Abstract
During the COVID-19 pandemic older subjects have been disproportionately affected by the disease. Vaccination is a fundamental intervention to prevent the negative consequences of COVID-19, but it is not known if the needs and vulnerabilities of older people are adequately addressed by their inclusion in randomized clinical trials (RCTs) evaluating the efficacy of vaccines for COVID-19. Given this background, we aimed to evaluate if current and ongoing phase II-III RCTs evaluating the efficacy of COVID-19 vaccines included a representative sample of older people. A systematic literature search in PubMed and Clinicaltrials.gov was performed until May 01st, 2021. Among 474 abstracts initially retrieved, 20 RCTs (ten already published, ten ongoing) were included. In the ten studies already published, the mean age of participants was 45.2 ± 11.9 years and only 9.83% of the participants were more than 65 years, 1.66% more than 75 years and less than 1% (0.55%) more than 85 years. In the ten ongoing RCTs, many of the studies aimed at including participants older than 18 years, with one study including participants between 18 and 84 years, and two between 21 and 100 years. In conclusion, our systematic review demonstrates that in published and ongoing phase II-III randomized clinical trials evaluating the efficacy of COVID-19 vaccines only a tiny fraction of the most vulnerable group of older people was included, although they clearly were the first population that had to be vaccinated.
Keywords: COVID-19, Vaccination, Older adults
1. Introduction
History will remember the year 2020 as the beginning of the most devastating pandemic after the Spanish flu, one century ago. Opposite to the influenza virus, the SARS-CoV-2 is a new virus for the human kind which contributed to its rapid spread and its ability to cause a pandemic which is still ongoing. The Coronavirus disease (COVID-19) most strongly affects older adults: they are more likely to experience severe disease, to be hospitalized and account for the vast majority of COVID-19 related deaths (Onder et al., 2020).
A rapid review performed to assess the magnitude of the association between risk factors and severity of COVID- 19 to inform the prioritization of vaccine administration in Canada concluded that older age seems the most important risk factor associated with higher probability of severe outcomes (Jentsch et al., 2021). Patients older than 60 years have an at least five times higher risk of hospitalization and mortality from COVID-19 in comparison with those younger than 45 years (Wingert et al., 2021).
Early in the pandemic, scientists began to evaluate potential treatments for the disease and to develop vaccines. After more than one year, while the search for a very effective drug is still ongoing, several vaccines have been developed and marketed, characterized by high efficacy and effectiveness in preventing COVID-19 disease (Koirala et al., 2020; Soiza et al., 2021). This remarkable achievement has been obtained within a time frame that was extremely reduced compared to usual time of a vaccine development, which typically takes 5–10 years (Mahase, 2020). In order to achieve such a result, the different phases of vaccine development have been performed in parallel, without compromising the accuracy of the studies (Agency, 2021). Therefore, several vaccines have been authorized, after a careful evaluation in order to assure that they fulfilled all the requirements of quality, safety and efficacy that are requested by drug regulatory agencies.
Since older subjects have the highest risk of developing severe disease and to die from COVID-19, they have been identified in several countries as the first group to be vaccinated (Cylus et al., 2021, Hasan et al., 2021). Furthermore, older subjects might have a blunted response to vaccination, due to immunosenescence (Crooke et al., 2019), as well as to common occurrence of diseases and conditions inducing immunological impairment and to pharmacological therapies that reduce immune response (Ciabattini et al., 2020). A previous work published by Helfand et al. suggested that RCTs for COVID-19, registered at clinicaltrials.gov, were likely to exclude older adults (Helfand et al., 2020). Finally, several Phase III clinical trials investigating vaccine safety and efficacy have been published and many others are ongoing but it was reported that participants enrolled in these studies are limited by trial selection criteria, including age limitation (Kwok, 2021).
Given this background, the aim of this systematic review was to investigate whether published and ongoing phase II-III randomized clinical trials evaluating the efficacy of COVID-19 vaccines included a representative sample of older adults.
2. Materials and methods
2.1. Data sources and literature search strategy
Two investigators (NV and AC) conducted a systematic literature search PubMed and Clinicaltrials.gov without language restriction, from database inception to 01st May 2021. The search syntax is fully reported in Supplementary Table 1.
