Abstract
Plasmodium sporozoites are the infectious, highly motile forms of the malaria parasite transmitted by Anopheles mosquitoes. Sporozoite motility can be assessed following the dissection of Anopheles salivary glands and isolation of sporozoites in vitro.
Keywords: Plasmodium, Plasmodium berghei, Sporozoites, Salivary gland isolation, Dissection, Gliding motility, Malaria, Mosquitoes, Anopheles
Background
Sporozoites of the phylum Plasmodium, the causative agent of malaria, are transmitted into the skin of their vertebrate host through the bite of an infectious mosquito. Sporozoite motility is a key prerequisite for parasite transmission and successful infection of the vertebrate host. Motility constitutes the first parasite mechanism that can be inhibited and is thus of interest for intervention strategies. Genetic modifications affecting gliding motility or motility modulating compounds can be readily investigated using 2D in vitro assays.
Part I: Isolation of salivary gland sporozoites
Materials and Reagents
15 ml conical centrifugation tube
Two 10 ml Petri dishes
Two 27 G needles
Two 1 ml syringes
Glass slide
1.5 ml plastic reaction tube
Disposable polypropylene pestles (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: F19923-0001)
Mosquitoes
Ice
RPMI (Thermo Fisher Scientific, GibcoTM, catalog number: 11835063)
70% ethanol
1x PBS (Thermo Fisher Scientific, GibcoTM, catalog number: 18912014)
Bovine serum albumin (BSA) (Carl Roth, catalog number: 8076)
BSA/RPMI 3% (see Recipes)
Equipment
Vacuum pump
Styrofoam box
Forceps
Micropipette with disposable tips
Binocular microscope (ZEISS, model: Stemi 305 or a comparable binocular microscope from any other manufacturer e.g., Nikon, Olympus, Leica)
Light microscope with phase contrast 40x objective (ZEISS, model: Axio Lab.A1 or a similar device from any other manufacturer e.g., Nikon, Olympus, Leica)
Hemocytometer
Procedure
Aspirate mosquitoes with the aid of a vacuum pump into a 15 ml conical centrifugation tube and place it on ice for five minutes in order to tranquilise the mosquitoes.
Prepare one reaction tube with 50-100 µl of RPMI for collection of the salivary glands. Place it on ice.
Fill one 10 ml Petri dish with 70% ethanol and a second Petri dish with 1x PBS.
Attach the 27 G needles to the syringes.
Transfer the cooled and therefore immobilised mosquitoes into the Petri dish filled with 70% ethanol for up to 1 min. This will on the one hand reduce bacteria contamination and on the other hand reduce their hydrophobicity. Afterwards, use forceps to transfer the mosquitoes into the Petri dish with 1x PBS.
-
For isolation of the salivary glands, place a female mosquito in a drop of 1x PBS on a glass slide under the binocular microscope. Gently immobilise the thorax of the mosquito with the help of one needle (needle 1), while the other needle (needle 2) is placed at the intersection of head and thorax (Figure 1).
Slowly, but firmly, pull the head apart from the body of the mosquito. Ideally, the salivary glands should stay connected to the head and are easily distinguished from the other tissue by their shining appearance (Figure 2).
While fixating the head, cut off the salivary glands and place them into the prepared 1.5 ml reaction tube. Proceed with the remaining mosquitoes and collect all isolated salivary glands in the same reaction tube.
Use the disposable polypropylene pestle to disrupt the tissue in the reaction tube and hence free the sporozoites. Grind gently for approximately 2 min until the solution is homogenous.
To determine the total number of sporozoites, make 20 µl of a 1:10 dilution in RPMI. Alternatively, set up the 1:10 dilution with activated sporozoites by using RPMI supplemented with 3% BSA. (see Note 1)
Transfer 10 µl of the dilution into a hemocytometer and allow the sporozoites to settle for 10 min at room temperature. Store the remaining sporozoites on ice.
Count the number of sporozoites in four big counting squares using a light microscope with phase contrast 40x objective (Figure 3).
-
Calculate the total number of sporozoites using following equation:
Ntotal= ([Ncounted/4] x 10 [dilution factor] x 10 [chamber factor]) x sporozoite solution (µl)
In addition, the number of sporozoites per salivary gland can be determined by dividing Ntotal by the amount of dissected female mosquitoes.
Part II: Motility assay
Figure 1. Scheme of a female mosquito with placement of needles and their movement indicated.
