Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is caused by germ line mutations in at least three ADPKD genes. Two recently isolated ADPKD genes, PKD1 and PKD2, encode integral membrane proteins of unknown function. We found that PKD2 upregulated AP-1-dependent transcription in human embryonic kidney 293T cells. The PKD2-mediated AP-1 activity was dependent upon activation of the mitogen-activated protein kinases p38 and JNK1 and protein kinase C (PKC) ɛ, a calcium-independent PKC isozyme. Staurosporine, but not the calcium chelator BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetate], inhibited PKD2-mediated signaling, consistent with the involvement of a calcium-independent PKC isozyme. Coexpression of PKD2 with the interacting C terminus of PKD1 dramatically augmented PKD2-mediated AP-1 activation. The synergistic signaling between PKD1 and PKD2 involved the activation of two distinct PKC isozymes, PKC α and PKC ɛ, respectively. Our findings are consistent with others that support a functional connection between PKD1 and PKD2 involving multiple signaling pathways that converge to induce AP-1 activity, a transcription factor that regulates different cellular programs such as proliferation, differentiation, and apoptosis. Activation of these signaling cascades may promote the full maturation of developing tubular epithelial cells, while inactivation of these signaling cascades may impair terminal differentiation and facilitate the development of renal tubular cysts.
Human autosomal dominant polycystic kidney disease (ADPKD), one of the most prevalent inherited disorders with an incidence of 1 in 500 to 1 in 1,000 individuals, is characterized by the development of gradually enlarging renal epithelial cysts that progressively impair renal function (16, 17). The vast majority of patients are affected by mutations in one of three ADPKD genes (7, 27, 43). PKD1 is mutated in more than 85% of ADPKD patients, and it encodes a large integral glycoprotein with multiple transmembrane domains and a large extracellular domain with significant homology to membrane proteins involved in cell-cell and/or cell-matrix interactions (1, 2, 20, 21, 45). PKD2 encodes a 968-amino-acid integral membrane protein with six transmembrane domains, and it is mutated in approximately 10 to 15% of all patients (35). Despite homologies to the family of voltage-gated calcium channel α1 subunits, the function of PKD2 remains elusive.
It has been hypothesized that PKD1 and PKD2 function in a common signaling pathway. Patients with PKD1 and those with PKD2 have a similar clinical phenotype, while both PKD1−/− and PKD2−/− mice develop kidney and liver cysts resembling the human phenotype (30, 54). Recent studies have shown that PKD1 and PKD2 interact via their C-terminal cytoplasmic domains (41, 52). Thus, PKD1 and PKD2 appear to work in conjunction with each other, but it remains unclear by which mechanism they control tubular proliferation and differentiation and thereby prevent cyst formation. Cystic epithelial cells are thought to be incompletely differentiated and persistently proliferative. Several proto-oncogenes, including c-erbB-2, c-Fos, c-Ki-ras, and c-myc, are abnormally regulated in cyst cells derived from ADPKD patients and animal models of cystic renal disease (6, 17, 28, 50, 51). It has been proposed that ADPKD gene products control proto-oncogenes and cellular programs directing cell cycle progression and cellular differentiation. Consistent with this hypothesis, we recently demonstrated that the C-terminal cytoplasmic domain of PKD1 stimulates protein kinase C (PKC) α-dependent and c-Jun N-terminal protein kinase (JNK)-dependent activation of AP-1 (1). We now report that PKD2 stimulates the phosphorylation of c-Jun and the induction of AP-1 activity through signaling molecules partially distinct from those involved in PKD1-mediated activation. Furthermore, a transcriptionally inactive PKD1 truncation greatly enhanced the PKD2-mediated activation of AP-1.
MATERIALS AND METHODS
Reagents and plasmids.
Genistein, staurosporine, wortmannin (Calbiochem, La Jolla, Calif.), and BAPTA-AM [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetate–acetoxymethyl ester] (Molecular Probe, Eugene, Oreg.) were used at various concentrations. A PKD2 expression vector was constructed in CDM8 by assembling the entire coding region of PKD2 from the clone K1-1 (kindly provided by S. Somlo) and the EST clone yj63h09 (Genome Systems, St. Louis, Mo.). The luciferase constructs, the CD16.7-PKD1 fusion protein, and the Cdc42, Rac1, RhoA, HA-p38, and PKC α constructs were recently described (1, 52). The hemagglutinin (HA)-tagged p42 was kindly provided by J. S. Gutkind, and a dominant-negative form of PKC ɛ was kindly provided by G. M. Cooper. Dominant-negative mutants of p42 and p44 were kindly provided by M. H. Cobb. Dominant-negative mutants of MKK3 and MKK6 were kindly provided by R. J. Davis.
Luciferase assay.
Human embryonic kidney (HEK) 293T cells seeded in 12-well plates were transiently transfected by the calcium phosphate method with a luciferase reporter construct, a β-galactosidase expression vector (kindly provided by C. Cepko), and a PKD2 expression vector. The total DNA amount was 1.0 or 1.5 μg/well. Cells were serum starved for 24 h, harvested in cold phosphate-buffered saline, and lysed in 100 μl of reporter lysis buffer (Promega, Madison, Wis.) for 15 min at room temperature. Lysates were centrifuged at 18,000 × g for 3 min to remove insoluble material. Luciferase activity was determined with a commercial assay system (Promega) following the manufacturer’s instructions and normalized for β-galactosidase activity to correct for the transfection efficiency. Pharmacological inhibitors at the indicated concentration were added for 6 h before the assay.
Western blot analysis.
Cells from one 10-cm dish were lysed 24 h after transfection in 1 ml of cold lysis buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, and a protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, Ind.), fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a polyvinylidene difluoride membrane. Western blot analysis was performed with an anti-PKD2 polyclonal antiserum, a polyclonal antibody against Jun family members (Santa Cruz Biotechnology, Santa Cruz, Calif.), or with a specific anti-phospho-c-Jun (Ser 63) (New England Biolabs, Beverly, Mass.) antibody, followed by incubation with the appropriate secondary antibodies. Immobilized antibodies were detected by enhanced chemiluminescence (Pierce, Rockford, Ill.). An MBP-PKD2 fusion protein containing amino acids 742 to 871 of PKD2 was utilized to generate a polyclonal rabbit antiserum. For Western blot analysis, the antiserum was protein A purified and used at a concentration of 1:2,000. This antiserum detects a specific band at approximately 110 kDa, the predicted size of PKD2. A comparison of HA-tagged PKD2 versions detected by the polyclonal rabbit antiserum and by a monoclonal antibody directed against the HA tag (Boehringer Mannheim) revealed that both versions have the same protein species.
AP-1 gel shift assay.
Electromobility shift assays were performed as previously described (4). Cells were transfected with plasmids encoding PKD2 and a vector control (CDM8), or they were stimulated with 100 nM phorbol myristate acetate (PMA) for 60 min. Cells were lysed in 500 μl of hypotonic buffer (HB) (20 mM HEPES [pH 7.9], 5 mM NaF, 1 mM Na2MoO4, 0.1 mM EDTA, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail) containing 0.2% Nonidet P-40. Nuclei were recovered through centrifugation and resuspended in 100 μl of HB containing 20% glycerol. Nuclear proteins were extracted for 30 min at 4°C in 200 μl of HB, 20% glycerol, and 0.8 M NaCl. Nuclear debris was removed by centrifugation; supernatants were aliquoted and stored at −80°C. Binding reactions were performed for 20 min with 5 μg of nuclear proteins in a final volume of 25 μl of binding buffer (2 mM HEPES [pH 7.9], 8 mM NaCl, 0.2 mM EDTA, 12% [vol/vol] glycerol, 5 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride), 1 μg of poly(dI-dC), and 2 × 104 cpm of 32P-labeled oligonucleotide containing the AP-1/tetradecanoyl phorbol acetate-responsive element (TRE) consensus sequence TGACTCA (Promega). For the supershift experiment, a monoclonal antibody that specifically recognized c-Jun (or polyclonal antisera that specifically recognized JunB) and JunD (Santa Cruz Biotechnology) were added. The mutant AP-1 oligonucleotide utilized in the gel shift assays contained a CA-to-TG substitution in the AP-1-binding motif (Santa Cruz Biotechnology). DNA-protein complexes were separated on a native 6% polyacrylamide gel and detected by autoradiography.
