Abstract
Root system architecture depends on nutrient availability. A symptom of iron (Fe) toxicity in plants is stunted root growth, yet little is known about the effects of excess Fe on lateral root (LR) development. To better understand how nutrient signals are integrated into root developmental programs, we investigated the morphological response of Arabidopsis thaliana root systems to Fe by testing homogeneous supply and localized Fe supply treatment.
Keywords: Arabidopsis, Fe toxicity, Lateral root, Localized iron supply, Homogeneous iron supply
Background
A localized supply of nutrients regulates lateral root elongation and/or lateral root density in Arabidopsis thaliana. Lateral root formation is affected by nutrients at different developmental stages (e.g., initiation versus elongation) and in a nutrient-specific manner ( Li et al., 2011 ; Giehl et al., 2012 ). Iron toxicity as a syndrome in plants is typically associated with an excessive amount of the ferrous form (the Fe2+ ion) in the soil (Vigani, 2012). Iron toxicity symptoms vary with cultivars and are characterized by a reddish-brown, purple bronzing, yellow, or orange discoloration of the lower leaves and a stunted root growth. The presence of the Fe2+ ion is increased by low pH and anoxic conditions, and there is an increasing presence in vertically lower strata (Becker and Asch, 2005; Li et al., 2016 ). We describe a detailed pipeline used for localized iron supply in Arabidopsis grown in vitro which we validated in Arabidopsis ( Li et al., 2015a ; 2015b and 2016).
Materials and Reagents
Eppendorf tubes (1.5 ml)
Sterile tips
Plastic wrap (Bemis, catalog number: PM996)
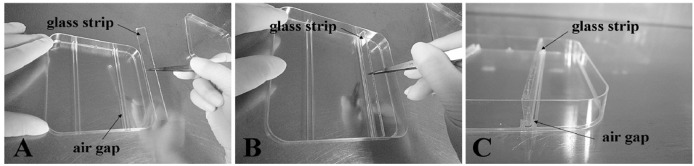
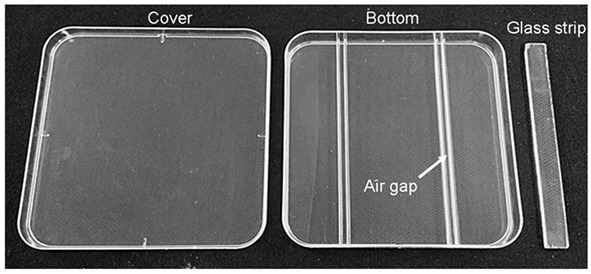
130 x 130 x 12 mm square plastic plates (self-made, see Figure 1)
130 x 13 x 2 mm glass strip (self-made, see Figure 1)
Arabidopsis thaliana seeds (DR5:GUS lines, Col-0 background)
Distilled water
Ethanol (Sinopharm Chemical Reagent, catalog number: 80176961)
Sodium hypochlorite (NaClO) (Sinopharm Chemical Reagent, catalog number: 80010428)
Sodium dodecyl sulfate, sodium salt (SDS) (Sinopharm Chemical Reagent, catalog number: 30166428)
Potassium phosphate monobasic (KH2PO4) (Sinopharm Chemical Reagent, catalog number: 10017618)
Sodium nitrate (NaNO3) (Sinopharm Chemical Reagent, catalog number: 10019918)
Magnesium sulfate (MgSO4) (Sinopharm Chemical Reagent, catalog number: 10013018)
Calcium chloride (CaCl2) (Sinopharm Chemical Reagent, catalog number: 20011160)
Ferrous sulfate (FeSO4.7H2O) (Sinopharm Chemical Reagent, catalog number: 10012116)
Ethylene diamine tetraacetic acid (EDTA) (Sinopharm Chemical Reagent, catalog number: 10009717)
Boric acid (H3BO3) (Sinopharm Chemical Reagent, catalog number: 10004818)
Manganese sulfate (MnSO4) (Sinopharm Chemical Reagent, catalog number: LB2208102)
Zinc chloride (ZnCl2) (Sinopharm Chemical Reagent, catalog number: 10023828)
Copper sulfate (CuSO4) (Sinopharm Chemical Reagent, catalog number: 10008216)
Sodium molybdate (Na2MoO4) (Sinopharm Chemical Reagent, catalog number: 10019818)
Sucrose (Sinopharm Chemical Reagent, catalog number: 10021418)
MES hydrate (Sigma-Aldrich, catalog number: M8250)
Agar-agar (Sigma-Aldrich, catalog number: A7002)
Seed sterilization solution (see Recipes)
Normal growth medium (see Recipes)
Fe-supplemented medium and the control medium (see Recipes)
Control medium (see Recipes)
Figure 1. Square plastic plates used for treatment.
