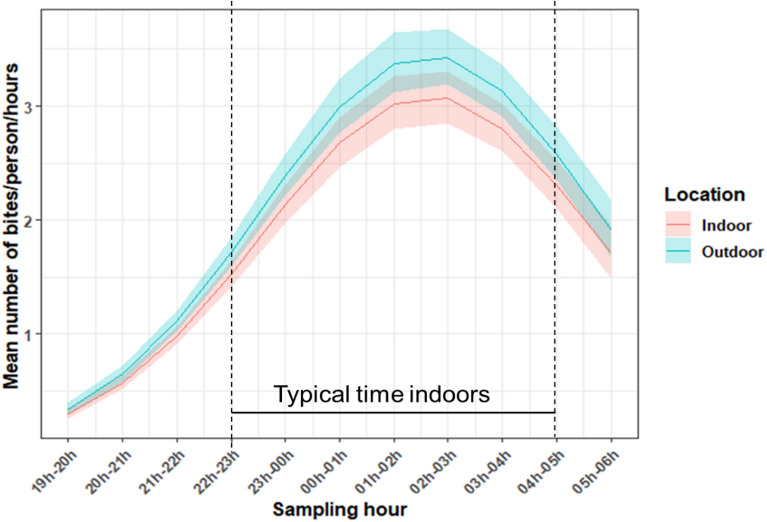
Anopheles gambiae s.l. biting activity was at its lowest in the early evening (7–8 pm) and steadily increased to reach to a peak between 00 and 04 h; with the median biting time between 01 and 02 h. Overall, about 54% [95% CI ~ 51–57%] of An. gambiae complex members were collected host-seeking outdoors. However, there was no long-term change in the biting location across the study (Supplementary Table 2). Further analysis of the subset of An. gambiae s.l. that were individually identified to species level indicated that the proportion of outdoor biting varied between An. gambiae and An. coluzzii (df = 1, χ2 = 6.82, p = 0.009). Specifically, An. gambiae was slightly more exophagic (% outdoor biting = 54.73%, [95% CI 52.35–57.12]) than An. coluzzii [% outdoor biting = 51.4%, 95% CI 48.9–53.9%]).
The timing of host-seeking activity was similar in the indoor and outdoor environment (Fig. 3). Although peak of biting occurred around midnight, some An. gambiae s.l. were still caught biting around 6 am (Fig. 3). No longitudinal change in median biting time was observed (Supplementary Table 2). Further analysis of the subsample of An. gambiae s.l. identified to species level indicated there was difference in the median biting time between An. coluzzii and An. gambiae (Supplementary Table 2). Anopheles coluzzii was found to bite earlier (11 pm–01 am) than An. gambiae (00 pm–02 am). The overall proportion of An. gambiae s.l. females resting outdoors was 46.97%. After controlling for the effect of season, there was no evidence of a longitudinal change in the proportion of female An. gambiae s.l. resting outdoors over the study period (df = 1, χ2 = 2.67, p = 0.32). Of the 94 s.l. females from which blood-meals could be identified, 52% were from human only, 35.11% were cattle only, and 12.77% contained a mixture of cattle and human blood (Table 3); corresponding to an HBI of 64.9%.
Figure 3.
Mean number of An. gambiae s.l. biting per hour (as assessed from Human Landing Catches) in 12 villages in southwestern Burkina Faso, from October 2016 to December 2018. Data are pooled over village and collection period. The period between the black vertical dashed lines indicates when most people are inside their dwelling and estimated to be sleeping under a nets. The blue and red curves indicate the predicted number of An. gambiae s.l. biting in outdoor and indoor settings, respectively; with the shaded areas indicating the 95% confidence intervals.
Table 3.
Total numbers of blood-fed female An. gambiae s.l. caught using Resting Bucket Traps (RBT) in the 12 villages, from October 2016 to December 2018. Results are displayed by species, trapping location (Indoor or outdoors) and host blood source, pooled over village. ‘HBI’ indicate the ‘Human Blood Index’; estimated as the proportion of blood meals taken from humans out of the blood meals whose source could be identified.
| Species | Location | Cattle | Human | Human–cattle | Total | HBI |
|---|---|---|---|---|---|---|
| An. arabiensis | Indoor | 1 | 0 | 0 | 1 | 0 |
| Outdoor | 1 | 0 | 0 | 1 | 0 | |
| An. coluzzii | Indoor | 18 | 23 | 4 | 45 | 0.6 |
| Outdoor | 2 | 7 | 2 | 11 | 0.82 | |
| An. gambiae | Indoor | 7 | 11 | 6 | 24 | 0.71 |
| Outdoor | 4 | 8 | 0 | 12 | 0.67 | |
| Total | 33 | 49 | 12 | 94 | 0.65 |
HBI indicate the proportion of mosquito that blood-fed on human.
Malaria transmission and LLIN effectiveness
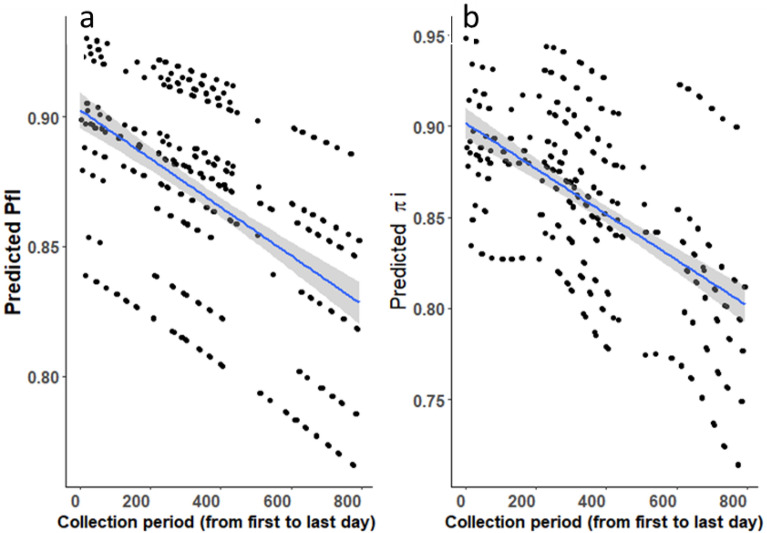
Overall, 86.81% [95% CI 83.6–90.02%] of s.l. were caught biting between 10 pm and 5 am (corresponding to time when most people are indoors, PfƖ). Furthermore, 85.45% [95% CI: 80.64–90.26%] of human exposure occurred when people are assumed to be indoors (πi). Thus, under the simplifying assumption that people remain under an LLIN between 10 pm and 5 am48, ~ 85% of exposure to An. gambiae s.l. could be preventable by LLIN use. Taking into account variations due to village and season, there was a modest but statistically signfiicant decline (of ~ 7%) in the proportion of biting taking place when people are expected to be indoors (Supplementary Table 3, Pfl: z = − 3.14, p = 0.002, Fig. 4a) over the study period. Similarly, there was also ~ 10% decrease in the proportion of human exposure estimated to occur indoors (Supplementary Table 3, πi: z = − 3.72, p = 0.0002, Fig. 4b).
