Introduction
Alopecia areata (AA) is a T-cell mediated autoimmune disease of the hair follicle with resultant nonscarring hair loss. It is an unpredictable condition with variation in disease duration and involvement. AA can present in several patterns, which are often more therapeutically challenging: entire scalp (alopecia totalis), entire body (alopecia universalis), or in a band-like distribution around the scalp (ophiasis).1 Recent studies have suggested significant molecular similarities in the disease process of AA with that of atopic dermatitis (AD).2, 3, 4, 5, 6, 7 This has led to the use of dupilumab, a novel human monoclonal antibody approved for treatment of moderate-to-severe AD, in AA. We present a patient who demonstrated marked improvement of worsening and recalcitrant ophiasis variant of AA as well as AD, with use of dupilumab.
Case report
A 34-year-old woman with a 21-year history of intermittent and worsening ophiasis and mild-to-moderate AD presented to the clinic with worsening hair loss. Her AA was recalcitrant to treatment with topical and intralesional steroids. The patient presented with a band-like distribution of nonscarring, confluent hair loss on the scalp with few exclamation point hairs and eczematous patches on her arms and leg (Fig 1). Initial therapy with topical fluocinonide, compounded topical cetirizine and caffeine daily, ketoconazole and ciclopirox shampoos, and intralesional triamcinolone injections at a dose of 10 mg/kg had no effect, and her disease worsened over the next 2 months. Cyclosporine 100 mg twice daily was added, which stabilized her alopecia and cleared her AD over a period of 14 months, but AA relapsed with attempts to wean. A trial of 2% topical tofacitinib again stabilized her disease, but minimal regrowth was noted after one year on this regimen; therefore, it was discontinued (Fig 2). Oral tofacitinib was not started due to lack of insurance coverage and high out-of-pocket cost to the patient. After stopping cyclosporine, there was a return of hair loss and eczema over the next several months, so therapy for AD with dupilumab 300 mg every other week was started. Within 3 months, there was notable hair growth with sparse vellus hairs present, as well as no new episodes of AD (Fig 3). After 10 months there was >90% regrowth with no signs of active AA or AD (Fig 4).
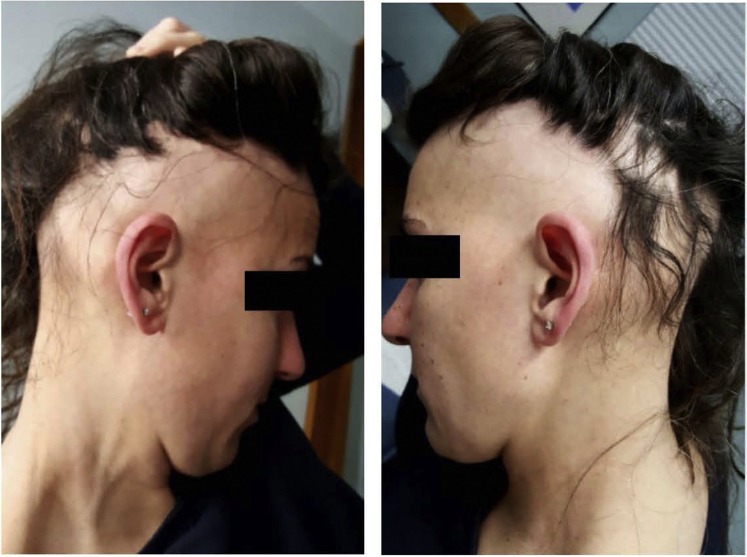
Fig 1.
A case of ophiasis in a young woman recalcitrant to topical and intralesional steroid treatment.
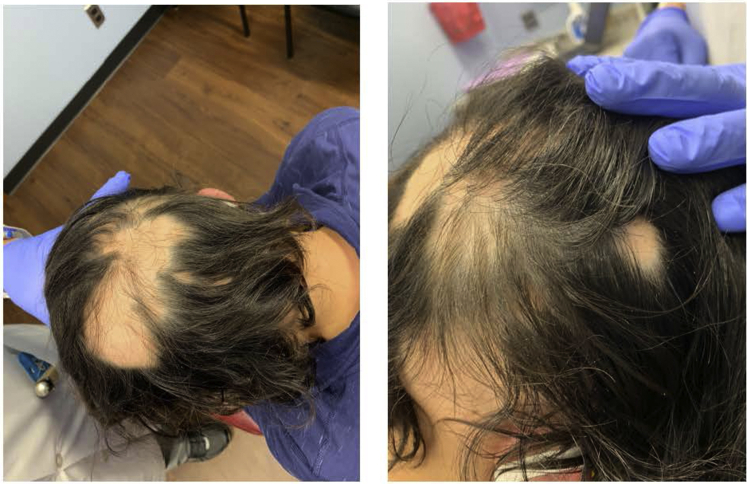
Fig 2.