In vitro kinase assays.
Immune complex kinase assays were carried out as previously described (18). Briefly, 293T cells were cotransfected with HA-tagged mitogen-activated protein kinases (MAPKs) and the PKD2 construct at a 1:1 ratio. Cells from one 10-cm dish were lysed 24 h after transfection in 1 ml of cold lysis buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, and a protease inhibitor cocktail (Boehringer Mannheim). After centrifugation for 15 min at 4°C, the HA-tagged kinases were immunoprecipitated from the cleared lysate with 1 μg of anti-HA monoclonal antibody (Boehringer Mannheim) for 2 h at 4°C. Immune complexes were immobilized by adding 30 μl of Gamma-Bind Sepharose (Pharmacia, Piscataway, N.J.) and washed three times with lysis buffer and twice with kinase reaction buffer (25 mM HEPES, 20 mM MgCl2, 2 mM DTT, 0.1 mM Na3VO4 [pH 7.6]). The immunoprecipitates were resuspended in 30 μl of kinase reaction buffer containing 3 μg of substrates, PHAS-1 (Stratagene, La Jolla, Calif.) for HA-p38 and HA-p42 or GST-c-Jun(1–79) (Stratagene) for HA-JNK1. The assay was carried out in the presence of 20 μM unlabeled ATP and 10 μCi of [γ-32P]ATP for 30 min at 37°C and stopped by the addition of 30 μl of SDS sample buffer. The reaction mixture was fractionated by SDS–12% PAGE. Phosphorylated substrates were visualized by autoradiography. HA-tagged kinase immunoprecipitates were analyzed by SDS-PAGE and Western blot analysis by using anti-HA polyclonal antiserum (Santa Cruz Biotechnology), a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin antibody (Amersham, Arlington Heights, Ill.), and an enhanced chemiluminescence kit (Pierce).
Measurement of PKC activity.
Total PKC activity was determined as previously described (1), using a colorimetric PKC assay with neurogranin as a dye-labeled synthetic peptide substrate (Pierce). Cells were harvested and lysed on ice for 10 min in 45 μl of cold hypotonic buffer (1 mM HEPES, 5 mM MgCl2, 25 μg of leupeptin per ml, 25 μg of pepstatin per ml). Isotonicity was reestablished by adding 5 μl of HEPES (200 mM, pH 7.4) and 25 μl of an equilibrium buffer (20 mM HEPES, 5 mM MgCl2, 1 mM NaF, 0.1 mM Na3VO4). The PKC reaction was performed at 30°C for 30 min with 10 μl of cleared lysates, following the instructions of the manufacturer. The absorbance of the phosphorylated substrates was spectrophotometrically determined at 570 nm, and a microprotein assay (Bio-Rad, Hercules, Calif.) was used to normalize the PKC activities for the protein content.
In vitro kinase assay for immunoprecipitated PKC isozymes.
To determine the activity of individual PKC isozymes, in vitro kinase assays were performed essentially as previously described (38). HEK 293T cells were lysed 24 h after transfection with cold lysis buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, and a protease inhibitor cocktail (Boehringer Mannheim). PKC isoforms were isolated from the cleared lysates by using a 1-μg/ml concentration of monoclonal antibodies against the different PKC isoforms as indicated (Transduction Laboratories, Lexington, Ky.). The immune complexes were immobilized with 30 μl of Gamma-Bind Sepharose (Pharmacia), followed by three washes in lysis buffer and two washes in a kinase reaction buffer containing 50 mM HEPES (pH 7.6), 75 mM KCl, 1 mM Na3VO4, 10 mM MgCl2, and 0.1 mM CaCl2. The kinase reaction was performed for 20 min at 30°C in 50 μl of kinase buffer in the presence of 5 μCi of [γ-32P]ATP (10 mCi/mmol), 20 μM unlabeled ATP, and 5 μg of neurogranin as an exogenous substrate. Adding 5% acetic acid terminated the reactions. The incorporated radioactivity was determined after two washes with 75 mM phosphoric acid by liquid scintillation spectrophotometry with a phosphocellulose membrane (Pierce). PKC immunoprecipitates were analyzed by SDS-PAGE and Western blot analysis by using specific anti-PKC monoclonal and polyclonal antibodies (anti-PKC δ, λ/τ, and ɛ from Transduction Laboratories; anti-PKC ζ from Upstate Biotechnology; and anti-PKC μ from Santa Cruz Biotechnology) in combination with a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin antibody (Dako, Carpinteria, Calif.) and enhanced chemiluminescence (Pierce).
Statistical analysis.
Results were expressed as means ± standard deviations (SD). Analysis of variance with subsequent Scheffe’s test was used to determine significant difference in multiple comparisons. Values of P less than 0.05 were considered to be significant.
RESULTS
PKD2 induces AP-1 activity and triggers phosphorylation of c-Jun.
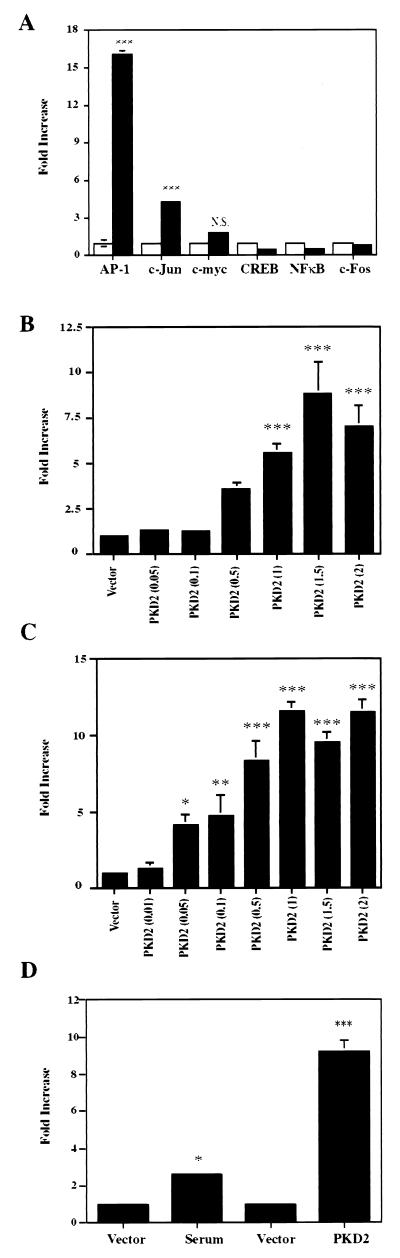
To identify potential signaling pathways of PKD2, we transiently coexpressed PKD2 in HEK 293T cells with the luciferase reporter constructs indicated in Fig. 1. PKD2 specifically induced AP-1 activity, activating AP-1 and the Jun2TRE reporter constructs (Fig. 1A). PKD2 transactivated both promoter constructs in a dose-dependent fashion (Fig. 1B and C). However, PKD2 had no effect on CREB, NF-κB, or c-Fos luciferase reporter constructs, while a weak but not significant activation was detectable for the c-myc promoter (<2-fold increase) (Fig. 1A). To demonstrate the physiological significance of the PKD2-mediated AP-1 activation, we tested the effect of PKD2 on a collagenase promoter construct containing only one AP-1 binding site. While serum induced a two- to threefold activation of this promoter, PKD2 induced a ninefold activation of the single AP-1-binding site (Fig. 1D). PKD2 protein levels were unaffected by the coexpression of different luciferase constructs; thus, variations in PKD2 levels could not account for the differences in transcriptional activities (data not shown).
FIG. 1.
