Abstract
A self-suppression mechanism of biofilm development in the cyanobacterium Synechococcus elongatus PCC 7942 was recently reported. These studies required quantification of biofilms formed by mutants impaired in the biofilm-inhibitory process. Here we describe in detail the use of chlorophyll measurements as a proxy for biomass accumulation in sessile and planktonic cells of biofilm-forming strains. These measurements allow quantification of the total biomass as estimated by chlorophyll level and representation of the extent of biofilm formation by depicting the relative fraction of chlorophyll in planktonic cells.
Keywords: Biofilm, Cyanobacteria, Synechococcus elongatus, Chlorophyll measurement, Sessile, Planktonic
Background
Several recently published studies indicate an emerging interest in the mechanisms that underlie cell-aggregation and biofilm development in cyanobacteria ( Fisher et al., 2013 ; Jittawuttipoka et al., 2013 ; Schatz et al., 2013 ; Enomoto et al., 2014 ; Schwarzkopf et al., 2014 ; Enomoto et al., 2015 ; Oliveira et al., 2015 ; Agostoni et al., 2016 ; Parnasa et al., 2016 ). We recently reported a self-biofilm-inhibitory mechanism that dictates planktonic growth of the model unicellular cyanobacterium Synechococcus elongatus PCC 7942 ( Schatz et al., 2013 ; Nagar and Schwarz, 2015). Abrogation of the biofilm-inhibitory process by inactivation of particular genes results in robust biofilm development in this otherwise planktonic strain ( Schatz et al., 2013 ; Nagar and Schwarz, 2015). These studies required quantification of the extent of biofilm development in various strains and under different conditions. Crystal violet is commonly used for quantification of biofilms in heterotrophic bacteria (O’Toole and Kolter, 1998). This staining procedure, however, quantifies only the sessile fraction of cells. Here we provide a detailed protocol for culture growth and quantification of cyanobacterial biofilms using chlorophyll measurement as a proxy for biomass accumulation in sessile as well as in planktonic cells. These measurements allow estimation of the total biomass accumulated and representation of the relative fraction of chlorophyll in sessile or in planktonic cells.
Materials and Reagents
-
Custom-made Pyrex glass tubes for bacterial liquid cultures (200 x 32 mm, made from Pyrex tubing, Corning, catalog number: 8510-32-D)
Note: Can order fromhttp://www.degroot.co.il/.
‘Sponge plug’ (Plastic foam stoppers, 27 x 34 mm) (Jaece Industies, catalog number: L800-C)
Pasteur pipettes (230 mm) (Romical, catalog number: 94-08401002)
0.45 µm syringe filter (Sartorius, catalog number: 16555)
Filter Stericup-GP 250 ml Express Plus PES (0.22 μm) (EMD Millipore, catalog number: SCGPU05RE)
Silicone tubing (6 x 9 mm, 4 x 6 mm) (Degania Silicone, catalog numbers: 2110600234, 2110400434, respectively)
-
Sterile pipettes 1 and 25 ml
1 ml pipettes (Corning, Costar®, catalog number: 4011)
25 ml pipettes (Corning, Costar®, catalog number: 4489)
Sterilized pipette tips (Corning, Axygen®, catalog numbers: T-200-C, T-1000-C)
Eppendorf tubes (1.5 ml) (Corning, Axygen®, catalog number: MCT-175-C)
Synechococcus elongatus PCC7942
Sodium nitrate (NaNO3) (Sigma-Aldrich, catalog number: S8170)
-
Magnesium sulfate heptahydrate (MgSO4.7H2O) (Merck, catalog number: K26364082)
Note: This product has been discontinued. Alternatively, Merck, catalog number: 105886 can be used.
-
Calcium chloride dihydrate (CaCl2.2H2O) (ICN, catalog number: 10035-04-8)
Note: This product has been discontinued. Alternatively, Bio-Lab, catalog number: 034205 can be used.
Potassium phosphate dibasic (K2HPO4) (Honeywell International, Riedel-de-Haen, catalog number: 04248)
-
Ethylenediaminetetraacetic acid (Na2Mg.EDTA) (Bio-Lab, catalog number: 05142359)
Note: This product has been discontinued. Alternatively, Biosolve, catalog number: 051423 can be used.