Figure 4.
Predicted mean of (a) Pfl = the proportion of An. gambiae s.l. bites occurring when most people are inside their dwellings and likely asleep (e.g. between 10 pm and 5 am; Pfl), (b) πi = total human exposure to An. gambiae s.l. bites occurring indoors (πi) based on Human Landing Catch from 6 villages over 2 years (Oct 1st, 2016 to Dec 4th, 2018) in southwestern Burkina Faso. Dots represent the predicted values (Pfl and πi) at each sampling night. The blue lines represent the regression lines from the models and the grey-shaded area the 95% confidence intervals.
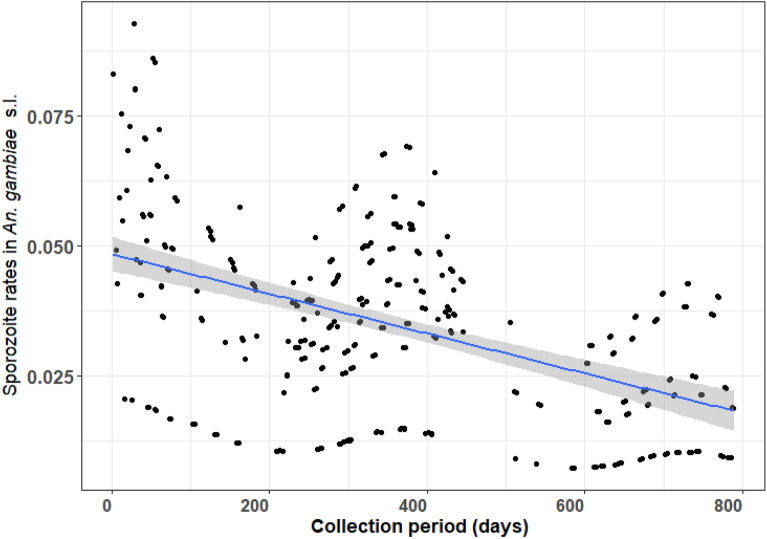
The mean sporozoite rate (SR) in An. gambiae s.l. across the study area was 3.48% [95% CI 1.51–5.26%]. After controlling for seasonal and spatial variations, their was evidence of a decline in mean SR ~ 5% to ~ 2% over the study period (z = − 2.5, p = 0.01, Supplementary Table 4, Fig. 5). There was no significant difference in the SR betweenAn. coluzzii and An. gambiae; or between An. gambiae s.l. captured host-seeking indoor versus outdoor (Supplementary Table 4). Annual Entomological Inoculation Rates (EIR) were calculated for the subset of 6 villages monitored in both year 1 (Oct 2016–Sept 2017) and year 2 (Oct 2017–Sept 2018). The annual EIR was considerably higher in year 1 (289.25 infective bites per person per year) compared to year 2 (94.81 infective bites per person per year). This decline was in accordance with the observed longitudinal declines in the human biting rate and SR across the study. Despite this decline, residents were still predicted to be exposed to ~29 infective bites per person per year after adjustment for the proportion of bites (~ 85%) that can be prevented through effective use of LLINs.
Figure 5.
Mean predicted longitudinal trend in sporozoite infection rates in An. gambiae s.l. (dots). Data are from An. gambiae s.l. collected in 12 villages in southwestern Burkina Faso using Human Landing Catches between October 2016 and December 2018. The blue line indicates the predicted temporal trend with the grey-shaded areas indicating the 95% confidence intervals.
Discussion
Here we tested for evidence of systematic changes in insecticide and behavioural resistance traits in malaria vectors over 2.5 years following a mass LLIN distribution in Burkina Faso. Consistent with expectation, there was a high prevalence of insecticide resistance (IR) within these malaria vector populations, which further intensified over the period of the study, with post-exposure mortality falling by ~ 50% (from 38% at the start to 17% at the end). In contrast, although baseline levels of “behavioural avoidance” traits were higher than expected (e.g. > 50% outdoor biting and resting), behavioural phenotypes were relatively stable across the study period. There was however a reduction in An. gambiae s.l. abundance (Human Biting Rate) and a shift in vector species composition across the study period; with a moderate reduction in the proportion of An. coluzzii relative to An. gambiae. The potential epidemiological impacts of these ecological changes are complex. On one hand, there was a significant drop in the Entomological Inoculation Rate between years, indicating a fall in human exposure and malaria transmission. On the other, the proportion of exposure to An. gambiae s.l. expected to be preventable by LLIN use also fell over the study period (πi, ~ 7% fall); indicating a moderate erosion of protection from residual transmission through time. We hypothesize that the fall in πi was due to a shift in vector species composition, with the slightly more exophagic An. gambiae increasing relative to An. coluzzii through time. Thus, we hypothesize that while both IR and avoidance behaviours are contributing to the limited impact of LLINs in this setting, behavioural traits appeared to be responding more slowly to selection from LLINs.