PKD2 activates AP-1- and c-Jun (TRE)-dependent transcription. (A) PKD2 triggers activation of an AP-1 reporter construct containing four tandem AP-1-binding sites and a c-Jun reporter construct containing three repeats of the second TRE of the c-Jun promoter (Jun2TRE). HEK 293T cells were transfected with 1 μg of vector (CDM8) (white bars) or PKD2 (black bars), as well as luciferase reporter constructs for AP-1, c-Jun, c-myc, CREB, NF-κB, or c-Fos. Transactivation was determined after 36 h of incubation, and luciferase activity was expressed after normalization for β-galactosidase activity as fold increase over the vector control. The results represent the means ± SD of transfections performed in triplicate. (B) PKD2 activates the AP-1 reporter in a dose-dependent fashion. Increasing amounts of PKD2 vector were transfected to transactivate the AP-1 reporter construct. The total amount of plasmid DNA (2 μg/transfection) was balanced with vector (CDM8). (C) PKD2 activates the Jun2TRE reporter in a dose-dependent fashion. Increasing amounts of PKD2 vector were transfected to transactivate the Jun2TRE reporter. The total amount of plasmid DNA (2 μg/transfection) was balanced with vector (CDM8). (D) PKD2 activates the collagenase promoter. HEK 293T cells were cotransfected with 1 μg of PKD2 or vector (CDM8) and a collagenase promoter construct containing only a single AP-1-binding site. Cells were stimulated with 10% serum for 8 h. The results represent the means ± SD of transfections performed in triplicate. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
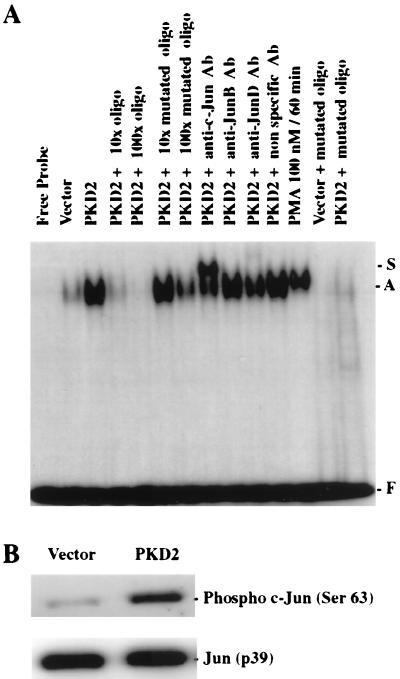
Since PKD2 activated the Jun2TRE but not the c-Fos promoter, the PKD2-induced AP-1 activity appeared to involve mainly Jun family members. Electromobility shift assays with a double-stranded AP-1/TRE oligonucleotide confirmed that nuclear proteins from HEK 293T cells transfected with PKD2 bind to this motif (Fig. 2A); a similar activity was observed after stimulation with PMA, a well-known AP-1 activator (15, 19). Preincubation of nuclear extract with specific antisera induced a significant supershift of the AP-1 band for c-Jun but not for JunB or JunD. The specificity of the AP-1/TRE DNA-binding protein was confirmed by the addition of increasing amounts of unlabeled AP-1 oligonucleotide that almost completely inhibited the formation of the radiolabeled DNA-protein complex at a 10-fold excess of unlabeled oligonucleotide. Conversely, the mutant AP-1 oligonucleotide failed to form a DNA-protein complex and had little effect on the AP-1 binding to the authentic AP-1 oligonucleotide. PKD2-dependent phosphorylation of c-Jun was demonstrated by using a specific antiserum against phosphorylated serine 63. As shown in Fig. 2B, PKD2, but not the control, induced c-Jun protein phosphorylation. Collectively, these data demonstrate that PKD2 stimulates the phosphorylation of c-Jun and the formation of transcriptionally active AP-1.
FIG. 2.
PKD2 triggers AP-1-binding activity. (A) PKD2 induces binding of nuclear proteins to the AP-1/TRE DNA-binding site. Nuclear extracts were isolated from HEK 293T cells transfected with either vector or PKD2 or from cells stimulated with 100 nM PMA for 60 min and analyzed by gel retardation assay for AP-1 binding. No nuclear extract was added to the radiolabeled AP-1 oligonucleotide in lane 1 (Free Probe). Addition of a 10- or 100-fold excess of unlabeled probe prevented the formation of a DNA-protein complex, while addition of a 10- or 100-fold excess of mutated AP-1 oligonucleotide had little effect. A significant supershift of the DNA-protein complex was observed after the addition of a monoclonal antibody to c-Jun but not an antibody to JunB or JunD or a nonspecific antibody. A mutated AP-1 oligonucleotide failed to bind nuclear proteins isolated from PKD2-transfected HEK 293T cells. The positions of the AP-1 complex (A), supershift (S), and free probe (F) are indicated. (B) Western blot analysis showing the phosphorylation of c-Jun after cotransfection of a His-tagged c-Jun plasmid with a construct encoding PKD2 or a vector control (CDM8). c-Jun phosphorylated on serine 63 was detected with a specific polyclonal antiserum. To demonstrate equal amounts of total c-Jun, the membrane was reprobed with an anti-Jun antiserum.
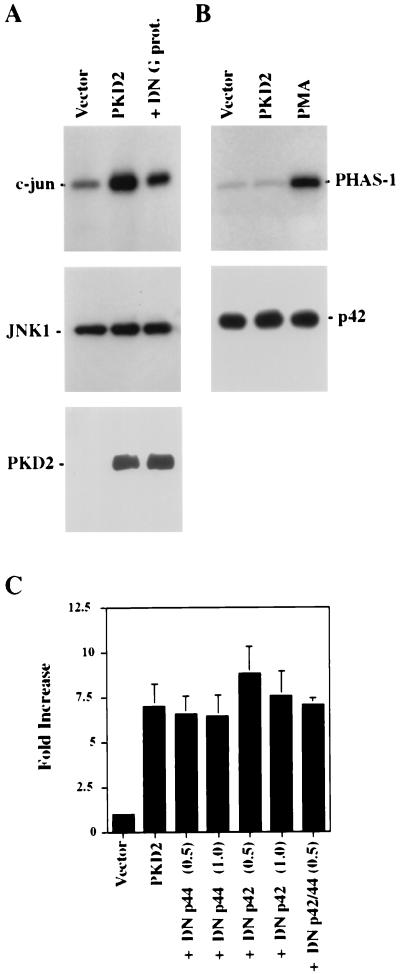
PKD2 activates JNK1 and p38 but not p42.
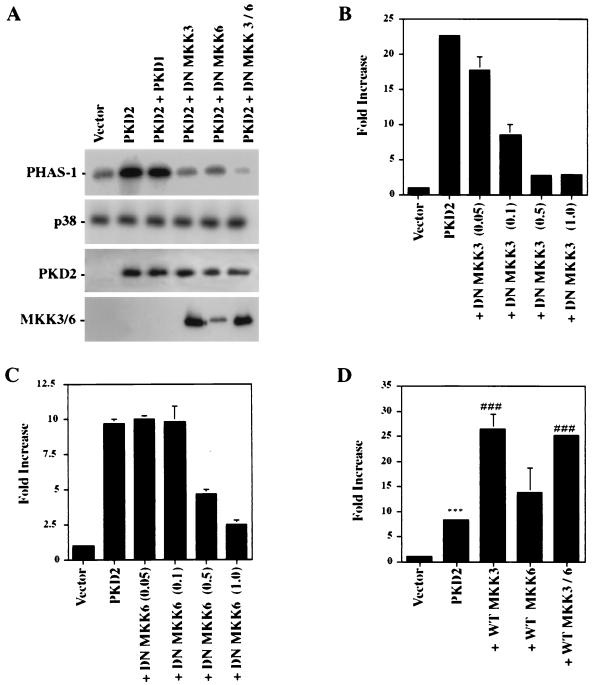
Several kinase cascades have been demonstrated to regulate AP-1 activity (31), including the Hog1p homologue p38, the MAPKs p42 and p44, and members of the JNK family (reviewed in reference 47). To further delineate the signaling pathway through which PKD2 triggers AP-1 activation, we examined the activity of these kinases in HEK 293T cells expressing PKD2. HA-tagged p38 and p42 and JNK1 were coexpressed with PKD2 or the vector control CDM8. After serum starvation for 16 h, HA-tagged kinases were immunoprecipitated, and the activity of each kinase was assessed by in vitro kinase assays. PKD2 activated JNK1 but not p42 (Fig. 3A and B). In addition, dominant-negative mutants of p42 and p44 had no effect on PKD2-mediated AP-1 activation (Fig. 3C), providing further evidence that PKD2-mediated AP-1 activation does not involve p42 or p44 MAPKs. In contrast, PKD2 induced MKK3- and MKK6-dependent activation of p38 (Fig. 4A). MKK3 and MKK6 are two MAPKs that are reported to selectively phosphorylate and activate p38 (10, 22, 23). Dominant-negative mutants of MKK3 and MKK6 significantly blocked the PKD2-mediated p38 activation as determined by in vitro kinase assays. To demonstrate the critical involvement of p38 in PKD2 signaling, we tested the effects of dominant-negative versions of MKK3 and MKK6 on PKD2-mediated AP-1 activation. As shown in Fig. 4B and C, coexpression of dominant-negative MKK3 or MKK6 resulted in a dose-dependent inhibition of PKD2-mediated AP-1 activation. Conversely, wild-type MKK3 and MKK6 augmented the PKD2-mediated AP-1 activation (Fig. 4D).