Ferric ammonium citrate (C6H11FeNO7) (MP Biomedicals, catalog number: 02158040)
-
Citric acid (C6H8O7) (Frutarom, catalog number: 2355511000)
Note: This product has been discontinued. Alternatively, Biosolve, catalog number: 030205 can be used.
-
Boric acid (H3BO3) (Bio-Lab, catalog number: 02010591)
Note: This product has been discontinued. Alternatively, Biosolve, catalog number: 020105 can be used.
Manganese chloride hexahydrate (MnCl2.6H2O) (Duchefa Biochemie, catalog number: M0533)
Zinc sulfate heptahydrate (ZnSO4.7H2O) (CARLO ERBA Reagents, catalog number: 494907)
Sodium molybdate dihydrate (Na2MoO4.2H2O) (MP Biomedicals, catalog number: 194863)
Copper sulphate pentahydrate (CuSO4.5H2O) (Honeywell International, Riedel-de-Haen, catalog number: 12849)
-
Cobalt(II) nitrate hexahydrate (Co(NO3)2.6H2O) (Honeywell International, Riedel-de-Haen, catalog number: 12922)
Note: This product has been discontinued. Alternatively, Sigma-Aldrich, catalog number: 239267 can be used.
N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H3375)
Sodium hydroxide (NaOH) (Frutarom, catalog number: 5553510)
Acetone ((CH3)2CO), A.C.S. reagent (Avantor Performance Materials, J.T. Baker®, catalog number: 9006-03)
Sodium bicarbonate (NaHCO3) (DAEJUNG CHEMICAL & METALS, catalog number: 7566-4100)
Sodium thiosulfate (Na2S2O3) (Sigma-Aldrich, catalog number: 217247)
Bacto agar (BD, BactoTM, catalog number: 214010)
LB agar (Lennox) (BD, DifcoTM, catalog number: 240110)
-
Antibiotics (added as appropriate according to the resistance of the particular strain)
Spectinomycin dihydrochloride pentahydrate (Duchefa Biochemie, catalog number: S0188)
Kanamycine sulphate monohydrate (Duchefa Biochemie, catalog number: K0126)
Gentamycin sulphate (Duchefa Biochemie, catalog number: G0124)
Chloramphenicol (Duchefa Biochemie, catalog number: C0113)
-
Liquid BG11-medium (see Recipes)
Stock I (100x concentrated)
Stock II (100x concentrated)
Stock III (100x concentrated)
Stock V (1,000x concentrated)
Solid BG11-medium (see Recipes)
Equipment
Laminar flow hood
Fume hood
Autoclave
Spectrophotometer (Agilent Technologies, model: Cary 100, catalog number: 10069000) and respective cuvettes (Cell type P.L = 10 mm/EA) (Starna Cells, catalog number: 9-SOG-10)
Benchtop centrifuge (MiniSpin, max. centrifugal force: 12,100 × g) (Eppendorf, model: MiniSpin®, catalog number: 5452000018)
Refrigerator (4-7 °C)
-
Growth rooms (30 ± 2 °C and 24 ± 2 °C for liquid cultures and cultures on solid medium, respectively)
Note: Growing cultures on solid medium at 24 °C rather than 30 °C allows maintaining the cultures for longer periods.