The rise in IR across this study is in line with similar increases observed over short time periods in other African settings49,50. This acceleration in IR appears to be a relatively recent phenomenon. For example, post-exposure mortality rates of An. gambiae s.l. to the DD of synthetic pyrethroids when first introduced in the 1970s (~ 95%51) was relatively similar to that in the 2010s52. The intensification of IR over the last decade is likely due to the initiation of mass LLIN distribution programmes in 2010. For example, post-exposure mortality of An. gambiae s.l. to the DD in one of our study sites (Tiefora) was ~ 39%53 in 2014 compared to only ~ 15% reported here (2016–18). Additionally, in Tengrela (another study village), post exposure mortality to DD declined from ~ 92 to ~ 19% between 2011 and 201337. In light of the rapid rise in IR, the government of Burkina Faso switched to new net products that combine pyrethroids with other insecticide classes in the most recent (2019) mass ITN distribution campaign (e.g. INTERCEPTOR G2: alpha-cypermethrin and chlorfenapyr; PBO-LLINs: deltamethrin and the synergist piperonyl butoxide).
While insecticide resistance increased significantly over the study, mosquito vector behaviours were generally static. However, some mosquito behavioural traits observed here differ substantially from historical reports in the study area; raising the possibility of more gradual long-term changes that may not be detectable over a few years. For example, previous studies from the Central, Plateau Central and West regions (2001–2015) of Burkina Faso reported An. gambiae s.l. was more likely to bite indoors than outdoors43,54, or had an even split between indoor and outdoor biting46. In contrast, here An. gambiae s.l. was more likely to host seek outdoors [~ 54%; 95% CI ~ 51–57%]. Additionally, a recent review of An. gambiae s.l. biting behaviour from a range of African countries concluded that in general > 80% of vector bites occur indoors28; substantially higher than observed here. Anopheles gambiae s.l. were also found in indoor and outdoor resting traps with relatively similar frequency (53% versus 47%); in contrast to earlier studies in south western Burkina Faso where indoor resting was more common (~ 67%55). The Human Blood Index (~ 65%) of these An. gambiae s.l. populations was also lower than previously reported in Burkina Faso (> 77%56) and Benin (> 90%57). Thus, although there was no clear evidence of mosquito behavioural change across the two years of this study, comparison with historical and wider regional data suggest that slower acting behavioural adaptations may be occuring in tandem with more rapid rises in insecticide resistance.
LLINs have also been hypothesized to generate selection on mosquito biting times; with vectors shifting to bite earlier in the evening before people go to bed58 to avoid this intervention. Here, An. gambiae s.l. still exhibited the characteristic “late night” pattern of biting, with no evidence of a shift over the study. The biting pattern observed here is consistent with early work on An. gambiae s.l. before mass LLIN use which also found biting rates peaked around 00 h12,59. Similarly, a study in western Kenya found An. gambiae s.l. and An. funestus continued biting late in the night even in the presence of LLINs60. However, other studies have detected substantial shifts in malaria vector biting times in association with interventions, including a recent report from Senegal of An. funestus biting earlier in the morning and even into daylight hours61. Adaptations in biting time may arise more rapidly in other settings due to variation in local ecology and background levels of IR.
Some previous studies that have reported shifts in malaria vector behaviour (e.g.27,62) did not identify An. gambiae s.l. to species level, thus preventing interpretation of whether changes were due to within-species adaptations or ecological shifts in species composition (e.g. reduction in the more endophagic An. gambiae relative to more exophagic An. arabiensis). While behavioural phenotypes remained relatively fixed within vector species in this study, there was evidence of a longitudinal shift in malaria vector species composition characterized by a reduction in An. coluzzii relative to An. gambiae. The proportion of outdoor biting was slightly higher in An. gambiae (55%) than An. coluzzii (51%), thus the relative decline in An. coluzzii is consistent with the prediction that LLINs have a greater impact on endophagic vector species. For example, highly anthropophagic and endophagic vector species like An. gambiae63 and An. quadriannulatus64 declined more substantially than the more zoophagic and exophagic An. arabiensis following ITN introduction in East Africa. This adds to the growing body of evidence from across Africa showing that LLINs can provoke shifts in malaria vector species communities. Such ecological shifts may occur more rapidly than longer-term behavioural adaptations within species.
Taken in combination, these results suggest that IR may be the first line of defence to LLINs compared to changes in mosquito behaviour, and/or that behavioural phenotypes have less capacity for adaptation than the traits underpinning IR. Differences in the rate of change in IR and mosquito behavioural traits also likely reflect their genetic basis. Mosquito behaviours are likely complex multigenic traits65, governed by many genes (some possibly antagonistic) that may limit capacity to respond quickly to selection. There is some evidence that behaviours like human host choice65 and outdoor/indoor biting or resting66 have a genetic basis in African malaria vectors. The genetic basis of IR traits is well established18,67, with some IR mechanisms linked to a single point mutation (e.g. Knock-down resistance16; indicating this type of resistance can respond rapidly and efficiently to selection. Vector populations in Burkina Faso have a high frequency of target site mutations (e.g. frequency of the L1014F type > 0.868) which could have provided the foundation for quicker adaptation to insecticides in contrast to slower development of behavioural resistance.
The potential epidemiological impacts of the vector adaptations observed here are complex. On one hand, results indicate that use of effective LLINs during typical sleeping hours (10 pm–5 am) can still effectively prevent the bulk of human exposure to malaria vectors (~ 85%) in this setting. Additionally, vector abundance, sporozoite infection rates and the associated Entomological Inoculation Rate fell between the two years of this study; suggesting a transmission decline. However, this degree of protection expected from LLIN use (85%) is somewhat lower than estimated in other parts of Burkina Faso (90%, in 2002–2004), and Africa (95–99%69). Furthermore, the longitudinal changes in vector populations described here could further diminish LLIN impact in several ways. First, the intensification of IR is likely to erode the community protection afforded to non-net users. Second the proportion of exposure preventable by using LLINs (πi) was predicted to decline over the study period. Even if the proportion of human exposure preventable by LLIN use remained at current levels, high levels of residual transmission will persist because of the relatively high baseline abundance and malaria infection rates in these vector populations. People in this area are estimated to be exposed to between ~ 29 infective bites per person per year even if they sleep under an LLIN between 10 pm–5 am. The small shifts in PfƖ and πi observed through time could further increase this exposure. Interventions that tackle both insecticide resistant and outdoor biting mosquitoes will thus be needed to tackle residual transmission.