FIG. 3.
PKD2 activates the JNK1 but not the MAPK p42. (A) HEK 293T cells were cotransfected with HA-tagged JNK1 and vector control (CDM8), PKD2, or PKD2 in combination with dominant-negative (DN) mutants of the small G proteins Cdc42, Rac1, and RhoA at equal ratios. Cells were harvested after 24 h, and immunoprecipitated JNK1 was incubated with GST-c-Jun(1–79) in the presence of [γ-32P]ATP. Incorporated radioactivity was visualized by SDS–10% PAGE and autoradiography. PKD2 increases JNK1 activity (top panel); this activation was inhibited by the DN Cdc42, Rac1, and RhoA. Western blot analysis revealed equal amounts of precipitated kinases (middle panel); the expression of PKD2 was not affected by the presence of the DN Cdc42, Rac1, and RhoA (bottom panel). (B) HEK 293T cells were cotransfected with HA-tagged p42 in combination with a vector control (CDM8) or PKD2 at equal ratios. Cells were harvested after 24 h, and immunoprecipitated p42 was incubated with PHAS-1 in the presence of [γ-32P]ATP. Incorporated radioactivity was visualized by SDS–12% PAGE and autoradiography. Only stimulation with 50 nM PMA for 30 min resulted in activation of p42. The lower panel demonstrates equal amounts of p42 kinase in each condition as determined by Western blot analysis. (C) PKD2-mediated AP-1 activation is unaffected by DN mutants of the MAPK p42 or p44. HEK 293T cells were transfected with the AP-1 reporter construct, vector control, PKD2, or PKD2 in combination with DN mutants of p42 and p44. Transactivation was determined after 36 h of incubation, and luciferase activity was expressed after normalization for β-galactosidase activity as fold increase over the vector control. The results represent the means ± SD of transfections performed in triplicate.
FIG. 4.
PKD2 activates p38 MAPK. (A) Dominant-negative (DN) mutants of MKK3 and MKK6 significantly inhibit PKD2-mediated p38 activation. In vitro kinase assays were performed after transfection of HEK 293T cells with HA-tagged p38 and PKD2 or a vector control (CDM8). Immunoprecipitated kinases were incubated with PHAS-1 as an exogenous substrate in the presence of [γ-32P]ATP. Incorporated radioactivity was visualized by SDS–12% PAGE and autoradiography. The amounts of immunoprecipitated p38, the expression of PKD2, and the expression of the DN MKK3 and MKK6 were monitored by Western blot analysis. PKD2 activates p38 in an MKK3- and MKK6-dependent fashion; coexpression of C-terminal PKD1 does not contribute to the PKD2-mediated p38 activation. (B) A DN mutant of MKK3 significantly inhibits PKD2-mediated AP-1 activation in a dose-dependent fashion. AP-1 luciferase assays were performed after transfection of HEK 293T cells with 1 μg of PKD2 and increasing amounts of a DN mutant of MKK3. The results represent the means ± SD of transfections performed in triplicate. (C) A DN mutant of MKK6 significantly inhibits PKD2-mediated AP-1 activation in a dose-dependent fashion. AP-1 luciferase assays were performed after transfection of HEK 293T cells with 1 μg of PKD2 and increasing amounts of a DN mutant of MKK6. The results represent the means ± SD of transfections performed in triplicate. (D) Wild-type (WT) MKK3 and MKK6 enhance PKD2-mediated AP-1 activation. AP-1 luciferase assays were performed after transfection of HEK 293T cells with 1 μg of PKD2 in combination with WT MKK3 or MKK6. The results represent the means ± SD of transfections performed in triplicate. ∗∗∗, significantly different from control vector (P < 0.001); ⋕⋕⋕⋕, significantly different from PKD2-transfected cells (P < 0.001).
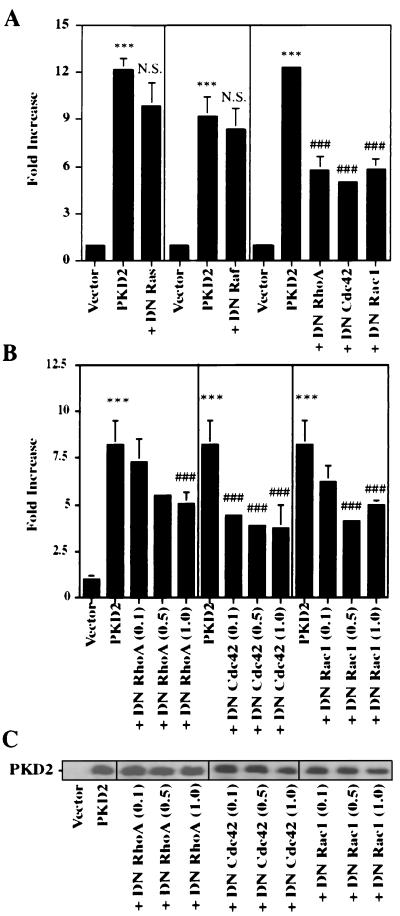
The small GTPases Rac and Cdc42 are important mediators of JNK and p38 activation (5, 33, 36). To determine the role of small GTP-binding proteins in the PKD2-mediated activation of AP-1, we coexpressed PKD2 with dominant-negative mutants of RhoA (N19), Cdc42 (N17), and Rac1 (N17). Dominant-negative RhoA, Cdc42, and Rac1 abrogated the PKD2-mediated JNK activation (Fig. 3A) and the PKD2-mediated AP-1 activation (Fig. 5A). The dominant-negative mutants inhibited the PKD2-mediated AP-1 activation in a dose-dependent fashion that was not related to a change in PKD2 expression (Fig. 5B and C). In contrast, dominant-negative forms of p21-Ras and Raf-1 had no effect on AP-1 activation (Fig. 5A). Thus, small GTP-binding proteins of the Rho family appear to play a central role in the signaling pathway triggered by PKD2.
FIG. 5.
Dominant-negative (DN) mutants of the GTP-binding proteins RhoA, Cdc42, and Rac1 inhibit PKD2-mediated AP-1 activation. (A) DN mutants of RhoA, Cdc42, and Rac1 but not Ras or Raf-1 blocked AP-1 activation in HEK 293T cells transfected with PKD2. Results represent the means ± SD of three independent experiments. (B) DN mutants of RhoA, Cdc42, and Rac1 inhibit PKD2-mediated AP-1 activation in a dose-dependent fashion. Results represent the means ± SD of three independent experiments. (C) DN mutants of RhoA, Cdc42, and Rac1 do not affect the expression of PKD2. PKD2 expression, monitored by Western blot analysis, was unaffected by increasing amounts of cotransfected DN RhoA, Cdc42, or Rac1. ∗∗∗, significantly different from control (P < 0.001); ⋕⋕⋕, significantly different from PKD2-transfected cells (P < 0.001); N.S., not significant.