2.2. Study selection
Inclusion criteria for this systematic review were: (i) being a RCT; (ii) double-blind design; (iii) participants treated with a SARS-CoV-2 vaccine; (iv) one group taking placebo; (v) being in a clinical phase, i.e., at least in phase II of an RCT. We have therefore excluded: (i) phase I RCTs; (ii) no original data, such as reviews and commentaries.
2.3. Data extraction
Two investigators (NV and AC) extracted key data from the included articles in a standardized Excel sheet. For each article, we extracted data about authors, year of publication, name and type of the vaccine, phase of the RCT, age criteria included in the protocol, health status of the participants included, reported mean age with standard deviation and overall sample size. When some information regarding age was missing, first and/or corresponding authors of the original article were contacted at least two times in one month to obtain unpublished data. Only one author of the published articles responded answered to our request.
2.4. Outcomes
The main outcome of our work was to evaluate the inclusion of older adults in RCTs of SARS-CoV-2 vaccines in both published and ongoing RCTs. To this purpose, we have extracted the data regarding mean age and the age criteria used in the protocol/published works, asking for other details if needed. Moreover, we calculated with standardized formulas the 95% and the 99% confidence intervals reporting the upper limit for the aims of our work. Finally, we have also reported data regarding the percentage of people older than 65, 75, and 85 years respectively, i.e. commonly used age cut-offs in geriatric medicine (Kowal and Dowd, 2001).
3. Results
3.1. Literature search
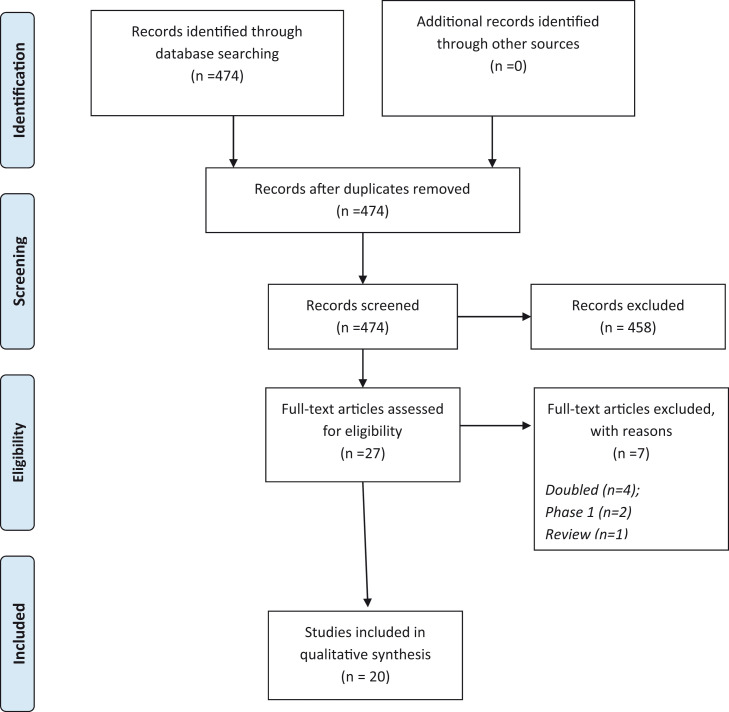
Fig. 1 shows the results of the literature search. Overall, 474 abstracts were retrieved, and 27 full texts were screened. Among them, 20 studies (ten already published, ten ongoing) were included for the current analysis.
Fig. 1.
PRISMA flow-chart.
3.2. Published works
Table 1 summarizes the data regarding all ten already published RCTs on SARS-CoV-2 vaccines (Baden et al., 2021, Keech et al., 2020, Logunov et al., 2021, Mulligan et al., 2020, Sadoff et al., 2021, Voysey et al., 2021, Xia et al., 2020, Xia et al., 2021, Zhang et al., 2021, Zhu et al., 2020). Different vaccine designs were tested. Overall, four studies used viral vector, three inactivated virus, two mRNA and one study protein sub-unit vaccines. Four RCTs were of phase II, three in phase I-II, two in phase III and one in phase II-III. Overall, the majority of the studies (n = 8) included only healthy individuals.
Table 1.