Conclusions
In a longitudinal study in Burkina Faso, we show that IR increases more rapidly than behavioural adaptations following the mass distribution of insecticidal nets. Although mosquito behaviours stayed relatively stable over the study, baseline traits indicated a higher capacity for ‘behavioural avoidance’ of nets (through outdoor biting and resting) than historical data. This highlights the possibility that behavioural traits are adapting but at a more gradual rate than physiological resistance. As most human exposure to infected mosquitoes still occurs indoors in this and other African settings, effective indoor interventions should still be prioritized including the use of novel insecticide classes on nets, sprays and non-insecticidal approaches. However, the proportion of exposure occurring outdoors is notable and increasing. Supplementary measures tackling human exposure in and outdoors will thus be required to make further progress and tackle residual transmission in this and other high burden African settings.
Methods
Study site
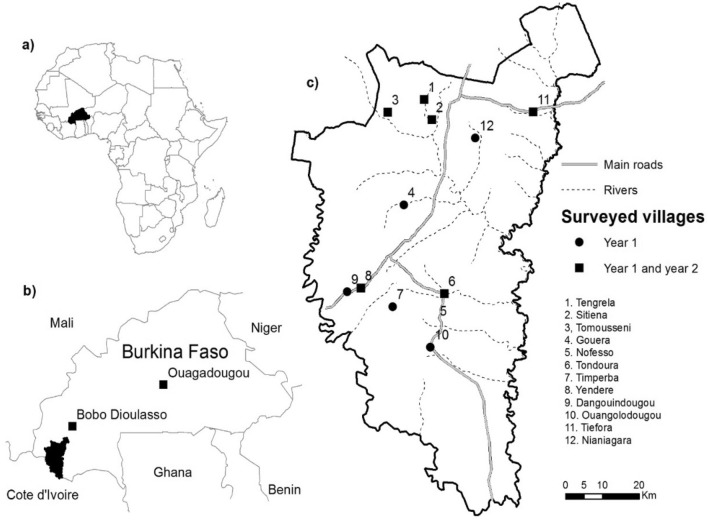
This study was conducted in 12 villages within the Banfora District, in the Cascades Region, south-western Burkina Faso (Supplementary Table 5, Fig. 6). In 2018, the population size of the region was 822,44570. The region has a humid savannah climate characterized by a rainy (May to October) and dry season (November to April). Annual rainfall ranged from 600 to 900 mm; with a mean temperature of ~ 26 °C [15.7 °C–38.84 °C] and humidity of ~ 62% [15.11–99.95%] during the study. Malaria is endemic in the region, with the incidence of Plasmodium falciparum being ~ 3 episodes per child (6–14 years old) during the six month annual transmission season32.
Figure 6.
Map of the 12 study villages. (a) location of Burkina Faso within Africa, (b) study area in the Cascades Region in Burkina Faso, and (c) villages where mosquito collection took place. Circles represent the villages sampled for 18 months and squares represent the longer-term study sites where sampling was extended to 26 months. The map was generated using QGIS 3.1671. Background layers were downloaded for OpenStreetMap72, villages were digitalised by the authors using GPS coordinates collected in the field using a GARMIN eTREX 10 GPS.
Insecticide resistance monitoring
We aimed to measure IR in all 12 original study sites at least once during the wet and dry season of each year. Due to the limited availability of larval habitats in some villages and months (especially during the dry season), IR monitoring was only possible in 9 villages (Supplementary Tables 6 and 7). Larvae were collected from aquatic habitats and reared to adulthood under standard conditions73. Insecticide resistance assays were performed by exposing groups of 22–27 adult female An. gambiae s.l. to the World Health Organization discriminating dose for deltamethrin (DD = 0.05%, a concentration that can kill 99.9% of a susceptible population74), 5 times (0.25%), 10 times (0.5%) and 15 times (0.75%) the DD respectively (Supplementary Table 6). Mosquitoes were exposed to insecticide-treated papers obtained from the WHO/Vector Control Research Unit, University Sains Malaysia75, following WHO guidelines for Tube bioassays74.
Assessment of human biting rate and behaviours
Vector surveillance was conducted to measure human biting rates (HBR) and mosquito resting behaviours bi-monthly at all 12 sites for 16 months (Oct 2016–Feb 2018, Fig. 6). Additionally, surveillance continued for a further 10 months (Feb–Dec 2018) in a subset of 6 villages to generate longer-term data. The longer-term study villages were selected to achieve a relatively broad spatial distribution.
Host-seeking mosquitoes were sampled twice a month in each village from two different households per day using Human Landing Catches (HLC). Here, men collected mosquitoes landing on their exposed leg using a mouth aspirator and flash torches76. Collection took place both inside houses and outdoors (the peridomestic area) from 7 pm to 6 am each night. During each hour of collection, participants caught mosquitoes for 45 min, followed by a 15-min rest break. Participants involved in mosquito collections rotated between indoor and outdoor trapping stations each hour to avoid confounding location with individual differences in attractiveness to mosquitoes.
In addition, Resting Box Traps (RBTs) were placed in and outside houses77 to sample resting mosquitoes. These RBTs were made locally using 20 L plastic buckets, with their inner surface covered with moistened black cotton cloth to create a high contrast and humid environment. On each night of collections, two RBTs were placed in the same households where HLCs took place (set within different houses in the compound). Inside houses, RBTs were placed on the floor in a relatively shaded corner of the sitting room. Two RBTs were also set outdoors at ~ 8 m from houses to capture outdoor resting mosquitoes. RBTs were set up at approximately 7 pm and emptied the following morning (~ 5 am) using electrical aspirators.