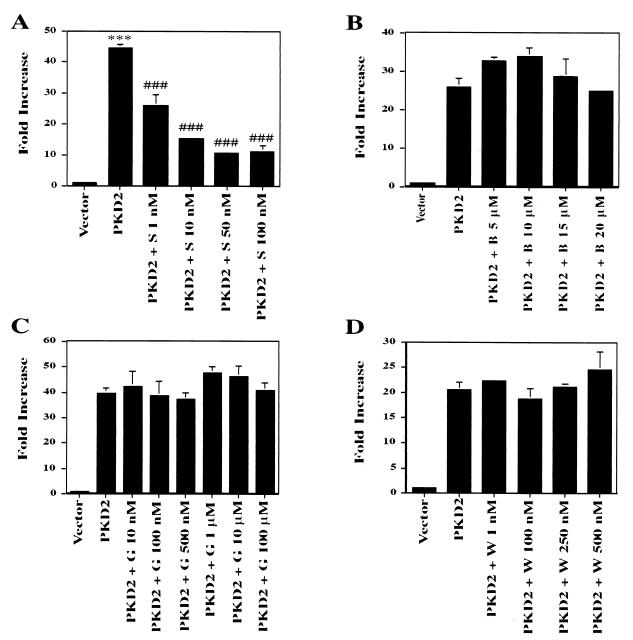
Staurosporine, but not BAPTA, genistein, or wortmannin, inhibits PKD2-induced AP-1 activity.
To further characterize the signaling pathway triggered by PKD2, we tested the effects of several pharmacological reagents on PKD2-mediated signaling. Only staurosporine, a broad-spectrum PKC inhibitor, decreased PKD2-induced AP-1 activity in a dose-dependent manner. This inhibition was evident at very low concentrations of staurosporine (1 to 10 nM) that did not impair cellular viability. No significant inhibition was observed with the phosphatidylinositol 3-kinase inhibitor wortmannin, the tyrosine kinase inhibitor genistein, or the intracellular calcium chelator BAPTA-AM (Fig. 6). The selective inhibitory effect of staurosporine implicated PKC as a downstream target of PKD2-mediated signaling, while the failure of BAPTA-AM to interfere with PKD2 signaling suggested that a calcium-independent PKC isozyme was involved in the AP-1 activity induced by PKD2.
FIG. 6.
Effects of staurosporine (S), BAPTA-AM (B), wortmannin (W), and genistein (G) on PKD2-mediated AP-1 activation. HEK 293T cells were transiently cotransfected with PKD2 or a vector control (CDM8) and were exposed to increasing concentrations of staurosporine (A), BAPTA (B), genistein (C), and wortmannin (D), added for the last 6 h of the incubation period. Transactivation of the AP-1 reporter construct was determined after 36 h of incubation, and luciferase activity was expressed as fold increase over the vector control after normalization for β-galactosidase activity. Representative results of two experiments, performed in triplicate, are expressed as means ± SD. ∗∗∗, significantly different from control vector (P < 0.001); ⋕⋕⋕, significantly different from PKD2-transfected cells (P < 0.001).
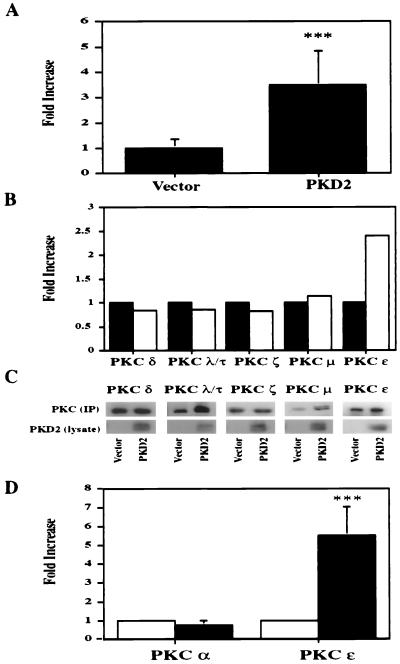
PKD2 increases total PKC activity and specifically activates PKC ɛ.
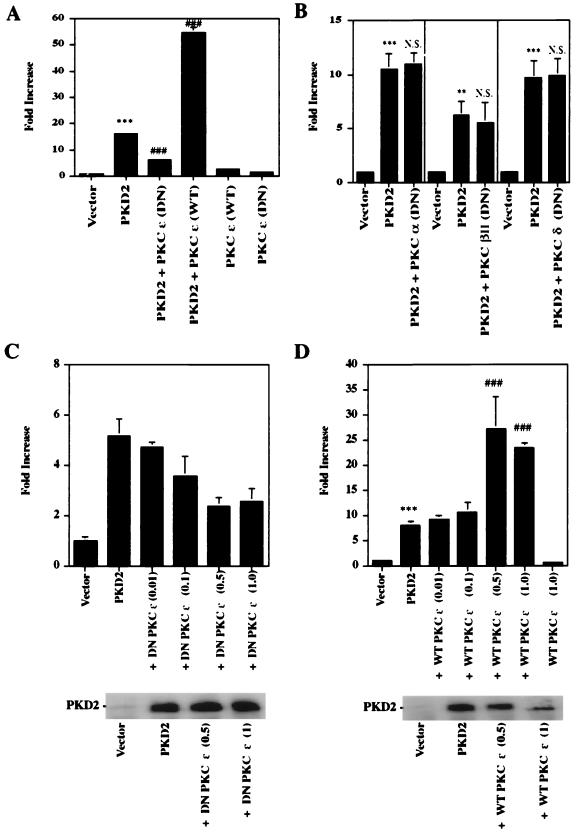
Consistent with the observed inhibitory effect of staurosporine, expression of PKD2 significantly elevated total PKC activity (P < 0.001, n = 6) (Fig. 7A). To determine the PKC isozyme activated by PKD2, we performed in vitro kinase assays of calcium-independent protein kinase isozymes isolated from PKD2-expressing HEK 293T cells. After immunoprecipitation of individual PKC isozymes with specific antibodies, their activity was quantitated by using neurogranin as an exogenous substrate. Only PKC ɛ was specifically activated by PKD2; the activities of most other isozymes appeared reduced in PKD2-expressing cells (Fig. 7B). The amount of immunoprecipitated PKC isozymes and the expression of PKD2 were monitored by Western blot analysis (Fig. 7C). Since PKD1 induces the activation of PKC α (1), we compared the effects of PKD2 on PKC α and ɛ activation. While PKD2 induced PKC ɛ activation, it had no effect on the activation of PKC α (Fig. 7D). As further confirmation that PKC ɛ is an essential component of PKD2-mediated signaling, PKD2-mediated AP-1 activation was significantly inhibited by a dominant-negative form of PKC ɛ (Fig. 8A and C) but not by other dominant-negative PKC isozymes, such as PKC α, βII, and δ (Fig. 8B). Wild-type PKCɛ potentiated the AP-1 activation triggered by PKD2 (Fig. 8A and D). Collectively, these data indicate that PKD2 induces AP-1 activity through a PKC ɛ-dependent mechanism.
FIG. 7.
PKD2 activates PKC ɛ. (A) PKD2 increases total PKC activity in HEK 293T cells. HEK 293T cells were transiently transfected with a vector encoding PKD2 or a vector control, and total PKC activity was measured by a colorimetric assay. Results are expressed as fold increase over the vector control for six independent experiments. (B) PKD2 activates PKC ɛ but not other calcium-independent PKC isozymes. HEK 293T cells were transiently transfected with PKD2 (black bars) or a vector control (CDM8) (white bars). The different PKC isozymes (PKC δ, λ/τ, ζ, μ, and ɛ) were immunoprecipitated with specific monoclonal antibodies and analyzed by in vitro kinase assays. (C) The amounts of immunoprecipitated PKC isozymes (IP) and expression of PKD2 were monitored by Western blot analysis. (D) PKD2 activates PKC ɛ but not PKC α. The differential activation of PKC α and ɛ by PKD2 was confirmed by in vitro kinase assays (three independent experiments) and expressed as fold increase over the vector control. ∗∗∗, P < 0.001.