Published Randomized controlled trials on the effect of SARS-CoV-2 Vaccination: characteristics and age distribution of participants.
| Vaccine | Vaccine type | Phase | N | Health status | Age criteria included in the protocol | Mean age | SD age | Calculated upper 95% CI | Calculated 99% upper CI | % ≥ 65 years | % ≥ 75 years | % ≥ 85 years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad26. CoV | Viral vector | Phase 1–2 | 805 | Healthy | 18–88 years | 52.6 | 7.1 | 53.09 | 53.25 | 50.00 | 7.00 | 0.25 |
| Ad5 | Viral vector | Phase 2 | 603 | Healthy | 18 Years and older | 39.7 | 12.5 | 40.70 | 41.01 | NA | NA | NA |
| BBIBP-CorV | Inactivated virus | Phase 2 | 448 | Healthy | 18–59 years | 41.7 | 9.9 | 42.62 | 42.91 | 0.00 | 0.00 | 0.00 |
| BNT162b2 | mRNA | Phase 1–2 | 76 | Healthy | 18–55 years | 35.4 | NA | NA | NA | 0.00 | 0.00 | 0.00 |
| ChAdOx1 (AZS1222) | Viral vector | Phase 2–3 | 11,636 | Healthy. at high risk of exposure | 18 Years and older | NA | NA | NA | NA | 3.81 | NA | NA |
| CoronaVac | Inactivated virus | Phase 1–2 | 673 | Healthy | 18–55 years | 42.6 | 9.4 | 43.31 | 43.53 | 0.00 | 0.00 | 0.00 |
| Gam-COVID-Vac (Sputnik V) | Viral vector | Phase 3 | 19,866 | Healthy | 18 Years and older | 45.3 | 12 | 45.47 | 45.52 | > 60 years: 10.8% | NA | NA |
| mRNA-1273 | mRNA | Phase 3 | 30,351 | No inclusion criteria pre-specified | 18–95 years | 51.4 | NA | NA | NA | 24.80 | 4.60 | 0.30 |
| NVX-CoV2373 | Protein subunit | Phase 2 | 131 | Healthy | 18–59 years | 30.8 | 10.2 | 32.55 | 33.10 | 0.00 | 0.00 | 0.00 |
| WIV04 | Inactivated virus | Phase 2 | 224 | Healthy | 18–59 years | 43.5 | 9.1 | 44.69 | 45.07 | 0.00 | 0.00 | 0.00 |
| Total | 64,813 | 45.2 | 11.9 | 45.29 | 45.32 | 9.83 | 1.66 | 0.55 |
Regarding the age criteria reported in the protocols, in the full-texts and asked to the authors, as shown in Table 1, three studies included participants between 18 and 59 years, three studies people aged > 18 years, two studies people aged between 18 and 55 years, and the other two studies had an upper age limit at 88 and 95 years, respectively.
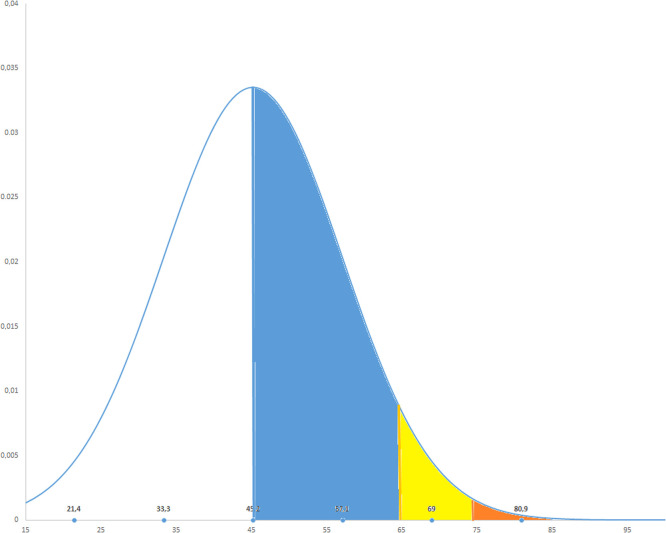
The mean age reported in the full-texts, in the regulatory documents or provided by the corresponding authors was 45.2 ± 11.9 years that leads to a 95% higher confidence of interval of 45.29 years and 99% of 45.32 years. Given these figures, only 9.83% of the subjects included were older than 65 years, 1.66% older than 75 years and less than 1% (0.55%) older than 85 years. These findings are reported in a graphical way in Fig. 2.
Fig. 2.
