Abstract
It is well-established that plants are able to acclimate to temperatures above or below the optimal temperature for their growth. Here, we provide protocols for assays that can be used quantitatively or qualitatively to assess the relative ability of plants to acquire tolerance to high temperature stress. The hypocotyl elongation assay described was developed to screen for mutants defective in the acquisition of tolerance to extreme temperature stress, and other assays were developed to further characterize mutant and transgenic plants for heat tolerance of other processes or at other growth stages. Although the protocols provide details for application to Arabidopsis thaliana, the same basic methods can be adopted to assay heat tolerance in other plant species.
Keywords: Thermotolerance, Greening, Arabidopsis, Heat shock proteins
Background
It is well-established that plants are able to acclimate to temperatures above or below the optimal temperature for their growth, and many studies have identified genes necessary for, or associated with temperature acclimation. Typically, acclimation requires a period of exposure to a non-damaging temperature treatment, either above optimal to induce heat tolerance, or below optimal to induce cold or freezing tolerance. In Arabidopsis and other plants, freezing tolerance can be achieved within a period of 24 h of cold treatment (cold hardening), with maximum freezing tolerance occurring after several days of hardening ( Gilmour et al., 1988 ; Thomashow, 1999). Tolerance to normally lethal or damaging high temperatures can develop more rapidly, within a few hours. This type of rapid heat acclimation was extensively documented by Yarwood (1967) and others in the 1960s using a variety of plant species. Research on high temperature acclimation accelerated when it was recognized in the early 1980s that all organisms, including plants, responded to heat stress with a ‘heat shock response’ that involves transcription and translation of a conserved set of ‘heat shock proteins’ (Lindquist, 1986; Vierling, 1991). Work with soybean seedlings, similar to the earlier work of Yarwood, defined a simple assay for hypocotyl elongation that allowed investigation of the relationship of heat acclimation to the heat shock response ( Lin et al., 1984 ). This assay was then extended to Arabidopsis and used to identify mutants altered in heat acclimation, in both forward and reverse genetic screens (Hong and Vierling, 2001; Larkindale et al., 2005 ; Kim et al., 2012 ). Other assays for heat acclimation were also developed and used for mutant screening in Arabidopsis, including heat acclimation of greening of dark grown seedlings ( Burke et al., 2000 ) and seedling viability ( Wu et al., 2013 ).
Because plants can experience rapid daily temperature cycles, it is perhaps not surprising that heat tolerance can be acquired on a time scale consistent with diurnal changes in temperature, and that acclimation treatments afford maximal protection if they are administered 24 h or less before the imposition of damaging heat stress. It is also important to recognize that plant responses to temperature vary significantly with the length and severity, as well as the developmental timing of the temperature treatment. A review by Yeh et al. (2012) provides an excellent description of how variations in temperature treatments can affect phenotypic outcomes. Here we describe in detail basic methods that have been used to assess the ability of plants to acclimate to severe high temperature using Arabidopsis thaliana. Done precisely, these assays can provide quantitative information on the relative heat tolerance of different plant genotypes or of plants grown under different conditions. They can also readily be developed to use with other plant species.