-
Light source
For liquid cultures–incandescent light producing a flux of 20-30 μmol photons m-2 sec-1; Cultures on solid-BG11 are illuminated by fluorescent light (5 μmol photons m-2 sec-1)
Pipet-aid
Single-channel pipettes 200 and 1,000 μl
-
Bubbling system
Air compressor (Oil free air compressor) (Assouline Compressors, model: vs 204 50)
Cylinder carbon dioxide (CO2, compr. 99.5%) (Maxima, catalog number: GCDCU2.527)
Flow meters (Tuttnauer company, 0-0.1 L/min, 1-10 L/min)
-
High-flow air pressure regulator (0-2 PSI) (Marsh Bellofram, model: Type 70, catalog number: 960-129-000)
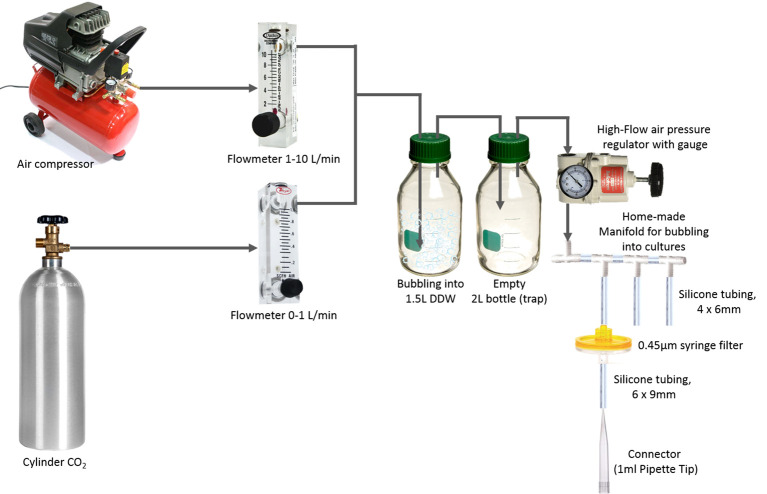
Note: To bubble cultures with 3% CO2 in air the following setup is used (Figure 1): CO2 gas is mixed with compressed air using flow meters to yield 3% CO2 in air. This mixture is humidified by bubbling into 1.5 L double distilled water (DDW) in a 2 L bottle to prevent water evaporation during bubbling of the cultures. The outlet from this bottle is passed through an empty 2 L bottle (serving to trap residual liquid) and through an air pressure regulator (200 mbar is appropriate for bubbling of ~300 culture tubes). Bubbling of CO2 enriched air from the latter into multiple cultures is obtained using home-made manifolds constructed from silicone tubing and appropriate T-bar connectors. The gas in each manifold channel is passed through a 0.45 µm filter (Figure 1). A short video is provided to demonstrate the flow rate in individual cultures tubes (Video 1).
Figure 1. A scheme describing the setup used to bubble cultures with CO2-enriched air .
Video 1. Cyanobacterial cultures under bubbling.

Procedure
-
Growing liquid cultures of Synechococcus elongatus PCC 7942 and biofilm-forming mutants thereof
Round-bottom Pyrex tubes (20 cm in length, 3 cm in diameter) are used as growth vessels.
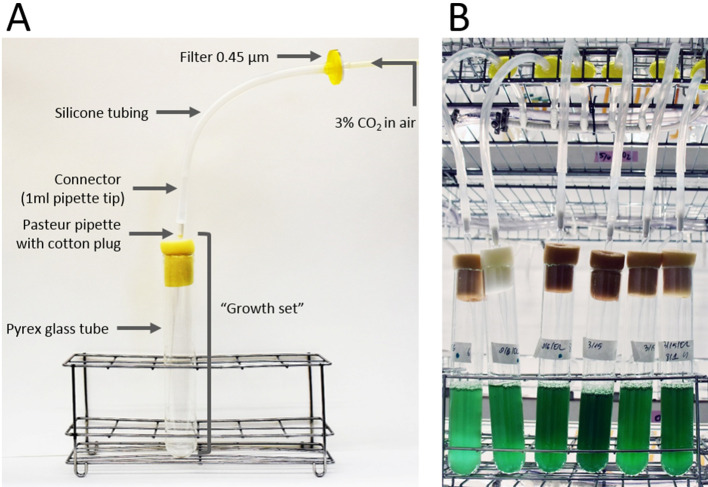
A cotton-plugged Pasteur pipette (23 cm long) is inserted through a sponge-plug such that the tip of the Pasteur pipette is placed 1-2 cm above the bottom of the tube (Figure 2A, ‘growth set’).
Growth sets are autoclaved in liquid cycle.
Sterile liquid BG11-medium (25-50 ml) (see Recipes) is transferred into the Pyrex tube. The culture volume is adjusted to the biomass required for the designated experiment.
-
Cells are scraped from solid-BG11 medium (see Recipes) with a 1 ml sterile plastic pipette and the biomass is shaken in the liquid medium (see Note 4).
Important: To reproducibly observe biofilm formation, it is crucial to add the ferric ammonium citrate and citric acid components from a freshly made stock (see note in Recipe 1f). Autoclaved BG11 should be used within 4 days to inoculate cultures.
Connect the growth set to the bubbling system (see description above) via 1 ml pipette tip (Figure 2A).
The use of manifolds allows simultaneous bubbling of multiple cultures (Figure 2B, see Note under Equipment).
-
Quantification of biofilm
Cells are cultured under continuous bubbling of 3% CO2 in air at 30 °C under continuous illumination with incandescent light (20-30 μmol photons m-2 sec-1).