Graphical representation of age distribution in the randomized controlled trials included. The Blue area represents people between 45.2 years (calculated mean) and 65 years; the yellow area participants between 65 and 75 years; the orange area those between 75 and 85 years; the white area participants with more than 85 years. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Ongoing works
Table 2 shows the 10 ongoing RCTs on SARS-CoV-2 vaccines. Overall, 8/10 studies were phase III, one in phase IV and a final one in phase II-III. The majority of the studies included participants older than 18 years, one study between 18 and 84 years, and another two between 21 and 100 years without more information about the age strata to date.
Table 2.
Characteristics of ongoing phase 3–4 randomized controlled trials on the effect of SARS-CoV-2 Vaccination.
| NCT Number | Title | Vaccine type | Phase | Age |
|---|---|---|---|---|
| NCT04439045 | Efficacy and Safety of VPM1002 in Reducing SARS-CoV-2 (COVID-19) Infection Rate and Severity | VPM1002 | Phase 3 | 18 Years and older |
| NCT04510207 | A Study to Evaluate The Efficacy, Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines (Vero Cell) in Healthy Population Aged 18 Years Old and Above | Inactivated SARS-CoV-2 Vaccine (Vero cell) | Phase 3 | 18 Years and older |
| NCT04583995 | A Study Looking at the Effectiveness, Immune Response, and Safety of a COVID-19 Vaccine in Adults in the United Kingdom | SARS-CoV-2 rS/Matrix M1-Adjuvant | Phase 3 | 18 Years to 84 Years |
| NCT04612972 | Efficacy, Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines (Vero Cell) in Healthy Adult Population In Peru | Inactivated SARS CoV 2 vaccine (Vero cell) | Phase 3 | 18 Years to 60 Years |
| NCT04640233 | Clinical Trial to Assess Safety and Immunogenicity of Gam-COVID-Vac Combined Vector Vaccine for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Сov-2) Infection | Gam-COVID-Vac | Phase 2–3 | 18 Years and older |
| NCT04646590 | A Phase III Clinical Trial to Determine the Safety and Efficacy of ZF2001 for Prevention of COVID-19 | Recombinant new coronavirus vaccine (CHO cell) group | Phase 3 | 18 Years and older |
| NCT04649515 | Efficacy and Safety of TY027, a Treatment for COVID-19, in Humans | TY027 | Phase 3 | 21 Years to 100 Years |
| NCT04651790 | Efficacy, Safety, and Immunogenicity of Two Vaccination Schedules of an Inactivated Vaccine Against COVID-19 in Adults | SARS-CoV-2 inactivated vaccine | Phase 3 | 18 Years and older |
| NCT04659239 | The Efficacy, Safety and Immunogenicity Study of Inactivated SARS-CoV-2 Vaccine for Preventing Against COVID-19 | Inactivated SARS-CoV-2 Vaccine (Vero cell) | Phase 3 | 18 Years and older |
| NCT04747821 | An Effectiveness Study of the Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine | Adsorbed COVID-19 (Inactivated) Vaccine | Phase 4 | 18 Years and older |
4. Discussion
In this systematic review including twenty studies, out of which ten are still ongoing, we found that the presence of older adults was extremely limited. In ten already published studies less than 10% of the participants included were older than 65 years and, only less than 1% of them were older than 85 years. In ongoing RCTs, the majority declared to include subjects older than 18 years without an upper age limit but also without prespecified strata.