Materials and Reagents
100 x 15 mm square Petri dish with grid (Simport, catalog number: D210-16)
Any brand sterile pipette tips that fit pipettors in 3 above
Parafilm (Bemis, catalog number: PM996)
Aluminum foil (any brand)
100 x 15 mm round Petri dish (Fisher Scientific, catalog number: S33580A)
Filter paper
Arabidopsis thaliana Columbia-0 seeds, hot1-3 mutant seeds (ABRC, catalog number: CS16284)
Household Bleach (Clorox or any brand that contains 5.25% sodium hypochlorite, 6 month shelf-life when stored at room temperature)
Triton X-100 (Sigma-Aldrich, catalog number: T8787)
MS basal salt mixture (Sigma-Aldrich, catalog number: M5524), store at 4 °C
2-(N-Morpholino) ethanesulfonic acid (MES) hydrate (Sigma-Aldrich, catalog number: M8250)
Phyto agar (Sigma-Aldrich, catalog number: A1296)
Sucrose (Fisher Scientific, catalog number: S5-500)
Potassium hydroxide (KOH) (Fisher Scientific, catalog number: P250-500)
Seed sterilization solution (see Recipes)
Half-strength MS agar media (see Recipes)
Equipment
Any brand of pipettors to dispense 2 to 20 μl, 20 to 100 μl, and 0.1 to 1.0 ml (e.g., Eppendorf, catalog numbers: 4924000037, 4924000061, 3123000063; or comparable)
Growth chamber (Percival Scientific, model: AR41L2)
Oven incubator (Precision, model: Thelco incubator 3DM, catalog number: 51221120)
Sharp pen
Scale loupe (CWJ, Peak, model: 1983 10x Scale Loupe)
Dissecting scope (Leica Microsystems, model: Leica MZ6 or comparable)
Leveling table (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: H18310-0000)
1 L glass bottles (Fisher Scientific)
Autoclave
Software
Microsoft Excel
ImageJ (optional)
Procedure
-
Hypocotyl elongation assay for testing acclimation to high temperature
The assay described here can provide quantitative data on the ability of seedlings to develop tolerance to a normally lethal/severely damaging high temperature stress.
-
Preparing plates with sterile seeds
Surface-sterilize Arabidopsis thaliana seeds of the genotype of interest with 50% Bleach solution containing 0.1% Triton X-100 (seed sterilization solution [see Recipes]) by soaking in the solution for 10 min. Using relatively fresh seeds (less than one year of harvest) is recommended because there is less variability in germination rate.
Rinse seeds with sterile water five times to remove any residual Bleach in a sterile environment.
In a sterile environment, place the seeds on half-strength MS agar media (see Recipes) in square plates with grids using sterile pipette tips that are cut appropriately (a couple of millimeters) at the tip to allow passage of the seeds, ideally one by one. We usually put 12-13 seeds on each line with even spacing between seeds (Figure 1). Stagger the seed placement from one line to the next in order to avoid contact when the hypocotyls elongate. It is important to put appropriate control samples (wild-type and a heat sensitive mutant) on every plate.
Wrap the plates with Parafilm and put the plates in 4 °C for 2-3 days, which helps synchronize germination.
-
Seedling growth and heat treatments
-
Growth prior to heat stress
Wrap the plates carefully with aluminum foil so that the seedlings are kept in the dark; hypocotyls elongate more in the dark. Mark the foil to indicate the bottom edge of the plate relative to expected upward growth of the seedlings. Let the seeds germinate and grow along the surface of the media by supporting the plates in a vertical orientation in a growth chamber for about 2.5 days at 22 °C. Plates could also be kept in any dark place at the appropriate temperature. The length of the hypocotyl should be roughly about 6 to 9 mm (for the wild-type Columbia accession) when ready for heat stress treatment.
-
Heat acclimation treatment
Unwrap the plates from aluminum foil and put them in an oven incubator in the dark set at 38 °C for the acclimation treatment for 1.5 h. Place plates horizontally for even heat transfer from the shelf to the media (Figure 2). Another replicate set of plates needs to be kept at 22 °C (or whatever the normal growth temperature is) as a control. Minimize the time the incubator is open in order to reduce temperature fluctuation and exposure of the seedlings to light. Many heat shock proteins are induced during this period ( Lin et al., 1984 ; Hong and Vierling, 2000).
-
Acclimation recovery period
Take the plates out of the incubator and keep at 22 °C for two hours in a dark place, again placing them upright. The plants recover from the mild heat treatment during this period and continue synthesizing heat shock proteins.