FIG. 8.
PKD2-mediated AP-1 activation requires PKC ɛ. (A) A dominant-negative (DN) PKC ɛ mutant blocks PKD2-mediated AP-1 activation. HEK 293T cells were transiently cotransfected with a vector control (CDM8) or PKD2 and a DN mutant of PKC ɛ or wild-type (WT) PKC ɛ at a ratio of 2:1. Transfections with 1 μg of the WT and DN PKC ɛ constructs alone are also shown. The experiment was performed in triplicate. (B) DN PKC α, βII, and δ mutants do not affect PKD2-mediated AP-1 activity. HEK 293T cells were transiently cotransfected with a vector control (CDM8) or PKD2, together with a DN mutant of PKC α, βII, or δ at a ratio of 2:1. The experiment was performed in triplicate. (C) A DN PKC ɛ mutant inhibits PKD2-mediated AP-1 activation in a dose-dependent fashion but does not affect PKD2 expression. HEK 293T cells were transiently cotransfected with a vector control (CDM8) or PKD2 and with increasing amounts of a DN mutant of PKC ɛ. PKD2 expression was monitored by Western blot analysis. (D) Wild-type (WT) PKC ɛ augments PKD2-mediated AP-1 activation. HEK 293T cells were transiently cotransfected with a vector control (CDM8) or PKD2 and with increasing amounts of WT PKC ɛ. PKD2 expression was monitored by Western blot analysis. ∗∗, significantly different from vector control (P < 0.01); ∗∗∗, significantly different from control vector (P < 0.001); ⋕⋕⋕, significantly different from PKD2-transfected cells (P < 0.001); N.S., not significantly different from PKD2-transfected cells.
PKD1 enhanced the AP-1 activation induced by PKD2.
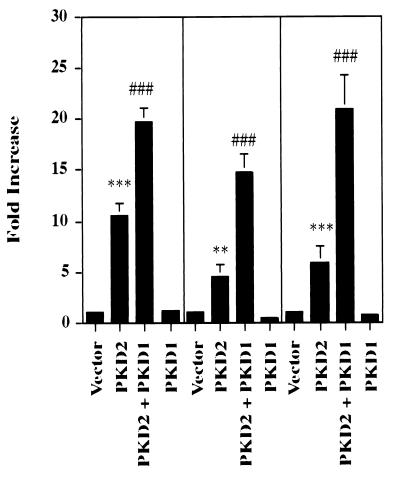
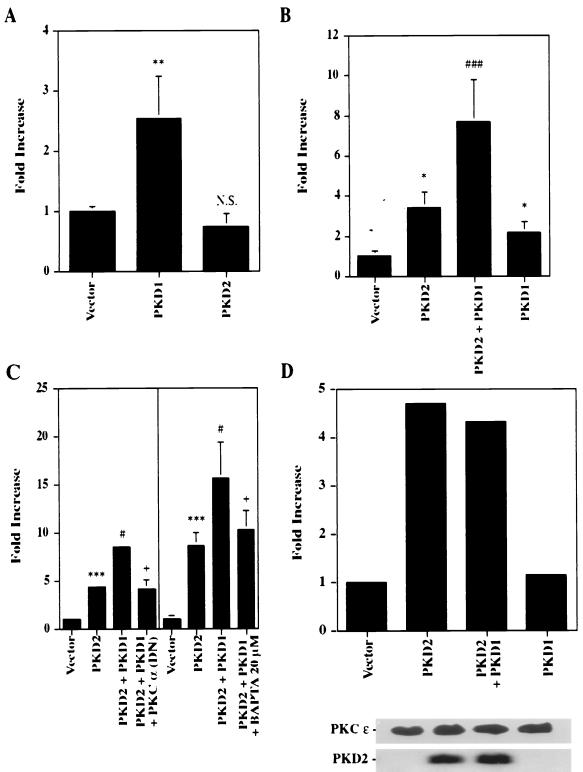
It has previously been shown that PKD1 and PKD2 interact directly via their C-terminal domains (41, 52), a finding suggestive of a common signaling pathway for PKD1 and PKD2. To analyze how PKD1 might modulate PKD2-mediated signaling, we cotransfected PKD2 with the C-terminal tail of PKD1. The 112 C-terminal amino acids of PKD1 were fused to the extracellular domain of CD16 and the transmembrane domain of CD7. The PKD1 fusion protein is well expressed at the plasma membrane, where it is capable of mediating protein-protein interactions and signaling events (1, 52). In three independent experiments performed in triplicate, expression of the C-terminal tail of PKD1 dramatically augmented PKD2-mediated AP-1 activity (Fig. 9). To further characterize the combinatorial effects of PKD1 and PKD2, we examined their influence on AP-1, PKC, and MAPK activity. The 112 C-terminal amino acids of PKD1 failed to activate AP-1 (Fig. 9) but stimulated PKC α (Fig. 10A). In contrast, PKD2 failed to activate PKC α, consistent with our observation that the PKD2-mediated signaling is calcium independent (Fig. 10A). Although PKD1 did not alter PKD2-mediated activation of p38 (Fig. 4A), it augmented PKD2-mediated total cellular PKC activity (Fig. 10B). Thus, we hypothesized that the synergism of PKD1- and PKD2-mediated AP-1 activity arose from the dual activation of two distinct PKC isozymes, PKC α and PKC ɛ, by PKD1 and PKD2, respectively. To test this possibility, we investigated whether a dominant-negative PKC α or the calcium chelator BAPTA would curtail the synergistic effect of PKD1 on PKD2-mediated AP-1 activation. Both dominant-negative PKC α and BAPTA-AM (20 μM) nearly abolished the PKD1-augmented activation of AP-1 while maintaining the baseline PKD2-mediated AP-1 activation (Fig. 10C). In contrast, PKD1 had no effect on the PKD2-mediated activation of PKC ɛ (Fig. 10D).
FIG. 9.
PKD1 augments PKD2-mediated AP-1 activation. HEK 293T cells were transfected with vector control (CDM8), PKD2 together with CD16.7 (or CD16.7 fused to the last 112 amino acids of the C-terminal domain of PKD1), or PKD1 alone. The means ± SD of three independent experiments performed in triplicate are shown. ∗∗, significantly different from control vector (P < 0.01); ∗∗∗, significantly different from control vector (P < 0.001); ⋕⋕⋕, significantly different from PKD2-transfected cells (P < 0.001).
FIG. 10.
PKD1-mediated augmentation of PKD2 activities requires PKC α. (A) The C-terminal domain of PKD1 but not PKD2 activates PKC α. The PKC α isozyme was immunoprecipitated from PKD1, PKD2, or vector control-transfected HEK 293T cells; the activity was determined by in vitro kinase assays. The means ± SD of three experiments were expressed as fold increase over the vector control. (B) PKD1 increases the total PKC activity triggered by PKD2. HEK 293T cells were transfected with PKD1, PKD2, or a vector control (CDM8). The means ± SD of the total PKC activity of six independent experiments were expressed as fold increase over the vector control. (C) A dominant-negative (DN) PKC α mutant and the calcium chelator BAPTA block the effect of PKD1 upon PKD2-mediated AP-1 activation. HEK 293T cells were transiently cotransfected with a vector control (CDM8), PKD1, and PKD2 in combination with a DN mutant of PKC α. In one experiment, BAPTA-AM (20 μM) was added for 6 h. Experiments were performed in triplicate. (D) PKD1 has no effect on the PKD2-mediated activation of PKC ɛ. The PKC ɛ isozyme was immunoprecipitated from HEK 293T cells transfected with vector control, PKD1, or PKD2. The PKC ɛ activity was determined by in vitro kinase assays. Immunoprecipitates of PKC ɛ and PKD2 expression were monitored by Western blot analysis. ∗, significantly different from vector control (P < 0.05); ∗∗, significantly different from vector control (P < 0.01); ∗∗∗, significantly different from control vector (P < 0.001); ⋕, significantly different from PKD2-transfected cells (P < 0.05); ⋕⋕⋕, significantly different from PKD2-transfected cells, P < 0.05; +, significantly different from PKD1- and PKD2-transfected cells (P < 0.05); N.S., not significantly different from control cells.