Equipment
Glass bottles (Schott Duran glass bottle, 500 ml capacity or higher)
Incubation chamber (23 ± 1 °C, under fluorescent lamps at 100 μmol/m2/sec, 16-h-light/8-h-dark)
pH meter (Mettler-Toledo International, model: FE20K)
Autoclave (TOMY DIGITAL BIOLOGY, model: SX-500)
Refrigerator (Haier, model: BCD-648WDBE)
Flow hood (Suzhou Antai Air-tech, model: SW-CJ-1F(D))
Vortex
Tweezers
Procedure
The following media are required (see Recipes section): (a) normal growth medium, for seed germination, (b) control medium which contains 50 μM Fe-EDTA and (c) Fe-supplemented medium which contains 350 μM Fe-EDTA.
-
Seed sterilization and plating of seeds.
Put 5 mg (200-300 seeds) of Arabidopsis seeds into an Eppendorf tube and add 750 µl sterilization solution (see Recipes).
Vortex for 1 min at room temperature.
Decant the sterilization solution (with sterile tips) under a flow hood and add 750 µl sterile water to wash the seeds.
Vortex briefly and decant the water.
Repeat the wash step 5 times.
Stratify the seeds in 750 µl sterile water at 4 °C for 2 days in the dark.
Sow seeds on sterile normal growth medium plates. Next close and seal the plates with plastic wrap.
Incubate plates in a vertical position in a growth chamber at 23 °C with a 16-h-light/8-h-dark cycle (150 μmol m-2 sec-1).
Grow seedlings for 5-7 days (with a primary root length of about 2 cm).
-
Whole root and localized root supply of excess Fe.
Prepare the plates (see the Figure 2), and at least three replicates (plates) are needed per treatment.
Preparation of agar media for uniform and localized Fe treatments. The agar media is firstly autoclaved for 20 min at 121 °C on the liquid cycle. The Fe-supplemented medium can be set up as follows. For (I) uniform-Fe supplemented medium, spread the treatment media evenly (Figure 3C). For (II) ‘root tip-Fe supplemented medium’, use segmented agar plates that are separated into upper and bottom parts with a 3-mm air gap that is made with 3 mm-wide movable glass strips. Pour the control medium into the upper part and Fe-supplemented medium into the bottom part (Figure 3D). For (III) ‘shoot-Fe supplemented medium’, pour control medium into the bottom part and pour Fe-supplemented medium into the upper part (Figure 3E). The composition of the Fe-supplemented medium (350 μM Fe-EDTA) and control medium (50 μM Fe-EDTA) is similar to the normal growth medium but the pH is 5.3.
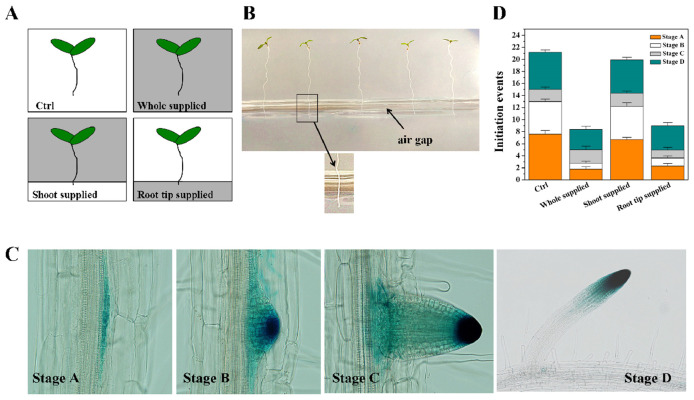
For Fe treatment of the seedlings. Germinate the seedlings (DR5:GUS lines) for 5 to 7 days and then transfer them to Fe-supplemented media with forceps (gently stir up the seedlings but do not pick them up) (Figure 4A). For ‘root tip-supplied’ plants, arrange so that only the primary root tip of the seedlings (~2 mm) is in contact with the bottom of the medium are referred to as ‘root tip-supplied Fe’ plants (Figure 4B). For ‘shoot-supplied Fe’ plants, arrange so that the shoot and mature primary root zone of the seedlings are in contact with the upper parts of the medium. The duration of the treatment is 5 days. All initiation events, including emerged LRs and LRP, are quantified using DR5:GUS lines (Figures 4C and 4D).
Figure 2. Prepare the plates for treatment.
The glass strip and tweezers are autoclaved. Place the glass bars in the Petri dishes with tweezers and the glass bar must be fixed tightly in the air gap.
Figure 3. Experimental setup for preparing control and Fe-supplemented medium.
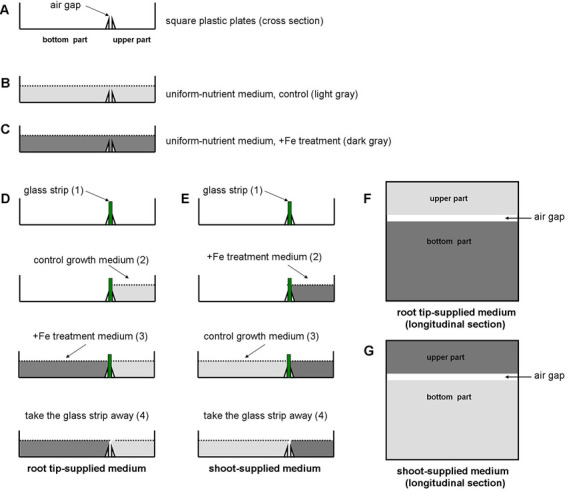
The control medium appears light gray; Fe-supplemented medium appears dark gray.