Overall, our work confirms the long-standing issue of underrepresentation of older adults in RCTs (Cherubini et al., 2010; Crome et al., 2014). While COVID-19 disease can affect subjects of any age, the majority of patients who suffer from severe disease including long-lasting syndromes (such as “Long COVID”) and experience high case fatality are older adults. (Onder et al., 2020) Moreover, several consequences of long COVID syndrome in older people are still not known (Reid et al., 2021). Therefore, in almost every country across the world, the first population that was vaccinated against COVID-19 were older adults and, in particular, the most vulnerable and frail, such as nursing home residents (Dooling et al., 2020). Although observational studies confirm effectiveness of vaccine also in nursing home residents with respect to symptomatic infection of COVID-19 disease (McEllistrem et al., 2021) and mortality (Wyller et al., 2021), no systematic reviews and controlled trials with a substantial proportion of older participants is available or in sight. To the contrary, mean age of included participants in Phase II/III RCTs was about 45 years. The main causes of the underrepresentation of older adults in clinical trials on COVID-19 vaccines are the selection criteria of the trials (Zhu et al., 2020). First of all, many of them had an upper age limit, that prevented the participation of older adults (Zhu et al., 2020). Even when an upper age limit was not present (Keech et al., 2020, Voysey et al., 2021, Xia et al., 2020, Zhang et al., 2021), the presence of other indirect criteria, such as the exclusion of specific diseases, e.g. chronic diseases or drugs, such as anticoagulants (Voysey et al., 2021, Zhu et al., 2020), disproportionately disadvantage older adults (Cherubini et al., 2011). While the exclusion of vulnerable subjects might be justifiable in the context of phase I or even phase 2 clinical trials, many published trials were phase 3 trials (Baden et al., 2021, Logunov et al., 2021, Voysey et al., 2021), which are performed in order to demonstrate efficacy and safety of a treatment in order to request authorization by regulatory agencies.
This evidence is a further proof of the concept that medications and vaccines commonly used in older adults have not been adequately evaluated in this population. The underrepresentation of older adults in RCTs can have important consequences as it prevents an adequate knowledge of the efficacy and safety of COVID-19 vaccines in a patient group with significant pharmacokinetic and dynamic changes, multimorbidity and polypharmacy, that all can affect efficacy and safety of drugs (Mangoni et al., 2015). This could lead to a higher susceptibility to develop adverse drug events (Davies and O'mahony, 2015, Zazzara et al., 2021), as for example in the case of spironolactone for heart failure which was clearly beneficial in younger (Pitt et al., 1999), but increased mortality in older adults (Juurlink et al., 2004, Pitt et al., 1999). It is also well known that older subjects tend to have a blunted response to vaccines, due to immunosenescence (Crooke et al., 2019), and this might also apply to vaccines used to prevent COVID-19 disease.
Another important aspect to discuss is that also ongoing RCTs that will lead to the approval of new anti-COVID-19 vaccines in the next future will likely not include a relevant percentage of older adults nor will they include any measure of physical frailty or other functional parameters needed. This is, again, in contrast with the strict indications of the most important international organization in the world, such as the World Health Organization (Girard et al., 2011) and the European Medicine Agency that strongly encourage the inclusion of older adults in the RCTs and propose also using frailty measures to better characterize this heterogeneous population (Agency, 2018; Cerreta et al., 2012).
Our findings have several implications. First of all, the underrepresentation of older people implies that the real efficacy and safety of vaccines in this large group, the one most severely affected by COVID-19, are not well characterized. Consequently, the use of vaccines in older people does not fulfill the criteria of evidence based medicine, which require that solid scientific evidence should be available to support the implementation of a new intervention, e.g. vaccination. Therefore, COVID-19 vaccines have been used in older subjects without a proper knowledge of their efficacy and safety in this heterogeneous population. This means also, from an ethical point of view, that older people are discriminated, being denied the right to receive evidence based treatment, as advocated by the Charter elaborated within the PREDICT study (Crome et al., 2014). Moreover, the lack of adequate scientific data in older adults, might undermine the public trust concerning using the vaccines in frail older adults. Finally, these results represent an important warning for researchers who are planning or conducting studies to evaluate preventive or therapeutic interventions against COVID-19 disease to include an adequate number of older subjects in order to assure the generalizability of their findings.
The findings of our review should be interpreted within certain limitations. First, several studies did not include sufficient information regarding age: even if we tried to reach the corresponding authors of the published works, only one answered to our request to provide the additional information. Second, we are not able to synthetize in case of inclusion of older people if they are representative of older population (e.g., adequate presence of people having comorbidities or other geriatric conditions of clinical importance, such as frailty and dementia).
In conclusion, our systematic review found that in published and ongoing phase II-III randomized clinical trials evaluating the efficacy of COVID-19 vaccines, a tiny fraction of older adults was included, even if older people were the most affected by COVID-19 disease and therefore the first population to be vaccinated. This finding further underlines the fact that we need an important change in attitude and policy actions to promote the inclusion in RCTs of all relevant groups of people in which medications and vaccines are used, including older adults.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.arr.2021.101455.
Appendix A. Supplementary material
Supplementary material
.