Mosquito processing
Mosquitoes collected in HLCs and RBTs were transferred to an insectary in Banfora town and sorted to species complex level using morphological keys78. Of those identified as belonging to the malaria vector group Anopheles gambiae s.l., a subset of 7852 females (~ 20% of total) were selected to provide a representative sample from each month, village, trapping location (indoor vs outdoor) for identification to species level by PCR79. The subsampling strategy is described elsewhere80. Furthermore, malaria infection in mosquitoes was assessed by testing for the presence of Plasmodium falciparum circumsporozoite protein (CSP) in their head and thoraxes using a monoclonal sandwich Enzyme Linkage Immuno-Sorbent Assay (ELISA) developed by81. Additionally, female An. gambiae s.l. from the RBT collections were visually graded according to their repletion status (abdominal condition) into categories of blood-fed, unfed, gravid, and half gravid82. Blood-meal identification was carried out on blood-fed An. gambiae s.l. to determine the origin of their blood-meal (human, cattle or both83) using a direct Enzyme Linkage Immuno-Sorbent Assay (ELISA84). The degree of “anthrophagy” in vectors was estimated in terms of the “human blood index” (HBI)85, defined as the proportion of identified blood meals taken from humans. In ELISA tests (for sporozoite and blood meal source identification), two technical replicates of each sample were run in two different microplates at the same time and retested in cases where the first result was ambiguous. The absorbance of the solutions/reactions at the end of each ELISA was measured using microplate reader (ELX808; BIO-TEK) at 450 nm. To avoid any false positives (due to background noise), a sample was considered positive for an assay when its optical density (OD) was twofold higher than the average of the OD of both negative controls. Positive controls were also used in all ELISAs to ensure the procedure was working.
Data analysis
Statistical analysis was conducted with the aim of testing for “longitudinal shifts” (over 26 months) in key indicators of vector demography, species composition, insecticide resistance (IR) and behaviour using Generalized Additive Mixed-Models (GAMMs) in the R statistical software (version 3.6.1 of 2019-07-05)86. Long-term trends in all ecological, behavioural and insecticide resistance variables were assessed after controlling for spatial (between-village) and environmental (season, temperature and humidity) variations. The key response variables were: (i) the proportion of mosquitoes dying within 24-h post-exposure to 1X DD, 5X DD and 10X DD, (ii) human biting rate (HBR) and species composition (proportion of An. coluzzii in An. gambiae s.l.), (iii) proportion of outdoor biting and resting, (iv) median biting time, (v) proportion of human exposure to bites, vi) the sporozoite rate and vii) the entomological inoculation rates.
A model was constructed for each response variable (Supplementary Table 8) to assess longer-term trends and seasonality occurring over the 26 months of the study. For the longer-term trend, a temporal variable was created by assigning a continuous value to each day of collections from the first (1 October 2016) until the last (4 December 2018), hereafter called “Longer_term” (Supplementary Table 8). This was incorporated into models as a continuous covariate to test for evidence of a consistent temporal rise or decline after accounting for seasonal and spatial variation. Seasonality was incorporated by fitting a smoothing spline where each day of the year was classified on a scale running from 1 (set as January 1st) to 365 (December 31st) hereafter called “Season” (Supplementary Table 8). Spatial variation was also assessed by fitting village as a fixed effect. The mean nightly temperature and humidity at collection households were derived from hourly values (7 pm–6 am) obtained from data loggers and included as additional explanatory variables (Supplementary Table 8).
However, due to the low number of mosquitoes caught in RBTs, it was not possible to build a model for testing for spatial and long-term variation in the abundance of resting mosquitoes. Mosquito host choice was assessed in terms of the Human Blood Index (HBI) defined as the proportion of blood-fed An. gambiae s.l. that tested positive for human blood out of the total from which blood-meals were identified (N = 94). We also calculated the Entomological Inoculation Rate (EIR); defined as the average number of infective bites a person (ibppn) would expect to receive from An. gambiae s.l. in a given location per year) for the six villages that were monitored over 2 years. Here EIR was calculated as the product of the mean nightly human biting rate (ma) and vector sporozoite infection rates (SR) multiplied by 365 days41 monitored over two years.
Mosquito mortality rate after exposure to insecticide
Insecticide resistance was measured in terms of mosquito mortality 24 h after exposure to deltamethrin (different doses). According to World Health Organisation (WHO) guidelines74, for a test to be validated the mortality should be less than 5% in the control groups. If, mortality in the control group ranges between 5 and 20%, the guidelines recommend using the Abbott formula74 to correct for anticipated background mortality. If the mortality rate in the control group exceeds 20%, results from the test should be discarded. Here, average mortality rates recorded in the control group on the same day as insecticide exposures were performed were all below 5%, and could thus be retained for use without correction. We constructed separate models of mosquito mortality for each of the insecticide concentrations used (Models 1 to 4, Supplementary Table 8). As the response variable is all these analyses was binary (e.g ‘0’ is alive, ‘1’ is dead); all models used a binomial distribution. Each model tested for seasonal and long-term variation in mosquito mortality following insecticide exposure (Supplementary Table 8), and variation between villages.
Anopheles gambiae s.l. species composition, biting and resting behaviours
Models were constructed to test for longer-term (systematic increase or decrease across the 26 month study period) and seasonal variation in species composition (Model 5), HBR (Model 6) and outdoor biting (Model 7 and 8) and resting (Model 12) as described in Supplementary Table 8. Additionally, long-term and seasonal variation in the mean mosquito biting times (both An gambiae s.l. overall, and by species; Model 10–11). Model 6 (Supplementary Table 8), for the HBR included an interaction term between village and year in addition to other main effects. Model for mosquito median biting time includes an interaction between village and location. Vector species composition was defined as the proportion of An. coluzzii within An. gambiae complex. This was defined as binary proportion as only two major vector species (An. coluzzii and An. gambiae) account for almost all within the An. gambiae complex in this region. The proportion of outdoor biting was defined as the proportion of An. gambiae s.l. collected in outdoor HLCs out of the total in outdoor and indoor HLCs. As described in Supplementary Table 8, different distributions were incorporated into models depending on the nature of the response variable (e.g count data fit to Poisson or Negative Binomial model; proportion data fit to a binomial distribution).