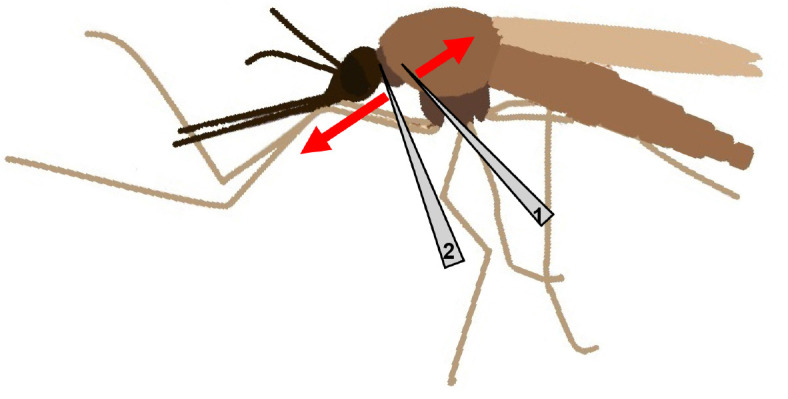
Figure 2. Salivary glands of a female Anopheles mosquito.
The glands on the left are still attached to the head (large dark object at lower left). Scale bar = 50 µm.
Figure 3. Neubauer hemocytometer with schematic sporozoites in the four big squares marked in red, which are counted.

Materials and Reagents
15 ml conical centrifugation tube
1.5 ml reaction tubes
96-well or 384-well optical flat bottom plate (NuncTM MicroWellTM 96 well plates [Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 267342]; NuncTM 384 well plates [Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 240074])
Latex bulb
Isolated Plasmodium sporozoites in a reaction tube (see Part I) on ice
Incomplete RPMI (Thermo Fisher Scientific, GibcoTM, catalog number: 11835063)
17% w/v solution of Accudenz (Accurate Chemical & Scientific, catalog number: AN7050/BLK) in distilled deionised water
Bovine serum albumin (Carl Roth, catalog number: 8076)
BSA/RPMI 6% (see Recipes)
17% Accudenz in distilled deionised water (see Recipes)
Equipment
Micropipette and disposable tips
Glass Pasteur pipette
Table top microcentrifuge (Eppendorf)
Heraeus Multifuge 1 SR (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM MultifugeTM 1 SR) or comparable centrifuge
Zeiss Axiovert 200M (ZEISS, model: Axiovert 200M) inverted microscope or equivalent device from any other manufacturer e.g., Nikon, Leica, Olympus; 25x (NA 0.8) or 10x (NA 0.25) objective
Software
Fiji (download at http://imagej.net/Fiji)
Procedure
Note: If using sporozoites from 5 or less well-infected female mosquitoes gliding assays can be performed directly. Accudenz purification of sporozoites (Kennedy et al., 2012) is needed when sporozoites derived from more than 5 salivary glands are used due to impurity with mosquito debris.
-
Accudenz purification
Adjust the volume of the undiluted sporozoite solution to 1 ml with RPMI.
Load the sporozoite solution onto a 3 ml Accudenz cushion in a 15 ml conical centrifugation tube.
Spin the tube at 2,500 × g without brake for 20 min at room temperature.
Collect the interface comprising of purified sporozoites with a glass Pasteur pipette and transfer the solution into a clean 1.5 ml reaction tube (see Figure 1A in Kennedy et al., 2012 ).
Spin for 3 min at 17,000 × g (maximum speed) at room temperature in a table top microcentrifuge.
Discard supernatant and resuspend the pelleted sporozoites in 50 µl incomplete RPMI.
Count sporozoites as described in Part I steps 8-11.
-
Motility assay
Prepare a 6% solution of bovine serum albumin in RPMI (see Note 1).
Determine an appropriate volume of the sporozoite solution and transfer it into a clean 1.5 ml reaction tube. A total number of about 2,500 sporozoites per 50 µl using a 96-well and 500 sporozoites in 25 µl in a 384-well plate is convenient for a motility assay. Adjust the volume using incomplete RPMI but consider the volume of the drugs, activators or inhibitors, which are to be tested.
If appropriate, add drug, activator, or inhibitor in the concentration to be tested (see Note 2)
Activate the sporozoites by adding 50 µl or 25 µl 6% BSA in RPMI, for the 96- or 384-well plate, respectively (final concentration of 3% BSA), into the reaction tube and transfer the solution into a well of the preferred plate.