-
Severe, acute heat stress
Put the plates in a 45 °C incubator horizontally for a desired length of time. Two hours are sufficient to completely inhibit the growth of hot1-3 (HSP101 knock-out Arabidopsis line that has a T-DNA insertion in AT1G74310) plants, while allowing recovery of wild-type plants (Hong and Vierling, 2001). Depending on the heat sensitivity of the plants being tested, the length of heat stress at 45 °C can be varied, e.g., 1.5 h, 2.0 h, 2.5 h, 3.0 h. You should also have as a control replicate plates that have received the acclimation treatment, but that have not been treated at 45 °C. Make sure you quickly open and close the incubator when taking out plates in order to minimize temperature drop of remaining plants inside.
-
Recovery after severe heat stress
After the heat stress treatment, mark on the plate the position of the tip of each hypocotyl with a sharp pen, and cover the plates again with foil, being sure to note the growth direction of the seedlings. Place the plates again in a vertical position and let the plants recover and grow at 22 °C for about 2.5 days. Minimize light exposure when the marking is done, as during the rest of the procedure. If the seedlings are exposed to light for a relatively long time, you will end up with cotyledon expansion instead of hypocotyl elongation.
-
Hypocotyl length measurement
After the period of recovery, remove the foil and mark the tip of the hypocotyls again. At this point the plates can be placed at 4 °C until measured. A typical result for wild-type Columbia is shown in Figure 3. The length of hypocotyl elongation can be measured by eye using a scale loupe. Alternatively, photograph the plates and use the measuring tool of ImageJ to perform the measurements ( Schneider et al., 2012 ).
-
-
-
Heat stress assay for light-grown 7-10 day old seedlings
Surface-sterilize seeds and plate them on round Petri dishes with MS agar media as described in section A1. Divide plates into equal sectors. If you divide 100 x 15 mm Petri dishes into six sectors, place 18-20 seeds on each sector. It is a good practice to put the same genotype in sectors on opposite sides of the plate for duplication.
Wrap the plates with Parafilm and put them at 4 °C for 2-3 days for stratification.
Move the plates to a growth chamber and let the seedlings grow for 7-10 days under long day conditions (22 °C/18 °C, 16-h-day/8-h-night cycle) with a light intensity at ~80 μmol m-2 sec-1. Short days can also be used if they provide better growth for a particular genotype.
Perform heat treatments in an oven incubator as described above for the hypocotyl elongation assay, varying the length of time as necessary in order to distinguish between genotypes. Acclimation treatment is the same as for the hypocotyl elongation assay. Again, try to minimize temperature drop by acting quickly when opening and closing the incubator. Make sure you have control plates with no acclimation treatment, as well as plates maintained continuously at optimal growth temperatures for comparison.
After the heat treatment, put the plates back in a growth chamber and let them recover for 5-8 days. You can take a picture when the phenotypic differences are optimal.
-
Heat tolerance of germination
Before germination seeds are significantly more heat tolerant and variation in heat tolerance of seeds between Arabidopsis accessions has been documented ( Clerkx et al., 2004 ).
It is not necessary to sterilize seeds for this test. However, because germination is sensitive to maternal effects, as well as seed storage conditions, only seeds grown and harvested at the same time and stored under the same conditions should be used in comparisons.
Sow seeds in Petri dishes on filter paper saturated with the same volume of water for all plates. Volume should be sufficient to completely wet the filter paper without leaving excess water. 30 to 50 seeds of each genotype to be tested are spread evenly on the filter paper, using a separate plate for each genotype. Seeds are allowed to imbibe for 18 h at room temperature. To retain moisture, Petri dishes should be enclosed in plastic boxes lined on the bottom with water saturated filter paper throughout the assay.
Perform heat treatment by direct transfer of the plates to 50 °C for 8 h, then return them to room temperature to score germination. Use identical plates maintained at room temperature as a control for germination potential of the seed lot.
Determine germination percentage at an end point after 7 days, or record germination daily for estimates of differences in germination rate. Score small seeds, such as Arabidopsis, under a dissecting scope, or alternatively by photographing and scoring enlarged pictures viewed on a computer screen. Score seeds as germinated at the first sign of radical emergence.