Starting cultures at the exponential phase are diluted (usually following 3 days of growth) to an optical density at 750 nm of 0.5.
Experiments are initiated by an additional dilution of the cultures to an optical density at 750 nm of 0.5 (see Note 5).
Biofilm development under this setup typically begins after 2-3 days.
To verify that cultures are axenic ‘spot’ a 20 μl aliquot on solid LB agar.
-
Percentage of chlorophyll in suspended cells serves to quantify biofilm formation as follows (Figure 3):
Extraction of chlorophyll in planktonic cells is performed in 1.5 ml Eppendorf tubes. Remove an aliquot from the upper part of the suspended culture (see Note 6). In cases where the suspended fraction appears especially dense (planktonic strains or poor biofilm formers), a 0.2 ml sample is used for extraction. When robust biofilms are formed, 15 ml of planktonic cells are removed and concentrated 3 to 6-fold by centrifugation (5,000 × g, 10 min) prior to the chlorophyll extraction in 80% acetone (final concentration).
Extraction of chlorophyll in the biofilm is performed in the growth tube. To separate the biofilm fraction, slowly remove the suspended cells. Biofilms tend to collapse during removal of the last few ml of liquid. Therefore, once clumps of biofilm detach and fall into the liquid, stop removing the culture. Measure the volume remaining in the tubes using a pipette (in our hands 2-5 ml are left) as well as the volume removed from the culture. The sum of these volumes (Vtotal) serves for calculation of the amount of chlorophyll in the suspended cells (see Data analysis). Extraction of chlorophyll is performed in 80% acetone at a final volume equal to Vtotal.
Extraction of chlorophyll is carried out overnight in the refrigerator and chlorophyll is quantified based on absorbance as previously described (Arnon, 1949; Ritchie, 2006). Chlorophyll may be extracted with methanol instead of acetone. A step-by-step protocol for measurement of chlorophyll a concentration in cyanobacteria is described by Zavřel et al. (2015) .
Figure 2. Growing cyanobacterial cultures.
A. Growth set connected to a single manifold channel; B. Cultures in the growth room.
Figure 3. Quantification of biofilm based on chlorophyll measurement.
Data analysis
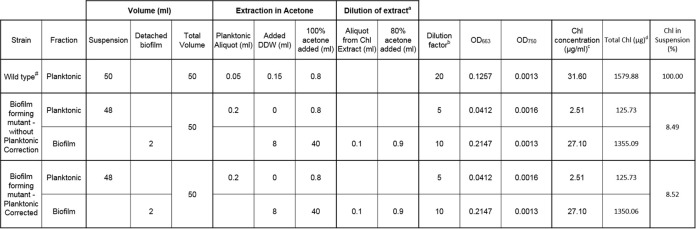
The percentage of chlorophyll in suspension is calculated from the amount of chlorophyll in the planktonic and biofilm fractions. These values are calculated based on measured chlorophyll absorbances, sample volumes, and dilution factors, as described below. An example calculation is provided in Table 1.
Table 1. An example of calculations of chlorophyll (Chl) in planktonic and biofilm cells.
#Grows planktonically.
aDilution of Chl extract is performed to reach the linear range of the assay.
bDilution factor is calculated from the dilution of the aliquot during acetone extraction and the dilution of the Chl extract for spectrophotometry.
cChl concentration = (OD663 - OD750) x 12.7 x (Dilution Factor); see Arnon, 1949.
dFor planktonic samples, (Total Chl) = (Chl concentration) x (Total Volume). For biofilm samples from robust strains, the contribution of Chl from planktonic cells is negligible, and thus (Total Chl) = (Chl concentration) x (Volume of Chl extract), where ‘Volume of Chl extract’ should be equivalent to 'Total Volume'. If correcting for the contribution of planktonic Chl to the biofilm fraction, then (Total Chl) = (Chl concentration in biofilm) x (Volume of Chl extract) - (Chl concentration in planktonic) x (Volume of Detached biofilm).
-
Calculate the amount of chlorophyll in suspended cells based on measured chlorophyll concentration and the volume of the suspended culture (Vtotal).
Total Chl Planktonic (µg) = (OD663 - OD750) x 12.7 x (Dilution Factor) x Vtotal
-
Calculate the amount of chlorophyll in the biofilm based on measured chlorophyll concentration and the volume of the acetone extraction, which should be Vtotal.