Human exposure to Anopheles gambiae s.l. bites
Data on the time and location of biting were combined for estimating two metrics of human exposure to bites from An. gambiae s.l.: the proportion of An. gambiae s.l. caught during hours when typically, ≥ 50% of people were indoors and likely to be in bed (PfƖ). Based on visual surveys described in80 over 672 households, we estimated that > 50% of residents were indoors between 10 pm and 5 am consistently across the study period; although more recent, detailed anthropological investigation indicates time indoors may vary substantially between individuals and seasons and be lower assumed here48. The Pfl (Model 14 in Supplementary Table 8) was estimated following42 by dividing the total number of s.l. collected indoors and outdoors when ≥ 50% of people were indoors (10 pm to 5 am) by the total number collected that night. The proportion of human exposure to bites that occur indoors (πi) was then calculated by dividing the number of An. gambiae s.l. collected indoors between 10 pm and 5 am by itself plus those collected outdoors when > 50% of residents were outdoors and thus unprotected by LLINs (between 7–10 pm and 5–6 am (Model 15, Supplementary Table 8). As πi and Pfl are proportions, they were modelled following a binomial distribution.
Within the R statistical software86 GAMMs within the ‘mgcv package’87 augmented with the lme4 package88 known as GAMM4 were used to test for associations between IR, all vector ecological and behavioural metrics and explanatory variables of village, season, “longer-term” trend and environmental factors. Details of fixed and random effects and distribution are given in Supplementary Table 8.
Ethical approval
Ethical clearance was obtained from the Ethical Committee for research in Health of the Ministry of Health of Burkina Faso (EC V3.0_CERS N°2016-09-097) and the Institutional Bioethical Committee of the local research institution (National Malaria Research and Training Centre, CNRFP) under EC V3.0_ N°2016-026/MS/SG/CNRFP/CIB) and the Liverpool School of Tropical Medicine (Certificate 16-038). Prior to starting the research, the project aims, and objectives were explained to community leaders in each village. Signed informed consent was also obtained from all household owners where mosquitoes were collected, and volunteers who took part in mosquito collections by HLC. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Supplementary Information
Acknowledgements
We thank all the head of the villages, all the communities, household owners and the volunteers for their collaboration and help in data collection. Thank also to all the field and lab teams for the great job in the data collection and processing.
Author contributions
A.S. designed the study, conducted the field data collection, did the molecular analysis, performed the data analysis, interpreted the results, and drafted the manuscript. W.M.G., N.F.S., H.R. and H.M.F. contributed to the design of the study and the sampling protocol and provided comments upon the manuscript. H.M.F. was a major contributor in writing this manuscript. M.T., F.C. and P.O. contributed to the molecular analysis in the labs. L.N., J.M., contributed to the data analysis. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Wellcome Trust under Grant Agreement Number [200222/Z/15/Z] MiRA through the “Improving the efficacy of malaria prevention in an insecticide resistant Africa (MiRA)”.
Data availability
All data are provided as Figures and Supplementary materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96759-w.
References
- 1.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Pluess B, et al. Indoor residual spraying for preventing malaria (Review) Cochrane Rev. 2010 doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Guidelines for malaria vector control. (World Health Organization, 2019). https://www.who.int/malaria/publications/atoz/9789241550499/en/. Accessed 08 Sept 2019. [PubMed]
- 4.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Killeen GF, et al. Made-to-measure malaria vector control strategies: Rational design based on insecticide properties and coverage of blood resources for mosquitoes. Malar. J. 2014;13(1):146. doi: 10.1186/1475-2875-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waite JL, et al. Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci. Rep. 2017;7:40551. doi: 10.1038/srep40551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke MK, et al. A bite before bed’: Exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar. J. 2015;14(1):259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milali MP, Sikulu-Lord MT, Govella NJ. Bites before and after bedtime can carry a high risk of human malaria infection. Malar. J. 2017;16(1):91. doi: 10.1186/s12936-017-1740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pates H, et al. Differential behaviour of Anopheles gambiae sensu stricto (Diptera: Culicidae) to human and cow odours in the laboratory. Bull. Entomol. Res. 2001;91(4):289–296. doi: 10.1079/BER200198. [DOI] [PubMed] [Google Scholar]
- 10.Costantini C, et al. Odor-mediated host preferences of West African mosquitoes, with particular reference to malaria vectors. Am. J. Trop. Med. Hyg. 1998;58(1):56–63. doi: 10.4269/ajtmh.1998.58.56. [DOI] [PubMed] [Google Scholar]
- 11.Gillies MT, DeMeillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) South African Institute for Medical Research; 1968. [Google Scholar]
- 12.Hamon, J. Les moustiques anthropophiles de la région de Bobo-Dioulasso (République de Haute-Volta): Cycles d'agressivité et variations saisonnières. Ann. Soc. Entomol. (1963).
- 13.Nyarango PM, et al. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar. J. 2006;5(1):33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO, World malaria report 2019, in Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019. Accessed 17 Dec 2019.