References
- Agency, E.M., 2018. Physical frailty: instruments for baseline characterisation of older populations in clinical trials.
- Agency, E.M., 2021. COVID-19 vaccines: development, evaluation, approval and monitoring.
- Baden L.R., Sahly El, Essink H.M., Kotloff B., Frey K., Novak S., Diemert R., Spector D., Rouphael S.A., Creech N., McGettigan C.B., Khetan J., Segall S., Solis N., Brosz J., Fierro A., Schwartz C., Neuzil H., Corey K., Gilbert L., Janes P., Follmann H., Marovich D., Mascola M., Polakowski J., Ledgerwood L., Graham J., Bennett B.S., Pajon H., Knightly R., Leav C., Deng B., Zhou W., Han H., Ivarsson S., Miller M., Zaks J., Group, C.S T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerreta F., Eichler H.-G., Rasi G. Drug policy for an aging population – the European Medicines Agency’s geriatric medicines strategy. N. Engl. J. Med. 2012;367:1972–1974. doi: 10.1056/NEJMp1209034. [DOI] [PubMed] [Google Scholar]
- Cherubini A., Oristrell J., Pla X., Ruggiero C., Ferretti R., Diestre G., Clarfield A.M., Crome P., Hertogh C., Lesauskaite V. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch. Intern. Med. 2011;171:550–556. doi: 10.1001/archinternmed.2011.31. [DOI] [PubMed] [Google Scholar]
- Cherubini A., Signore S.D., Ouslander J., Semla T., Michel J.P. Fighting against age discrimination in clinical trials. J. Am. Geriatr. Soc. 2010;58:1791–1796. doi: 10.1111/j.1532-5415.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- Ciabattini A., Garagnani P., Santoro F., Rappuoli R., Franceschi C., Medaglini D. Shelter from the cytokine storm: pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin. immunopathol. 2020:1–16. doi: 10.1007/s00281-020-00821-0. (Springer) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crome P., Cherubini A., Oristrell J. The PREDICT (increasing the participation of the elderly in clinical trials) study: the charter and beyond. Expert Rev. Clin. Pharmacol. 2014;7:457–468. doi: 10.1586/17512433.2014.922864. [DOI] [PubMed] [Google Scholar]
- Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing. 2019;16:1–16. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cylus J., Panteli D., van Ginneken E. Who should be vaccinated first? Comparing vaccine prioritization strategies in Israel and European countries using the Covid-19 Health System Response Monitor. Isr. J. Health Policy Res. 2021;10:1–3. doi: 10.1186/s13584-021-00453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., O’mahony M. Adverse drug reactions in special populations – the elderly. Br. J. Clin. Pharmacol. 2015;80:796–807. doi: 10.1111/bcp.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling K., McClung N., Chamberland M., Marin M., Wallace M., Bell B.P., Lee G.M., Talbot H.K., Romero J.R., Oliver S.E. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine – United States, 2020. Morb. Mortal. Wkly. Rep. 2020;69:1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, M.P., Katz, J.M., Pervikov, Y., Hombach, J., Tam, J.S., 2011. Report of the 7th meeting on evaluation of pandemic influenza vaccines in clinical trials, World Health Organization, Vaccine 29, Geneva, 17–18 February, 2011, pp. 7579–7586. [DOI] [PubMed]
- Hasan T., Beardsley J., Marais B.J., Nguyen T.A., Fox G.J. The implementation of mass-vaccination against SARS-CoV-2: a systematic review of existing strategies and guidelines. Vaccines. 2021;9:326. doi: 10.3390/vaccines9040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand B.K., Webb M., Gartaganis S.L., Fuller L., Kwon C.-S., Inouye S.K. The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019—missing the target. JAMA Intern. Med. 2020;180:1546–1549. doi: 10.1001/jamainternmed.2020.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch P.C., Anand M., Bauch C.T. Prioritising COVID-19 vaccination in changing social and epidemiological landscapes: a mathematical modelling study. Lancet Infect. Dis. 2021;21:1097–1106. doi: 10.1016/S1473-3099(21)00057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink D.N., Mamdani M.M., Lee D.S., Kopp A., Austin P.C., Laupacis A., Redelmeier D.A. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M.B., Haupt R.E., Logue J., McGrath M., Weston S., Piedra P.A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J.D., Griffin P., Wilkinson B., Glenn G.M. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala A., Joo Y.J., Khatami A., Chiu C., Britton P.N. Vaccines for COVID-19: the current state of play. Paediatr. Respir. Rev. 2020;35:43–49. doi: 10.1016/j.prrv.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal P., Dowd J.E. World Health Organization; Geneva: 2001. Definition of An Older Person. Proposed Working Definition of An Older Person in Africa for the MDS Project. doi 10, 5188.9286. [Google Scholar]