DISCUSSION
Our findings demonstrate that PKD2 strongly stimulates AP-1 activity in HEK 293T cells. The functional relevance of this activity can be extrapolated from its effect on a physiological promoter, the collagenase promoter, which contains only a single AP-1-binding site. While serum induced a modest 2- to 3-fold activation of this promoter, PKD2 increased its transcriptional activity nearly 10-fold. Our experiments indicate that PKD2 activates AP-1 through a pathway involving activation of JNK1, p38, and PKC ɛ. The observed inhibitory effects of staurosporine and dominant-negative mutants of PKC ɛ, MKK3, MKK6, and Rho family members support a critical role of these kinases in PKD2-mediated AP-1 activation.
The transcription factor AP-1 is composed of homodimers and heterodimers of Jun (v-Jun, c-Jun, JunB, and JunD), Fos (v-Fos, c-Fos, FosB, Fra1, and Fra2), or activating transcription factor (ATF2, ATF3, and B-ATF) that bind to a consensus DNA sequence, the TRE (reviewed in reference 26), and thereby regulate target genes involved in cellular proliferation, differentiation, and apoptosis. Its critical role in embryogenesis is evident in Xenopus, Drosophila (13, 44), and mammalian development. Targeted deletion of c-Jun in mice disrupts normal organogenesis and is embryo lethal at E12 to E14 (24), suggesting that AP-1 could play a role in nephrogenesis.
AP-1 activity can be generated through a variety of signaling events. Gel shift and supershift analysis revealed that PKD2 increased the binding of Jun-containing AP-1 to the TRE. Moreover, PKD2 increased the amount of c-Jun phosphorylated on serine 63. Activated JNKs phosphorylate c-Jun on serines 63 and 73, two positively regulatory sites in the transcriptional activation domain of c-Jun (9, 34), while activated p38 phosphorylates the corresponding positively regulatory sites on ATF2 but not on c-Jun (42). The inhibition of PKD2-induced AP-1 activity by dominant-negative MKK3 and MKK6, specific abrogators of p38 activity, is consistent with a role for p38 in PKD2-mediated signaling, perhaps through the formation of transcriptionally active ATF family members.
Dominant-negative mutants of Cdc42, RhoA, and Rac1 significantly reduced PKD2-mediated AP-1 activation. Since small GTP-binding proteins of the Rho family members activate p38 and JNKs (2, 5, 49, 55) and enterotoxin-mediated inactivation of these small G proteins inhibits p38 activation (25, 53), these small G proteins may represent an upstream target of PKD2 signaling. These small GTP-binding proteins are known to regulate complex cellular programs, including the organization of the actin cytoskeleton, cell motility, shape, adhesion, and polarity (reviewed in references 39 and 48). Interestingly, Cdc42-dependent p38 activation appears to inhibit cell cycle progression, arresting cells at the G1/S transition (36), consistent with reports that increased levels of p38 can inhibit the mitogenic induction of G1 cyclins (29). These findings suggest that PKD2 may modulate cell cycle progression through activation of Cdc42 and p38.
PKD1 and PKD2 have been hypothesized to be components of a common signaling pathway. Studies of PKD1−/− and PKD2−/− mice have confirmed an essential role for PKD1 and PKD2 mutations in cystogenesis that resembles human ADPKD (30, 54), and the gene products of PKD1 and PKD2 are known to interact (41, 52). Despite the homologies of PKD1 and PKD2 to adhesion molecules and voltage-gated sodium channels (21, 35), respectively, the functions of these integral membrane proteins remain unknown. Our analysis reveals that both PKD1 and PKD2 induce signaling events that converge on the activation of AP-1. The synergism between the C-terminal PKD1 and PKD2 seems to depend on the comlementary activation of two different PKC isozymes, PKC α by the 112 C-terminal amino acids of PKD1 and PKC ɛ by PKD2. Activation of PKC α inhibits c-Jun phosphorylation on negatively regulatory DNA-binding sites, but it fails to generate transcriptionally active AP-1 (3). In contrast, PKC ɛ alone induces a powerful AP-1 response in several tissues, although the precise signaling cascade remains unclear (8, 15, 37).
Although AP-1 is a clearly defined target of converging PKD1 and PKD2 signaling, other cellular programs triggered by the combined activation of PKC α and ɛ should be examined. Numerous studies document the growth-promoting properties of PKC; however, there is increasing evidence that some PKC isozymes are involved in cell growth inhibition and differentiation (reviewed in reference 13). In renal tissue, PKC α, δ, and ζ are the most abundant isozymes, while PKC ɛ is weakly but uniformly expressed in both the cortex and the medulla during ontogeny (40). Previous studies have shown that activated PKC ɛ inhibits cell growth in some tissues (32, 46). Moreover, the combinatorial activation of PKC α and ɛ appears to cause induction of the cyclin-dependent kinase inhibitors p21waf1/cip1 and p27kip1, hyperphosphorylation of the retinoblastoma protein, and an inhibition of cell cycle progression in G1 of intestinal epithelial cells (14). Thus, the ability of PKD1 and PKD2 to activate PKC α and ɛ, respectively, may enable the two ADPKD gene products to inhibit cell cycle progression and promote the cellular differentiation of tubular epithelial cells during renal development.
The mechanism by which PKD2 triggers the activation of PKC ɛ remains unknown. In vivo activation of phosphatidylinositol 3-kinase and phospholipase Cγ has been reported to induce the activation of PKC ɛ (37). Since wortmannin had no effect on PKD2-mediated AP-1 activation, it would be of interest to determine whether PKD2 can activate phospholipase Cγ in vivo. PKD2 contains several putative PKC phosphorylation sites that could represent a target of either PKD1-mediated activation of PKC α or PKD2-mediated activation of PKC ɛ. To further characterize these signaling events, it will be important to identify PKD2 motifs and PKD2-binding adapter proteins involved in the PKD2-mediated cellular activation. Modeling the observed signaling events in a more complex cellular or animal system would facilitate the design of therapeutic approaches to mimic the normal signaling of PKD1 and PKD2 and thereby reduce cyst progression to prevent renal failure in patients with ADPKD.
ACKNOWLEDGMENTS
We are grateful to Bertrand Knebelmann for a critical reading of the manuscript.
E.K. was supported by Public Health Service grant MH-01147. T.B. was supported by the Deutsche Forschungsgemeinshaft, Germany, Be 2212/1-1. L.T. was supported by Public Health Service grant DK-09625. This work was supported by NIH-RO1 DK 52897 (G.W.) and by a grant from the Polycystic Kidney Research Foundation (G.W.).