- 15.Hancock PA, et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020;18(6):e3000633. doi: 10.1371/journal.pbio.3000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Torres D, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae ss. Insect Mol. Biol. 1998;7(2):179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 17.Balabanidou V, et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2016;113(33):9268–9273. doi: 10.1073/pnas.1608295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edi CV, et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PloS Genet. 2014;10(3):e1004236. doi: 10.1371/journal.pgen.1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreppel K, et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-71187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moiroux N, et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS ONE. 2014;9:e104967. doi: 10.1371/journal.pone.0104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy MR, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 2011;10(1):184–184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozendaal J, et al. Behavioral responses of Anopheles darlingi in Suriname to DDT residues on house walls. J. Am. Mosq. Control Assoc. 1989;5(3):339–350. [PubMed] [Google Scholar]
- 23.Mwangangi JM, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar. J. 2013;12(1):1. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sougoufara S, et al. Shift in species composition in the Anopheles gambiae complex after implementation of long-lasting insecticidal nets in Dielmo, Senegal. Med. Vet. Entomol. 2016;30(3):365–368. doi: 10.1111/mve.12171. [DOI] [PubMed] [Google Scholar]
- 25.Moiroux N, et al. Changes in Anopheles funestus biting behaviour following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- 26.Matowo NS, et al. Using a new odour-baited device to explore options for luring and killing outdoor-biting malaria vectors: A report on design and field evaluation of the Mosquito Landing Box. Parasit. Vectors. 2013;6(1):1–16. doi: 10.1186/1756-3305-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell TL, et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011;10:1. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherrard-Smith E, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. 2019;116(30):15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dambach P, et al. Reduction of malaria vector mosquitoes in a large-scale intervention trial in rural Burkina Faso using Bti based larval source management. Malar. J. 2019;18(1):311. doi: 10.1186/s12936-019-2951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayili K, et al. Evaluation of efficacy of Interceptor (R) G2, a long-lasting insecticide net coated with a mixture of chlorfenapyr and alpha-cypermethrin, against pyrethroid resistant Anopheles gambiae s.l. in Burkina Faso. Malar. J. 2017;16:9. doi: 10.1186/s12936-017-1846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oumbouke WA, et al. Evaluation of an alpha-cypermethrin + PBO mixture long-lasting insecticidal net VEERALIN® LN against pyrethroid resistant Anopheles gambiae s.s.: an experimental hut trial in M’bé, central Côte d’Ivoire. Parasit. Vectors. 2019;12(1):544. doi: 10.1186/s13071-019-3796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaro JB, et al. A cohort study to identify risk factors for Plasmodium falciparum infection in Burkinabe children: Implications for other high burden high impact countries. Malar. J. 2020;19(1):371. doi: 10.1186/s12936-020-03443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabire K, et al. Anopheles funestus (Diptera: Culicidae) in a humid savannah area of western Burkina Faso: Bionomics, insecticide resistance status, and role in malaria transmission. J. Med. Entomol. 2007;44(6):990–997. doi: 10.1093/jmedent/44.6.990. [DOI] [PubMed] [Google Scholar]
- 34.Badolo A, et al. Three years of insecticide resistance monitoring in Anopheles gambiae in Burkina Faso: Resistance on the rise? Malar. J. 2012;11(1):1. doi: 10.1186/1475-2875-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamon J, et al. Présence dans le Sud-Ouest de la Haute-Volta d'une Population d'Anopheles gambiae" A" résistante au DDT. Organisation mondiale de la Santé; 1968. [PubMed] [Google Scholar]
- 36.Hamon J, et al. Présence dans le Sud-Ouest de la Haute-Volta de populations d'Anopheles funestus Giles résistantes à la dieldrine. Med. Trop. 1968;28(2):222–226. [PubMed] [Google Scholar]
- 37.Toe H. Characterisation of Insecticide Resistance in Anopheles gambiae from Burkina Faso and Its Impact on Current Malaria Control Strategies. University of Liverpool; 2015. [Google Scholar]
- 38.Namountougou M, et al. Insecticide resistance mechanisms in Anopheles gambiae complex populations from Burkina Faso, West Africa. Acta Trop. 2019;197:105054. doi: 10.1016/j.actatropica.2019.105054. [DOI] [PubMed] [Google Scholar]
- 39.Toe KH, et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 2014;20:10. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beier JC, Killeen G, Githure JI. Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 41.Smith DL, et al. The entomological inoculation rate and Plasmodiumfalciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Killeen GF, et al. Quantifying behavioural interactions between humans and mosquitoes: Evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect. Dis. 2006;6:161. doi: 10.1186/1471-2334-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huho B, et al. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int. J. Epidemiol. 2013;42(1):235. doi: 10.1093/ije/dys214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robert V, Carnevale P. Influence of deltamethrin treatment of bed nets on malaria transmission in the Kou valley, Burkina Faso. Bull. World Health Organ. 1991;69(6):735. [PMC free article] [PubMed] [Google Scholar]
- 45.Dabire K, et al. Year to year and seasonal variations in vector bionomics and malaria transmission in a humid savannah village in west Burkina Faso. J. Vector Ecol. 2008;33(1):70–75. doi: 10.3376/1081-1710(2008)33[70:YTYASV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Epopa PS, et al. Seasonal malaria vector and transmission dynamics in western Burkina Faso. Malar. J. 2019;18(1):113. doi: 10.1186/s12936-019-2747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dambach P, et al. Nightly biting cycles of anopheles species in rural northwestern Burkina Faso. J. Med. Entomol. 2018;55(4):1027–1034. doi: 10.1093/jme/tjy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guglielmo, F. et al. Quantifying variation in exposure risk to mosquito bites at the individual level in Burkina Faso. Malar. J. (2020) [DOI] [PMC free article] [PubMed]
- 49.Edi CA, et al. Long-term trends in Anopheles gambiae insecticide resistance in Côte d’Ivoire. Parasit. Vectors. 2014;7(1):500. doi: 10.1186/s13071-014-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuseini G, et al. Evaluation of the residual effectiveness of Fludora fusion WP-SB, a combination of clothianidin and deltamethrin, for the control of pyrethroid-resistant malaria vectors on Bioko Island, Equatorial Guinea. Acta Trop. 2019;196:42–47. doi: 10.1016/j.actatropica.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Rongsriyam Y, Busvine J. Cross-resistance in DDT-resistant strains of various mosquitoes (Diptera, Culicidae) Bull. Entomol. Res. 1975;65(3):459–471. doi: 10.1017/S0007485300006131. [DOI] [Google Scholar]
- 52.Chandre F, et al. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull. World Health Organ. 1999;77(3):230–234. [PMC free article] [PubMed] [Google Scholar]
- 53.Bagi J, et al. When a discriminating dose assay is not enough: measuring the intensity of insecticide resistance in malaria vectors. Malar. J. 2015;14(1):210. doi: 10.1186/s12936-015-0721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert, V., et al., Etude des taux de parturité et d’infection du complexe Anopheles gambiae dans la rizière de la vallée du Kou, Burkina Faso. Le paludisme en Afrique de l’Ouest, 1991: p. 17.