Figure 4. Effect of local supply of Fe on LR initiation in Arabidopsis.
A. Schematic diagram of the experimental setup for applying excess Fe to the whole root versus the root tip and the shoot-mature root continuum. White sections indicate the basal growth medium with the control medium, and gray sections indicate the Fe-supplemented medium (350 μM Fe-EDTA). B. A picture of seedlings after transfer to the final media; C. Images of lateral roots observed under the microscope ( Zhang et al., 1999 ); D. The effect of locally supplied Fe on LR initiation at 5 day. Values are means of 6 plants ± SE. Ctrl, Control medium.
Data analysis
After treatment count the number of lateral root initiation events under a dissecting microscope. The developmental stage of each lateral root primordia (LRP) is classified according to Zhang et al. (1999) into the following stages: stage A, up to three cell layers; stage B, unemerged, more than three cell layers; stage C, emerged LRs, less than 0.5 mm in length; and stage D, LRs, greater than 0.5 mm. The emerged but not activated LRP are still referred to as LRP, and only mature LRs (exceeding 0.5 mm in length) are denoted as LRs (Figure 4C). The number of lateral root initiation events is stage A + B + C + D (Figure 4D).
Notes
From the seed sterilization step, all the operations must be performed at a clean bench.
For the step of placing the glass bar in the Petri dishes: Firstly, the glass bar and forceps must be sterile; Secondly, the glass bar must be placed in the groove (air gap) of the Petri dishes using forceps. All the operations must be performed at a clean bench.
In Figures 3D and 3E, before moving on to step 3, you have to wait for the agar from step 2 to solidify in the upper part. Similarly, before moving on to step 4, wait for the agar from step 3 to solidify in the bottom part.
Recipes
-
Seed sterilization solution
75% (v/v) ethanol
10% (v/v) NaClO
0.1% (w/v) SDS
-
Normal growth medium
Components Final concentration Stock concentration Volume of stock solution/weight for 1 L KH2PO4 2 mM 40 mM 50 ml NaNO3 5 mM 100 mM 50 ml MgSO4 2 mM 40 mM 50 ml CaCl2 1 mM 500 mM 2 ml Fe-EDTA 50 μM 10 mM 5 ml H3BO3 50 μM 50 mM 1 ml MnSO4 12 μM 12 mM 1 ml ZnCl2 1 μM 1 mM 1 ml CuSO4 1 μ M 1 mM 1 ml Na2MoO4 0.2 μM 0.2 mM 1 ml Sucrose 1% 10 g MES 0.5 g/L 10 g Agar 0.8% 8 g pH 5.7 (adjust with 1 N NaOH) -
Fe-supplemented medium
Normal growth medium supplemented with 350 μM Fe-EDTA
Adjust pH to 5.3 with 1 N NaOH
-
Control medium
Normal growth medium supplemented with 50 μM Fe-EDTA
Adjust pH to 5.3 with 1 N NaOH
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31430095) and the Chinese Academy of Sciences Innovation Program (CAS ISSASIP1604). This protocol was adapted from Li et al., 2015a .
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Becker M. and Asch F.(2005). Iron toxicity in rice-conditions and management concepts. J Plant Nutr Soil Sci 168: 558-573. [Google Scholar]
- 2.Giehl R. F., Lima J. E., von Wiren N.(2012). Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution . Plant Cell 24(1): 33-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B., Li Q., Su Y., Chen H., Xiong L., Mi G., Kronzucker H. J. and Shi W.(2011). Shoot-supplied ammonium targets the root auxin influx carrier AUX1 and inhibits lateral root emergence in Arabidopsis . Plant Cell Environ 34(6): 933-946. [DOI] [PubMed] [Google Scholar]
- 4.Li G., Kronzucker H. J. and Shi W.(2016). The response of the root apex in plant adaptation to iron heterogeneity in soil. Front Plant Sci 7: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Song H., Li B., Kronzucker H. J. and Shi W.(2015). Auxin resistant1 and PIN-FORMED2 protect lateral root formation in Arabidopsis under iron stress . Plant Physiol 169(4): 2608-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G., Xu W., Kronzucker H. J. and Shi W. M.(2015). Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis . J Exp Bot 66, 2041-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigani G.(2012). Discovering the role of mitochondria in the iron deficiency-induced metabolic responses of plants. J Plant Physiol 169(1): 1-11. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., Jennings A., Barlow P. W. and Forde B. G.(1999). Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci U S A 96(11): 6529-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]