-
If robust biofilm formation is observed, then the contribution from the remaining planktonic cells in the biofilm fraction is negligible and the total chlorophyll is calculated as follows:
Total Chl Biofilm (µg) = (OD663 - OD750) x 12.7 x (Dilution Factor) x Vtotal
-
To account for the contribution of remaining planktonic cells in the biofilm fraction, subtract the planktonic chlorophyll present in the fraction’s volume from the above value:
Total Chl Biofilm Corrected (µg) = (Chl concentration in biofilm) x Vtotal - (Chl concentration in planktonic) x (Volume of Detached biofilm)
-
-
Total chlorophyll in the culture is the sum of the amount of chlorophyll in planktonic cells and in the biofilm.
Total Chl (µg) = Total Chl Biofilm + Total Chl Planktonic
Or
Total Chl (µg) = Total Chl Biofilm Corrected + Total Chl Planktonic
-
The extent of biofilm formation is presented as percent of chlorophyll in planktonic cells.
Chl in suspension (%) = (Total Chl Planktonic)/(Total Chl) x 100%
Notes
All work with cyanobacterial cultures is carried out under sterile conditions using a laminar flow hood.
The sponge plug is punched using an awl.
For bubbling of a small number of cultures, a mini aquarium pump (e.g., JBL ProAir a50, Art. No. 6054600) may be used instead of a compressor. As an alternative to mixing air and CO2 one may purchase 3% CO2 cylinders.
A previous study revealed that a biofilm inhibitory substance is present in extracellular fluids from wild type culture as well as in old cultures of the biofilm forming mutant, T2SEΩ ( Schatz et al., 2013 ). To avoid possible inhibitor accumulation in cultures of biofilm-forming mutants, cultures are initiated by inoculation of cells grown from solid agar. Make sure to dilute these starter cultures for ‘biofilm quantification assays’ while yet at exponential phase of growth.
Starter cultures are inoculated from cultures grown on solid agar. Due to variations in the inoculum, the resulting liquid cultures vary in cell density. The dilution steps are important to ensure that experiments are initiated at the same density and a similar physiological state.
First, take an aliquot from the upper part of the suspended culture and then remove the rest of the suspended fraction to avoid contamination of the extraction aliquot with biofilm cells.
Recipes
-
Liquid BG11-medium (based on Stanier et al., 1971 )
-
Stock I, 100x concentrated (autoclave)
150.00 g/L NaNO3
6.50 g/L MgSO4.7H2O
3.60 g/L CaCl2.2H2O
-
Stock II, 100x concentrated (autoclave)
3.05 g/L K2HPO4
0.10 g/L Na2Mg.EDTA
-
Stock III, 100x concentrated (prepare fresh, no need to sterilize)
0.60 g/L C6H11FeNO7
0.60 g/L C6H8O7
-
Stock V, 1,000x concentrated (sterilize by filtration using Filter Stericup [0.22 μm])
2.86 g/L H3BO3
1.84 g/L MnCl2.4H2O
0.22 g/L ZnSO4.7H2O
0.39 g/L NaMoO4.2H2O
0.08 g/L CuSO4.5H2O
0.05 g/L Co(NO3)2.6H2O
HEPES 119.15 g/L (25x concentrated) titrated to pH 8.0 with 10 N NaOH (399.97 g/L) (autoclave)
-
Preparation of 1 L liquid-BG11:
Start with ~500 ml DDW, add the required volume from the stock solutions and complete with DDW up to 1 L. Solution is sterilized by autoclaving (sterilization time: 40 min). Immediately remove from autoclave once the cycle is over (Long exposure to the high temperature may affect precipitation of minerals). Do not autoclave the medium more than once. Mix before aliquoting the medium to growth tubes
Note: To reproducibly observe biofilm formation, it is crucial to use freshly made stock III each time liquid BG11 is prepared. Autoclaved BG11 is stored at room temperature. The medium should be used within 4 days to inoculate cultures.