-
Heat tolerance of seedling greening
The ability of dark grown seedlings to accumulate chlorophyll can be tested for heat tolerance either with or without an acclimation treatment. The assay below does not employ acclimation.
Prepare plates with seedlings as for the hypocotyl elongation assay and grow seedlings for 2.5 to 3 days, vertically, in the dark.
Treat plates with seedlings at 43 °C for 120 min in the dark. Leave a duplicate set of plates untreated. Different lengths of time at 43 °C can also be tested to provide resolution between genotypes if necessary.
Place the plates in the light, horizontally or vertically, either at room temperature in room light or low light (50 to 80 µE m-2 sec-1) in a growth chamber at 22 °C. Any day length or continuous light can be used. Record the light intensity at the plate level for reference in future experiments.
Record cotyledon greening daily, judged by eye as number of seedlings with green, light green, yellow green, or yellow cotyledons. Score again at an end point after three days in the light. Cotyledons can also be counted daily for expansion/opening.
-
Other heat stress treatments
The heat stress treatments described above have been used extensively in studies of Arabidopsis. However, the exact times and temperatures can also be varied to assay different phenotypes. For example, acclimation pretreatments can be performed by raising the temperature gradually from 20 to 22 °C to the stress temperature of 45 °C over the course of 3 to 4 h (Larkindale and Vierling, 2008). This requires an appropriate chamber with temperature ramping capabilities, which is a limiting resource for most laboratories. However, this gradual acclimation treatment was documented to provide better seedling viability after recovery from stress than the acclimation treatment described above. Another parameter than can be varied is the time between the acclimation treatment and the heat stress treatment, which can assess differences in the ability of different genotypes to maintain acclimation. Yeh et al. (2012) describe other parameters that can be modified in assays for heat tolerance.
-
Adapting assays to other plant species
As noted in the background section, the original assay for hypocotyl elongation was adapted from experiments with soybean ( Lin et al., 1984 ), and other plant species can be similarly assayed for acclimation to high temperatures. For this purpose, it is simply necessary to perform careful preliminary experiments to establish the rate of seedling germination and extent of hypocotyl growth or other parameters, as well as the time and temperature that results in heat killing or seedling growth arrest for each species at different growth stages.
Figure 1. Diagram of a plate with seeds placed evenly on each line.
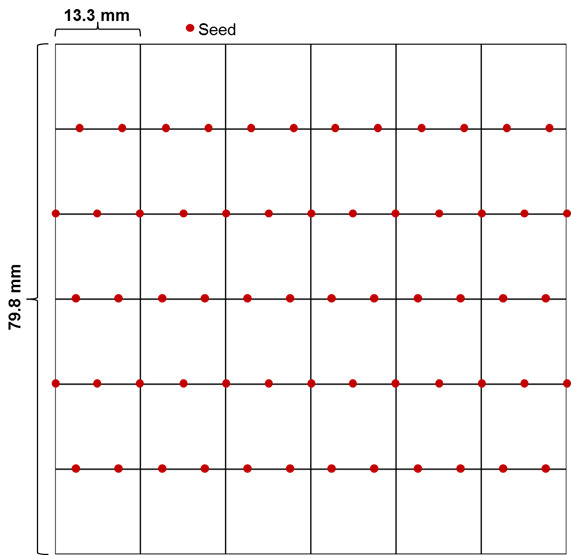
Figure 2. Plates are placed horizontally in an oven incubator.

Figure 3. Heat stress phenotype of wild-type A. thaliana (Columbia accession) seedlings.
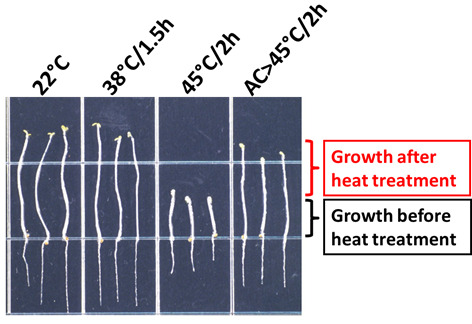
After the treatments indicated, seedlings were transferred to a new plate and photographed (Modified from Hong and Vierling, 2000). AC: Acclimation treatment: 38 °C/1.5 h followed by 22 °C/2 h.