Spin the 96- or 384-well plate for 3 min at 200 × g in a centrifuge at room temperature.
Transfer the plate to the Axiovert 200M microscope and start recording 1 image every 3 sec for 3 min using the 25x objective for a 96-well plate (Video 1) and a 10x objective for a 384-well plate. Record at least 3 movies each from 3 independent experiments to get an appropriate number of sporozoites to analyse. Be aware that it is absolutely necessary to keep the same settings between different experiments as otherwise results cannot be compared.
Video 1. Representative video of gliding sporozoites on glass recorded with a frame rate of 1 image every 1.5 sec for a time frame of 90 sec.

Scale bar = 10 µm.
Data analysis
Analysis of individual sporozoite speeds can be performed by manual tracking using the manual tracking plugin in Fiji ( Carey et al., 2013 ). Alternatively the number of circles a gliding sporozoite accomplishes in 100 sec can be determined and used as a proxy for speed ( Hegge et al., 2010 ).
For sporozoite movement pattern classification, z-projection of individual movies can be performed using Fiji (Image->Stacks->z-Project->Max intensity->OK), followed by manually assigning the pattern into attached, waving or gliding (Figure 4).
For in-depth analysis, movies have to be investigated manually in full-length to additionally determine other forms of motion or whether sporozoites are gliding clockwise or counterclockwise.
Movies of fluorescent sporozoites recorded with a 10x objective can also be analysed with the automated tracking plugin ToAST ( Hegge et al., 2009 ) also present in Fiji. The output data record speed of individual sporozoites, average speed as well as detailed movement patterns for all tracked sporozoites.
Figure 4. Motility assay.

A. First slide of movie and B. z-projection; Examples of C. continuous gliding (red squares), D. Waving (blue squares) and E. patch gliding (orange squares). Max projection = maximum projection.
Notes
Always prepare fresh 3% or 6% BSA/RPMI solution before starting the experiment.
When testing different compounds or concentrations, plan at least 10-15 min at the microscope to assess the gliding for each reaction mix. To ensure identical incubation times, the addition of compounds and/or BSA can be delayed until the measurement of the previous sample is completed.
Generally, sporozoites from rodent infecting malaria model species such as P. berghei or P. yoelii show robust motility and are mostly investigated in order to understand the underlying molecular mechanisms. Sporozoites expressing fluorescent proteins in their cytoplasm are the most easy to handle. However, also non-fluorescent sporozoites from human infecting species can be analysed.
It is important to dissect salivary glands as pure as possible given that impurities like mosquito debris can influence gliding and complicate image analysis.
Recipes
-
BSA/RPMI 3%
Weigh out 0.03 mg BSA, ideally directly in the reaction tube
Add 1 ml RPMI
Vortex until the albumin crystals are completely dissolved
Store on ice during the experiment
-
BSA/RPMI 6%
Weigh out 0.06 mg BSA, ideally directly in the reaction tube
Add 1 ml RPMI
Vortex until the albumin crystals are completely dissolved
Store on ice during the experiment
-
17% Accudenz in distilled deionised water
Weigh out 1.7 mg Accudenz in a 15 ml conical centrifugation tube
Add 10 ml distilled deionised water
Vortex until complete dissolving of the powder
The solution can be stored at 4 °C for at least a week
Acknowledgments
Funding: collaborative research center SFB 1129 of the Deutsche Forschungsgemeinschaft.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Carey A. F., Menard R. and Bargieri D. Y.(2013). Scoring sporozoite motility. Methods Mol Biol 923: 371-383. [DOI] [PubMed] [Google Scholar]
- 2.Hegge S., Kudryashev M., Smith A. and Frischknecht F.(2009). Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection . Biotechnol J 4(6): 903-913. [DOI] [PubMed] [Google Scholar]
- 3.Hegge S., Munter S., Steinbuchel M., Heiss K., Engel U., Matuschewski K. and Frischknecht F.(2010). Multistep adhesion of Plasmodium sporozoites . FASEB J 24(7): 2222-2234. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy M., Fishbaugher M. E., Vaughan A. M., Patrapuvich R., Boonhok R., Yimamnuaychok N., Rezakhani N., Metzger P., Ponpuak M., Sattabongkot J., Kappe S. H., Hume J. C. and Lindner S. E.(2012). A rapid and scalable density gradient purification method for Plasmodium sporozoites . Malar J 11: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]



