Abstract
The innate fear response is an emotional response that does not require any previously acquired conditioning. One of the standard methods to analyze the innate fear response is a 2,4,5-trimethylthiazoline (TMT)-induced freezing test. TMT is an odor originally isolated from anal secretion of the red fox. Acute TMT exposure has been shown to induce robust freezing behavior in rats and mice (Wallace and Rosen, 2000; Galliot et al., 2012 ). Here, I show how to expose mice to TMT and how to analyze their freezing behavior.
Keywords: Fear, Freezing, Innate, Predator, TMT, Odor
Background
To escape detection by predators, many mammalian species, including rodents, have developed innate fear responses triggered by odor stimuli that indicate the presence of predators ( Takahashi et al., 2005 ). The predator’s odorous substance, such as excretion and fur particles, triggers anxiety in the rodent without direct contact and induces avoidance or freezing behavior, depending on the circumstances. For example, if a mouse is not able to run away from the source of the odor (e.g., confined to a small box), the mouse freezes. If the mouse can run away, they will avoid the source of the odor rather than freeze ( Hacquemand et al., 2010 ; Johnston et al., 2012). TMT (2,4,5-trimethylthiazoline), a component of fox feces, is the most used synthesizable reagent for inducing innate fear in rodents (Vernet- Maury et al., 1984 ). Wallace et al. found that innate fear responses of rats can be quantified by measuring the freezing duration when the animals are exposed to TMT in a small confined space. They also found that innate fear responses to TMT do not induce conditioned learning. This finding indicates that different neural pathways are activated during TMT exposure from those activated during conventional footshock-induced fear responses. Lesion studies have shown that the regions associated with the innate fear responses include the medial/central nucleus of the amygdala and the bed nucleus of the stria terminalis (BNST) ( Fendt et al., 2003 ; Müller and Fendt, 2006). Here, I present conventional methods for measuring TMT-induced fear responses in the mouse.
Materials and Reagents
Kimwipe (1.5 x 2 cm)
Plastic bag
Gloves
-
Disposable circular test chamber with a transparent lid (13 cm in diameter, 10 cm in height)
Note: Transparent lid is required for video tracking. I purchased the opaque chambers (B-313, Tokyo Garasu Kikai) and modified their lids to be transparent. However totally transparent chamber could be utilized alternately.
C57BL/6J from Charles River Laboratories, 14 weeks old
-
2,5-Dihydro-2,4,5-trimethylthiazoline (TMT) (Contech, catalog number: 13267)
Note: Contech no longer manufactures TMT. Obtain from other vendors (e.g., ChemSpider, catalog number: 231591).
80% ethanol
5% ammonium hydroxide
Equipment
Charge-coupled device (CCD) camera (Logicool, model: HD WEBCAM C270)
Fume hood (Safety cabinet)
Walls (Cardboard, 30 x 35 cm)
Pipetman pipette (P10, Gilson)
Software
ImageFZ (ImageJ plugin; Shoji et al., 2014 ; http://www.mouse-phenotype.org/) Note: Although I had used ImageFZ in the work, this software supports for only old OS (Windows XP/7 and Mac OS 9). For the compatibility with later Windows OS, a standalone software, FreezeAnalyzerForAVI (http://www.yuzaki-lab.org/publication/software) can be used as an alternative (compatible with Windows XP/7/8.1/10).
Procedure
Notes:
Although TMT is not a deleterious substance, it can cause irritations at high concentrations so it should be treated in a fume hood.
TMT is colorless but has a distinct, strong smell. Thus, it is not easy to perform the experiments with the experimenter blinded to the exposure condition. However, if the fume hood has a minimal opening and there is no leakage of TMT, performing this experiment in a blinded manner may be possible.
Sex-and age-matched littermate controls should be used (sex, age, genetic background, and parental nurturance could affect animal behavior).
The experimenter should opt for either single- or group-housing, since the number of cage mates might affect the results. I kept 2-4 mice per cage (136 x 208 x 115 mm).
Avoid cage changing 24 h before all the procedures of the experiment.
Genotype/treatment should be blinded to avoid experimenter bias.
Handle all animals twice for 5 min each in a fume hood the day before the TMT exposure to habituate them to the experimenter and the fume hood (Video 1).
-
Move animal housing cages in the experimental room from the breeding area at least 30 min before start of the experiment.
Note: If possible, experimental animal cages should be placed in a next room to experimental room to minimize an effect of leaking TMT during the experiment.
Stick a piece of Kimwipe (1.5 x 2 cm) to the top edge of the wall of a disposable circular test chamber (Figure 1A).
Place the test chamber in the fume hood and locate the CCD camera in a suitable position so that the test chamber is kept within its visual field (Figure 1B).
Place each mouse gently into the test chamber, put on a transparent lid with vents, and close the door with a blinder to hide the experimenter from the mouse. Start video capture. This ‘no-odor’ interval lasts for 5 min, in order to record a baseline level of freezing.