Data analysis
-
Hypocotyl elongation assay
Using Microsoft Excel, plot genotypes on the x-axis and corresponding hypocotyl lengths on the y-axis. When there are mutants that are defective in growth, normalize the length relative to the control (no heat treatment) growth for each genotype. An example is shown in Figure 4. Typical standard deviation from 45 °C/2 h treatment after acclimation is about 10% for wild-type Columbia, when the lengths are normalized to the control growth. Statistical significance can be tested by performing a Student’s t-test. At least three independent experiments must be performed with similar results for reproducibility.
-
Heat stress assay for light-grown 7-10 day old seedlings
Take a picture when the phenotypic differences are optimal, which happens usually 5 to 8 days after heat treatment. An example is shown in Figure 5. To make the assay more quantitative, count seedlings that have survived and graph the percentage of survival as in Larkindale et al. (2005) . As detailed in the procedure, it is also good practice to plant sectors of the same genotype on opposite sides of the plate, to account for differences in heat distribution. Even small temperature differences can result in different outcomes. It is also critical to have genotypes to be compared on the same plate. At least three independent experiments must be performed with similar results for reproducibility.
-
Heat tolerance of germination
Express results of seed germination as a percentage of untreated seeds of the same seed lot. If data are collected daily, results can be plotted as a line graph. If data are collected only at an end point, use a table to display the data.
-
Heat tolerance of seedling greening
Express results of cotyledon greening as a bar graph showing for each day the percentage of cotyledons at different stages of greening compared to seedlings that were not heat treated. Similarly, percent of open cotyledons can also be graphed as a percentage of untreated seedlings. Statistical significance can be tested by performing a Student’s t-test. At least three independent experiments should be performed with 20 to 30 seedlings per genotype and treatment.
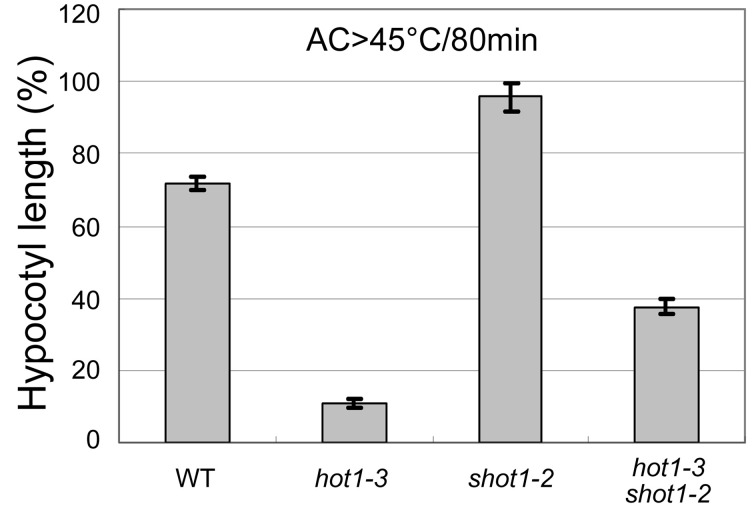
Figure 4. An example plot showing hypocotyl elongation of various Arabidopsis genotypes after heat treatment (45 °C/80min followed by heat acclimation, modified from Kim et al., 2012 ).
The shot1-2 mutant grows more slowly than the wild-type and hot1-3, so the hypocotyl growth was normalized by the growth during the same time period for seedlings that had not been heat treated. Error bars indicate SE; n = 12.
Figure 5. 10 day old seedlings were heat-stressed as shown above the picture.