- Kwok H.F. Review of Covid-19 vaccine clinical trials – a puzzle with missing pieces. Int. J. Biol. Sci. 2021;17:1461–1468. doi: 10.7150/ijbs.59170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V., Naroditsky B.S., Gintsburg A.L. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: what do we know so far about a vaccine? BMJ. 2020:m1679. doi: 10.1136/bmj.m1679. [DOI] [PubMed] [Google Scholar]
- Mangoni A.A., Jansen P.A., Jackson S.H. Pharmacokinetic and pharmacodynamic studies in older adults. Clin. Trials Older Adults. 2015:79–94. doi: 10.1586/ecp.12.75. [DOI] [PubMed] [Google Scholar]
- McEllistrem M.C., Clancy C.J., Buehrle D.J., Lucas A., Decker B.K. Single dose of a mRNA SARS-CoV-2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID-19. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2021 doi: 10.1093/cid/ciab263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Onder, G., Rezza, G., Brusaferro, S., 2020. Characteristics of patients dying in relation to COVID-19 in Italy JAMA Published online March 23. [DOI] [PubMed]
- Pitt B., Zannad F., Remme W.J., Cody R., Castaigne A., Perez A., Palensky J., Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Reid H., Miller W.C., Esfandiari E., Mohammadi S., Rash I., Tao G., Simpson E., Leong K., Matharu P., Sakakibara B., Schmidt J., Jarus T., Forwell S., Borisoff J., Backman C., Alic A., Brooks E., Chan J., Flockhart E., Irish J., Tsukura C., Dispirito N., Mortenson B. The impact of COVID-19 related restrictions on social and daily activities of parents, people with disabilities and older adults: protocol for a longitudinal, mixed-methods study. JMIR Res. Protoc. 2021;10:28337. doi: 10.2196/28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., Berghmans P.J., Kimmel M., Van Damme P., de Hoon J., Smith W., Stephenson K.E., De Rosa S.C., Cohen K.W., McElrath M.J., Cormier E., Scheper G., Barouch D.H., Hendriks J., Struyf F., Douoguih M., Van Hoof J., Schuitemaker H. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert A., Pillay J., Gates M., Guitard S., Rahman S., Beck A., Vandermeer B., Hartling L. Risk factors for severity of COVID-19: a rapid review to inform vaccine prioritisation in Canada. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyller T.B., Kittang B.R., Ranhoff A.H., Harg P., Myrstad M. Nursing home deaths after COVID-19 vaccination. Tidsskr. Den. Nor. Laege.: Tidsskr. Prakt. Med., Ny. raekke. 2021;141 doi: 10.4045/tidsskr.21.0383. [DOI] [PubMed] [Google Scholar]
- Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., Yang Y., Chen W., Gao X., You W., Wang X., Wang Z., Shi Z., Wang Y., Yang X., Zhang L., Huang L., Wang Q., Lu J., Yang Y., Guo J., Zhou W., Wan X., Wu C., Wang W., Huang S., Du J., Meng Z., Pan A., Yuan Z., Shen S., Guo W., Yang X. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang H., Wang W., Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang X., Liu Y., Wang Q., Huang L., Yang Y., Xu G., Luo B., Wang W., Liu P., Guo W., Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazzara M.B., Palmer K., Vetrano D.L., Carfì A., Graziano O. Adverse drug reactions in older adults: a narrative review of the literature. Eur. Geriatr. Med. 2021:1–11. doi: 10.1007/s41999-021-00481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Y., Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., Wu S.-P., Wang Z., Wu X.-H., Xu J.-J., Zhang Z., Jia S.-Y., Wang B.-S., Hu Y., Liu J.-J., Zhang J., Qian X.-A., Li Q., Pan H.-X., Jiang H.-D., Deng P., Gou J.-B., Wang X.-W., Wang X.-H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material