-
-
Solid BG11-medium
Stock solutions as for liquid BG11
Sodium bicarbonate 42 g/L (100x concentrated)
Sodium thiosulfate 470 g/L (100x concentrated)
Prepare two-fold concentrated liquid BG11 (BG11X2) by diluting appropriate volumes from stock solutions
Prepare 3% Bacto agar in DDW
Autoclave BG11X2 and 3% agar in separate containers
Immediately following sterilization, combine equal volumes of BG11X2 and 3% agar to get 1.5% agar in BG11
-
Cool to ~50-55 °C, dilute 100-fold NaHCO3 and Na2S2O3 stock solutions (at this step antibiotics may be added):
Spectinomycin dihydrochloride pentahydrate (final concentration: 50 μg/ml)
Kanamycine sulphate monohydrate (final concentration: 50 μg/ml)
Gentamycin sulphate (final concentration: 7.5 μg/ml)
Chloramphenicol (final concentration: 7.5 μg/ml)
Pour ~50 ml into 90 mm Petri-dish
Acknowledgments
This protocol is modified from previous studies ( Schatz et al., 2013 ; Parnasa et al., 2016 ). Growth medium is based on the protocol by ( Stanier et al., 1971 ). Chlorophyll measurements are based on previous protocols (Arnon, 1949; Ritchie, 2006). Rakefet Schwarz and Susan Golden are supported by the program of the National Science Foundation and the US-Israel Binational Science Foundation (NSF-BSF 2012823). This study was also supported by a grant from the Israel Science Foundation (ISF 1406/14) to Rakefet Schwarz.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Agostoni M., Waters C. M. and Montgomery B. L.(2016). Regulation of biofilm formation and cellular buoyancy through modulating intracellular cyclic di-GMP levels in engineered cyanobacteria. Biotechnol Bioeng 113(2): 311-319. [DOI] [PubMed] [Google Scholar]
- 2.Arnon D. I.(1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol 24(1): 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enomoto G., Ni Ni W., Narikawa R. and Ikeuchi M.(2015). Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation. Proc Natl Acad Sci U S A 112(26): 8082-8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enomoto G., Nomura R., Shimada T., Ni Ni W., Narikawa R. and Ikeuchi M.(2014). Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus . J Biol Chem 289(36): 24801-24809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M. L., Allen R., Luo Y. and Curtiss R. 3rd(2013). Export of extracellular polysaccharides modulates adherence of the Cyanobacterium synechocystis . PLoS One 8(9): e74514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jittawuttipoka T., Planchon M., Spalla O., Benzerara K., Guyot F., Cassier-Chauvat C. and Chauvat F.(2013). Multidisciplinary evidences that Synechocystis PCC6803 exopolysaccharides operate in cell sedimentation and protection against salt and metal stresses . PLoS One 8(2): e55564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagar E. and Schwarz R.(2015). To be or not to be planktonic? Self-inhibition of biofilm development. Environ Microbiol 17(5): 1477-1486. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira P., Pinto F., Pacheco C. C., Mota R. and Tamagnini P.(2015). HesF, an exoprotein required for filament adhesion and aggregation in Anabaena sp. PCC 7120 . Environ Microbiol 17(5): 1631-1648. [DOI] [PubMed] [Google Scholar]
- 9.O’Toole G. A. and Kolter R.(1998). Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis . Mol Microbiol 28(3): 449-461. [DOI] [PubMed] [Google Scholar]
- 10.Parnasa R., Nagar E., Sendersky E., Reich Z., Simkovsky R., Golden S. and Schwarz R.(2016). Small secreted proteins enable biofilm development in the cyanobacterium Synechococcus elongatus . Sci Rep 6: 32209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie R. J.(2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89(1): 27-41. [DOI] [PubMed] [Google Scholar]
- 12.Schatz D., Nagar E., Sendersky E., Parnasa R., Zilberman S., Carmeli S., Mastai Y., Shimoni E., Klein E., Yeger O., Reich Z. and Schwarz R.(2013). Self-suppression of biofilm formation in the cyanobacterium Synechococcus elongatus . Environ Microbiol 15(6): 1786-1794. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzkopf M., Yoo Y. C., Huckelhoven R., Park Y. M. and Proels R. K.(2014). Cyanobacterial phytochrome2 regulates the heterotrophic metabolism and has a function in the heat and high-light stress response. Plant Physiol 164(4): 2157-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanier R. Y., Kunisawa R., Mandel M. and Cohen-Bazire G.(1971). Purification and properties of unicellular blue-green algae(order Chroococcales) . Bacteriol Rev 35(2): 171-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zavřel T., Sinetova M.A., and Červený J.(2015). Measurement of chlorophyll a and carotenoids concentration in Cyanobacteria . Bio-protocol 5: 1-5. [Google Scholar]