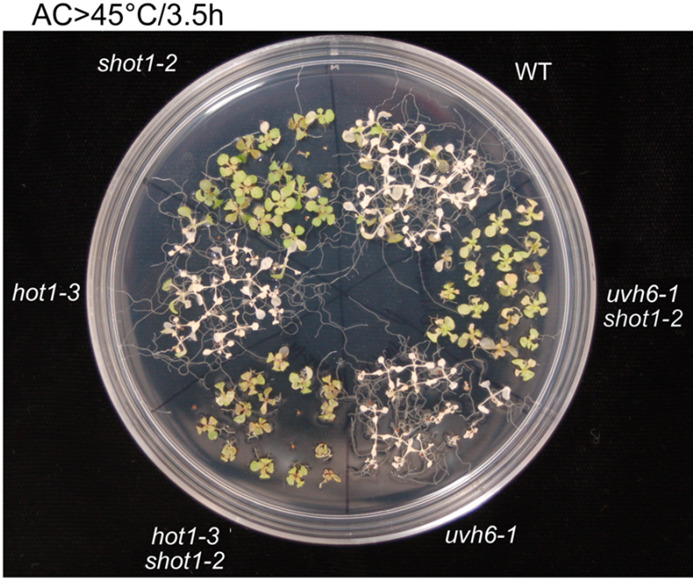
Picture was taken 7 days after the treatment (modified from Kim et al., 2012 ).
Notes
Be careful not to damage the aluminum foil covering for dark grown seedlings. Also, minimize light exposure of seedlings during handling. Light leakage makes plants undergo photomorphogenesis with cotyledons opening and expanding, while preventing hypocotyl elongation.
It is important to not overload the incubator and to be aware of any dramatic differences in temperature in different parts of your incubator. It is best to use a thermometer to check the incubator temperature in case the calibration of the incubator is not accurate, and also to compare different incubators. Seedlings are very sensitive to the exact temperature, and differences of as little as ± 1 °C can alter the outcome significantly.
We use an oven incubator for our dark heat treatment. However, other researchers put tightly sealed plates in a water bath, which may help even heat transfer and prevent temperature fluctuation ( Charng et al., 2006 ).
Marking seedling growth is important, as it allows you to separate growth before heat stress from growth after heat stress. This can normalize for not only differences in hypocotyl length between genotypes, but also differences in germination rates.
When making plates with MS agar media, pour the same amount of media (e.g., 10 ml for the 100 x 15 mm square Petri dish) in each plate on a leveling table. This will reduce temperature variability between plates and also within a plate.
When measuring heat tolerance of germination, all genotypes should be tested in three replicates for statistical analysis. Results should be expressed relative to germination of the unheated controls. The temperature or time of heat stress can be varied to increase resolution of differences between genotypes as necessary. If seeds have germinated after the 18 h imbibition period, shorten the time of imbibition prior to heat treatment.
For all assays described here, comparisons between genotypes are made by comparing percentages calculated based on untreated seeds or seedlings of the same genotype. In addition, whenever possible, it is preferable to use seeds from plants that were grown at the same time and under the same conditions, and to store the seeds under the same conditions. This is important for determining if there are significant differences in heat tolerance between genotypes, especially if differences are small. Repeating with more than one batch of seeds is critical for confirming small differences between genotypes.
For assays of heat stressed light grown seedlings or for heat tolerance of cotyledon greening, quantitative results can also be obtained by extracting chlorophyll from equal numbers of seedlings from control and heat stressed samples as developed by Burke et al. (2000) .
Recipes
-
Seed sterilization solution (for 100 ml)
50 ml Bleach
50 ml distilled water
500 µl 20% Triton X-100 (0.1% final concentration)
Note: The solution can be kept in a dark place for up to 1 month at room temperature.