- 55.Pombi M, et al. The Sticky Resting Box, a new tool for studying resting behaviour of Afrotropical malaria vectors. Parasit. Vectors. 2014;7(1):247. doi: 10.1186/1756-3305-7-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnémé, A. et al. Anopheline occurrence and the risk of urban malaria in the city of Ouagadougou, Burkina Faso. (2019).
- 57.Akogbéto MC, et al. Blood feeding behaviour comparison and contribution of Anopheles coluzzii and Anopheles gambiae, two sibling species living in sympatry, to malaria transmission in Alibori and Donga region, northern Benin, West Africa. Malar. J. 2018;17(1):307. doi: 10.1186/s12936-018-2452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wamae PM, et al. Early biting of the Anopheles gambiae s.s. and its challenges to vector control using insecticide treated nets in western Kenya highlands. Acta Trop. 2015;150:136–142. doi: 10.1016/j.actatropica.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Gillies M. Age-groups and the biting cycle in Anopheles gambiae: A preliminary investigation. Bull. Entomol. Res. 1957;48(3):553–559. doi: 10.1017/S0007485300002728. [DOI] [Google Scholar]
- 60.Bayoh M, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit. Vectors. 2014;7:380. doi: 10.1186/1756-3305-7-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sougoufara S, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: A new challenge to malaria elimination. Malar. J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyers JI, et al. Increasing outdoor host-seeking in Anopheles gambiae over 6 years of vector control on Bioko Island. Malar. J. 2016;15(1):1. doi: 10.1186/s12936-016-1286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayoh MN, et al. Anopheles gambiae: Historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 2010;9(1):62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chinula D, et al. Proportional decline of Anopheles quadriannulatus and increased contribution of An. arabiensis to the An. gambiae complex following introduction of indoor residual spraying with pirimiphos-methyl: An observational, retrospective secondary analysis of pre-existing data from south-east Zambia. Parasit. Vectors. 2018;11(1):544. doi: 10.1186/s13071-018-3121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Main BJ, et al. The genetic basis of host choice and resting behavior in the major African malaria vector Anopheles arabiensis. BioRxiv. 2016;2016:044701. doi: 10.1371/journal.pgen.1006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coluzzi M, et al. Behavioral divergences between mosquitos with different inversion karyotypes in polymorphic populations of Anopheles gambiae complex. Nature. 1977;266:832–833. doi: 10.1038/266832a0. [DOI] [PubMed] [Google Scholar]
- 67.Elanga-Ndille E, et al. The G119S Acetylcholinesterase (Ace-1) target site mutation confers carbamate resistance in the major malaria vector Anopheles gambiae from cameroon: A challenge for the coming IRS implementation. Genes. 2019;10(10):790. doi: 10.3390/genes10100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toé KH, et al. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16(1):146. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finda MF, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14(6):e0217414. doi: 10.1371/journal.pone.0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.INSD. Annuaire sttistique 2018 Burkina Faso: Institut national de la statistique et de la démographie (2019).
- 71.QGIS.org. QGIS Geographic Information System. QGIS Association. http://www.qgis.org. (2021).
- 72.OpenStreetMap. OpenStreetMap contributors. https://www.openstreetmap.org. (2021).
- 73.Benedict MQ. The Molecular Biology of Insect Disease Vectors. Springer; 1997. Care and maintenance of anopheline mosquito colonies; pp. 3–12. [Google Scholar]
- 74.WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes: 2nd edition. (World Health Organization, 2016). http://www.who.int/malaria.
- 75.WHO. Supplies for Monitoring Insecticide Resistance in Disease Vectors. (World Health Organization: Parasitic Diseases and Vector Control (PVC)/Communicable Disease Control, Prevention and Eradication (CPE), 2001).
- 76.M.W. Service . Mosquito Ecology Field Sampling Methods. Elsevier Science Publishers; 1993. [Google Scholar]
- 77.Kreppel KS, et al. Comparative evaluation of the Sticky-Resting-Box-Trap, the standardised resting-bucket-trap and indoor aspiration for sampling malaria vectors. Parasit. Vectors. 2015;8(1):1–5. doi: 10.1186/s13071-015-1066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gillies, M. & Coetzee, M. A Supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). (1987).
- 79.Fanello C, Santolamazza FD, DellaTorre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Medi. Vet. Entomol. 2002;16(4):461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 80.Sanou A, et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar. J. 2019;18(1):386. doi: 10.1186/s12936-019-3030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wirtz R, et al. ELISA method for detecting Plasmodium falciparum circumsporozoite antibody. Bull. World Health Organ. 1989;67(5):535. [PMC free article] [PubMed] [Google Scholar]
- 82.WHO. Manual on Practical Entomology in Malaria. Part II. Methods and Techniques. 84–85 (WHO Division of Malaria and other Parasitic Diseases, 1975).
- 83.Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull. World Health Organ. 1964;30(2):241. [PMC free article] [PubMed] [Google Scholar]
- 84.Beier JC, et al. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. J. Med. Entomol. 1988;25(1):9–16. doi: 10.1093/jmedent/25.1.9. [DOI] [PubMed] [Google Scholar]
- 85.Kiszewski A, et al. A global index representing the stability of malaria transmission. Am. J. Trop. Med. Hyg. 2004;70(5):486–498. doi: 10.4269/ajtmh.2004.70.486. [DOI] [PubMed] [Google Scholar]
- 86.Team, R.C. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2018). https://www.R-project.org/.
- 87.Wood, S. & Wood, M.S. The mgcv package.https://www.R-project.org/ (2007).
- 88.Bates, D. et al. Fitting linear mixed-effects models using lme4.http://arxiv.org/abs/1406.5823 (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided as Figures and Supplementary materials.