-
Open the door and apply the neat 5 μl TMT to the Kimwipe with a Pipetman pipette. Close the door and continue observing for 12 min to monitor TMT-induced freezing.
Note: Pay attention to avoid potential leakage of TMT from the fume hood to the experimental room. If TMT is spilled out, wipe out as soon as possible with 80% ethanol or 5% ammonium hydroxide.
Remove the mouse from the test chamber and place it into the temporary holding chamber next to the experiment room, to prevent untested mice from being disturbed by TMT on the surface of the analyzed mouse.
Replace the test chamber with a new one and seal off the used test chamber tightly in a plastic bag and place it at a separate room (Each test chamber is used only once).
Renew gloves and repeat from step 5.
When all experimental procedures are completed, wipe fume hood with 80% ethanol or 5% ammonium hydroxide and discard all experimental apparatus such as cardboard walls and test chambers.
Video 1. An example of mice handling in the fume hood.

Figure 1. Equipment for a TMT-induced freezing test.
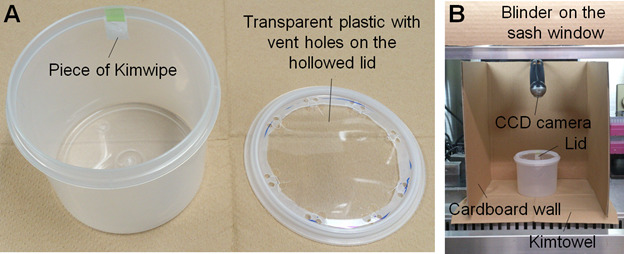
A. Disposable circular test chamber and lid; B. The placement of equipment. The chamber is placed at the center of the walled area and illuminated at 500 lux in my equipment.
Data analysis
Freezing behavior is defined as immobility with the exception of breathing and percentages of the summed freezing durations in each bin were calculated. The duration of freezing is considered to reflect a degree of fear. Longer freezing is interpreted as larger fear. Measure freezing duration of each animal by use of an automated freeze analyzing software (e.g., ImageFZ or FreezeAnalyzerForAVI), or manually in a blinded fashion. A video-rate of 2 frames/sec is sufficient for the automated analysis. A representative result of wild-type mice is shown in Video 2 and Figure 2. Two-way repeated-measures ANOVA (Genotype/treatment x 1-min time bin) was used to determine statistical difference ( Wallace et al., 2000 ).
Video 2. An example of TMT-induced freezing.
The mouse showed freezing behavior after the administration of TMT.
Figure 2. Representative result of wild-type mice in a TMT-induced freezing test.
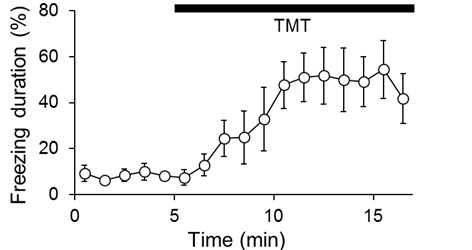
Each dot represents the percentage of average freezing duration of six female wild-types (C57BL/6J from Charles River Laboratories, 14 weeks old). Error bars show SEM. The mice were exposed to TMT for 5 min after the start of acclimation.
Acknowledgments
This protocol was modified from Galliot et al. (2012). I thank Dr. Michisuke Yuzaki for his continuous support. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (16H06461 to MY).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Galliot E., Laurent L., Hacquemand R., Pourie G. and Millot J. L.(2012). Fear-like behavioral responses in mice in different odorant environments: Trigeminal versus olfactory mediation under low doses. Behav Processes 90(2): 161-166. [DOI] [PubMed] [Google Scholar]
- 2.Fendt M., Endres T. and Apfelbach R.(2003). Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci 23(1): 23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston R., Müller-Schwarze D. and Sorenson P.(1999). Advances in Chemical Signals in Vertebrates. Springer. [Google Scholar]
- 4.Müller M. and Fendt M.(2006). Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res 167(1): 57-62. [DOI] [PubMed] [Google Scholar]
- 5.Hacquemand R., Jacquot L. and Brand G.(2010). Comparative fear-related behaviors to predator odors(TMT and natural fox feces) before and after Intranasal ZnSO4 treatment in mice . Front Behav Neurosci 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoji H., Takao K., Hattori S. and Miyakawa T.(2014). Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp (85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi L. K., Nakashima B. R., Hong H. and Watanabe K.(2005). The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev 29(8): 1157-1167. [DOI] [PubMed] [Google Scholar]
- 8.Vernet-Maury E., Polak E. H. and Demael A.(1984). Structure-activity relationship of stress-inducing odorants in the rat. J Chem Ecol 10(7): 1007-1018. [DOI] [PubMed] [Google Scholar]
- 9.Wallace K. J. and Rosen J. B.(2000). Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci 114(5): 912-922. [DOI] [PubMed] [Google Scholar]