-
Half-strength MS agar media (for 1,000 ml)
2.15 g MS basal salt mixture (stored at 4 °C)
0.5 g 2-(N-Morpholino) ethanesulfonic acid (MES) hydrate
5 g sucrose
Add 900 ml of distilled water and stir to dissolve
Adjust pH to 5.7 using 1 N KOH
Add distilled water to the final volume of 1,000 ml
Divide the media into two 1 L glass bottles. Add 4 g of phyto agar into each bottle
Autoclave for 30 min at 121 °C, 15 psi
Note: After the solution is autoclaved, let it cool to approximately 60 °C and pour a fixed amount of media (10-30 ml) in each plate on a leveling table in a sterile environment. The amount of agar influences the rate at which the temperature increases in the interior of the plate, and it is therefore important to add the exact same amount of media per plate. After pouring the plates, let them solidify and dry for 20 min in a sterile environment. Unused media plates can be stored upside down at 4 °C for months in a sealed container.
Acknowledgments
Assays reported here have been developed with support of grants from the US Department of Energy’s Basic Biosciences program, the US Department of Agriculture’s National Research Initiative Competitive Grants program, and the National Science Foundation Molecular Biosciences Division in the Directorate for Biological Sciences to EV. Seed germination assays were developed with support of a Guggenheim Fellowship to EV. These protocols were adapted and modified from Hong and Vierling, 2000 and Larkindale et al., 2005 .
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Burke J. J., O’Mahony P. J. and Oliver M. J.(2000). Isolation of Arabidopsis mutants lacking components of acquired thermotolerance . Plant Physiol 123(2): 575-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charng Y. Y., Liu H. C., Liu N. Y., Hsu F. C. and Ko S. S.(2006). Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation . Plant Physiol 140(4): 1297-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerkx E. J., El-Lithy M. E., Vierling E., Ruys G. J., Blankestijn-De Vries H., Groot S. P., Vreugdenhil D. and Koornneef M.(2004). Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population . Plant Physiol 135(1): 432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilmour S. J., Hajela R. K. and Thomashow M. F.(1988). Cold acclimation in Arabidopsis thaliana . Plant Physiol 87(3): 745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong S.W., and Vierling E.(2000). Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress . Proc Natl Acad Sci U S A 97: 4392-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong S.W., and Vierling E.(2001). Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J 27(1): 25-35. [DOI] [PubMed] [Google Scholar]
- 7.Kim M., Lee U., Small I., des Francs-Small C. C. and Vierling E.(2012). Mutations in an Arabidopsis mitochondrial transcription termination factor-related protein enhance thermotolerance in the absence of the major molecular chaperone HSP101 . Plant Cell 24(8): 3349-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkindale J., Hall J. D., Knight M. R. and Vierling E.(2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance . Plant Physiol 138(2): 882-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkindale J. and Vierling E.(2008). Core genome responses involved in acclimation to high temperature. Plant Physiol 146(2): 748-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C. Y., Roberts J. K. and Key J. L.(1984). Acquisition of thermotolerance in soybean seedlings: synthesis and accumulation of heat shock proteins and their cellular localization. Plant Physiol 74(1): 152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindquist S.(1986). The heat-shock response. Annu Rev Biochem 55: 1151-1191. [DOI] [PubMed] [Google Scholar]
- 12.Schneider C. A., Rasband W. S. and Eliceiri K. W.(2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7): 671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomashow M. F.(1999). PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50: 571-599. [DOI] [PubMed] [Google Scholar]
- 14.Vierling E.(1991). The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biolo 42: 579-620. [Google Scholar]
- 15.Wu T. Y., Juan Y. T., Hsu Y. H., Wu S. H., Liao H. T., Fung R. W. and Charng Y. Y.(2013). Interplay between heat shock proteins HSP101 and HSA32 prolongs heat acclimation memory posttranscriptionally in Arabidopsis . Plant Physiol 161(4): 2075-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarwood C. E.(1967). Adaptation of plants and plant pathogens to heat. In: Prosser, C. L.(Ed.). Molecular mechanisms of temperature adaptation. Amer Ass Adv Sci pp: 75-89. [Google Scholar]
- 17.Yeh C. H., Kaplinsky N. J., Hu C. and Charng Y. Y.(2012). Some like it hot, some like it warm: phenotyping to explore thermotolerance diversity. Plant Sci 195: 10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]