REFERENCES
- 1.Arnould T, Kim E, Tsiokas L, Jochimsen F, Gruning W, Chang J D, Walz G. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem. 1998;273:6013–6018. doi: 10.1074/jbc.273.11.6013. [DOI] [PubMed] [Google Scholar]
- 2.Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 3.Boyle W J, Smeal T, Defize L H, Angel P, Woodgett J R, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Takeshita A, Ozaki K, Kitano S, Hanazawa S. Transcriptional regulation by transforming growth factor beta of the expression of retinoic acid and retinoid X receptor genes in osteoblastic cells is mediated through AP-1. J Biol Chem. 1996;271:31602–31606. doi: 10.1074/jbc.271.49.31602. [DOI] [PubMed] [Google Scholar]
- 5.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 6.Cowley B D, Jr, Chadwick L J, Grantham J J, Calvet J P. Elevated proto-oncogene expression in polycystic kidneys of the C57BL/6J (cpk) mouse. J Am Soc Nephrol. 1991;1:1048–1053. doi: 10.1681/ASN.V181048. [DOI] [PubMed] [Google Scholar]
- 7.Daoust M C, Reynolds D M, Bichet D G, Somlo S. Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics. 1995;2535:733–736. doi: 10.1016/0888-7543(95)80020-m. [DOI] [PubMed] [Google Scholar]
- 8.Decock J B, Gillespie-Brown J, Parker P J, Sugden P H, Fuller S J. Classical, novel and atypical isoforms of PKC stimulate ANF- and TRE/AP-1-regulated-promoter activity in ventricular cardiomyocytes. FEBS Lett. 1994;356:275–278. doi: 10.1016/0014-5793(94)01283-0. [DOI] [PubMed] [Google Scholar]
- 9.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 10.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 11.Dong Z, Xu R H, Kim J, Zhan S N, Ma W Y, Colburn N H, Kung H. AP-1/jun is required for early Xenopus development and mediates mesoderm induction by fibroblast growth factor but not by activin. J Biol Chem. 1996;271:9942–9946. doi: 10.1074/jbc.271.17.9942. [DOI] [PubMed] [Google Scholar]
- 12.The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 13.Fishman D D, Segal S, Livneh E. The role of protein kinase C in G1 and G2/M phases of the cell cycle. Int J Oncol. 1998;12:181–186. doi: 10.3892/ijo.12.1.181. [DOI] [PubMed] [Google Scholar]
- 14.Frey M R, Saxon M L, Zhao X, Rollins A, Evans S S, Black J D. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem. 1997;272:9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- 15.Genot E M, Parker P J, Cantrell D A. Analysis of the role of protein kinase C-alpha, -epsilon, and -zeta in T cell activation. J Biol Chem. 1995;270:9833–9839. doi: 10.1074/jbc.270.17.9833. [DOI] [PubMed] [Google Scholar]
- 16.Grantham J J. Polycystic kidney disease: a predominance of giant nephrons. Am J Physiol. 1983;244:F3–F10. doi: 10.1152/ajprenal.1983.244.1.F3. [DOI] [PubMed] [Google Scholar]
- 17.Harding M A, Gattone II V H, Grantham J J, Calvet J P. Localization of overexpressed c-myc mRNA in polycystic kidneys of the cpk mouse. Kidney Int. 1992;41:317–325. doi: 10.1038/ki.1992.44. [DOI] [PubMed] [Google Scholar]
- 18.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 19.Huang T S, Lee S C, Lin J K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci USA. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes J, Ward C J, Peral B, Aspinwall R, Clark K, San Millan J L, Gamble V, Harris P C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 21.The International Polycystic Kidney Disease Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Chen C, Li Z, Guo W, Gegner J A, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch R J, Han J. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R S, van Lingen B, Papaioannou V E, Spiegelman B M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 25.Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 26.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 27.Kimberling W J, Kumar S, Gabow P A, Kenyon J B, Connolly C J, Somlo S. Autosomal dominant polycystic kidney disease: localization of the second gene to chromosome 4q13-q23. Genomics. 1993;18:467–472. doi: 10.1016/s0888-7543(11)80001-7. [DOI] [PubMed] [Google Scholar]
- 28.Lanoix J, D’Agati V, Szabolcs M, Trudel M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD) Oncogene. 1996;13:1153–1160. [PubMed] [Google Scholar]
- 29.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 30.Lu W, Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targeted Pkd1 mutation. Nat Genet. 1997;17:179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 31.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 32.Mihalik R, Farkas G, Kopper L, Benczur M, Farago A. Possible involvement of protein kinase C-epsilon in phorbol ester-induced growth inhibition of human lymphoblastic cells. Int J Biochem Cell Biol. 1996;28:925–933. doi: 10.1016/1357-2725(96)00020-9. [DOI] [PubMed] [Google Scholar]
- 33.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 34.Minden A, Lin A, Smeal T, Dérijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mochizuki T, Wu G, Hayashi T, Xenophontos S L, Veldhuisen B, Saris J J, Reynolds D M, Cai Y, Gavow P A, Pierides A, Kimberling W J, Breuning M H, Deltas C C, Peters D J, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 36.Molnar A, Theodoras A M, Zon L I, Kyriakis J M. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–13235. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 37.Moriya S, Kazlauskas A, Akimoto K, Hirai S, Mizuno K, Takenawa T, Fukui Y, Watanabe Y, Ozaki S, Ohno S. Platelet-derived growth factor activates protein kinase C epsilon through redundant and independent signaling pathways involving phospholipase C gamma or phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:151–155. doi: 10.1073/pnas.93.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musial A, Mandal A, Coroneos E, Kester M. Interleukin-1 and endothelin stimulate distinct species of diglycerides that differentially regulate protein kinase C in mesangial cells. J Biol Chem. 1995;270:21632–21638. doi: 10.1074/jbc.270.37.21632. [DOI] [PubMed] [Google Scholar]
- 39.Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- 40.Ostlund E, Mendez C F, Jacobsson G, Fryckstedt J, Meister B, Aperia A. Expression of protein kinase C isoforms in renal tissue. Kidney Int. 1995;47:766–773. doi: 10.1038/ki.1995.117. [DOI] [PubMed] [Google Scholar]
- 41.Qian F, Germino F J, Cai Y, Zhang X, Somlo S, Germino G G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 42.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 43.Reeders S T, Breuning M H, Davies K E, Nicholls R D, Jarman A P, Higgs D R, Pearson P L, Weatherall D J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985;317:542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 44.Riesgo-Escovar J R, Hafen E. Common and distinct roles of DFos and DJun during Drosophila development. Science. 1997;278:669–672. doi: 10.1126/science.278.5338.669. [DOI] [PubMed] [Google Scholar]
- 45.Sandford R, Sgotto B, Aparicio S, Brenner S, Vaudin M, Wilson R K, Chissoe S, Pepin K, Bateman A, Chothia C, Hughes J, Harris P. Comparative analysis of the polycystic kidney disease 1 (PKD1) gene reveals an integral membrane glycoprotein with multiple evolutionary conserved domains. Hum Mol Genet. 1997;6:1483–1489. doi: 10.1093/hmg/6.9.1483. [DOI] [PubMed] [Google Scholar]
- 46.Sasaguri T, Kosaka C, Hirata M, Masuda J, Shimokado K, Fujishima M, Ogata J. Protein kinase C-mediated inhibition of vascular smooth muscle cell proliferation: the isoforms that may mediate G1/S inhibition. Exp Cell Res. 1993;208:311–320. doi: 10.1006/excr.1993.1251. [DOI] [PubMed] [Google Scholar]
- 47.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 48.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 49.Teramoto H, Crespo P, Coso O A, Igishi T, Xu N, Gutkind J S. The small GTP-binding protein rho activates c-Jun N-terminal kinases/stress-activated protein kinases in human kidney 293T cells. Evidence for a Pak-independent signaling pathway. J Biol Chem. 1996;271:25731–25734. doi: 10.1074/jbc.271.42.25731. [DOI] [PubMed] [Google Scholar]
- 50.Trudel M, D’Agati V, Constantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 51.Trudel M, Lanoix J, Barisoni L, Blouin M J, Desforges M, L’Italien C, D’Agati V. C-myc-induced apoptosis in polycystic kidney disease is Bcl-2 and p53 independent. J Exp Med. 1997;186:1873–1884. doi: 10.1084/jem.186.11.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsiokas L, Kim E, Arnould T, Sukhatme V P, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesselborg S, Bauer M K A, Vogt M, Schmitz M L, Schulze-Osthoff K. Activation of transcription factor NF-kappaB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J Biol Chem. 1997;272:12422–12429. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 54.Wu G, D’Agati V, Cai Y, Markowitz G, Park J H, Reynolds D M, Maeda Y, Le T C, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevitch R J, Bokoch G M. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]