Stable ophiasis without regrowth in a patient treated with topical tofacitinib for one year.
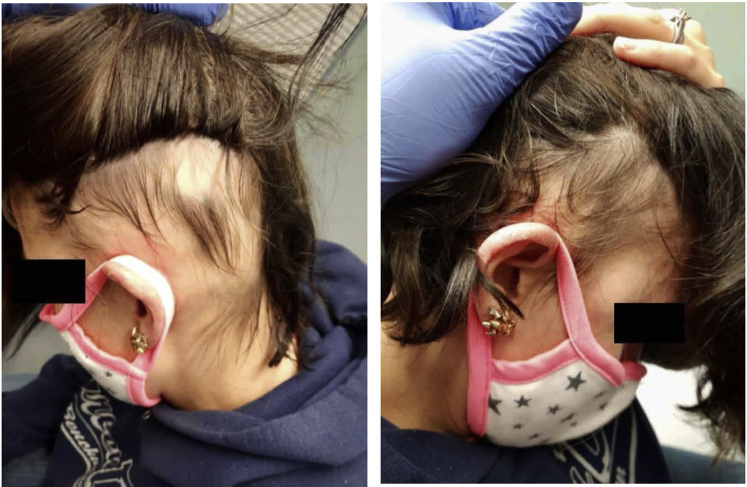
Fig 3.
Substantial hair regrowth in a patient with ophiasis treated with 3 months of dupilumab.
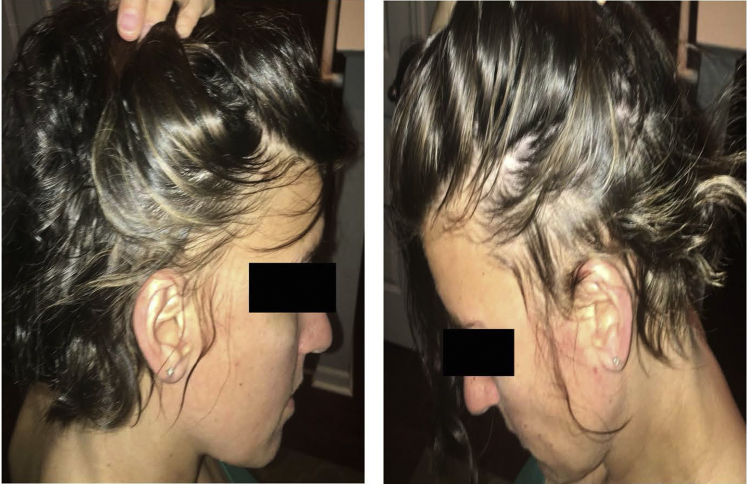
Fig 4.
Continued substantial regrowth in a patient with ophiasis treated with 10 months of dupilumab.
Discussion
Dupilumab is a human monoclonal antibody targeting the alpha subunit of the interleukin (IL) 4 receptor and is an inhibitor of IL-4 and IL- 13 signaling. It was approved in 2017 by the Food and Drug Administration for the treatment of adult patients with moderate-to-severe AD.2, 3, 4, 5, 6 The pathogenesis of AA is not fully understood; however, some studies suggest a strong Th2 component, which provides a possible explanation for the effectiveness of dupilumab treatment in this disease process.
Polymerase chain reaction analysis of AA scalp samples compared with controls demonstrate increased Th2, Th1, IL-23, and IL-9 cytokine activation, and genome-wide association studies reveal that IL-13 is a susceptibility locus for AA, suggesting that patients with AA may have genetic susceptibility that overlaps with AD whose cytokine profile involves the Th2 response.7,8,9
There are several clinical presentations and subtypes of AA including alopecia totalis, alopecia universalis, and ophiasis subtype. Case reports of each have appeared in recent literature noting a response to dupilumab.2, 3, 4, 5, 6 Ophiasis is notorious for its treatment resistance, and its improvement in this case is anecdotal but encouraging.1 Additionally, AA disease duration over 5 years portends a worse prognosis.9 In the case of this patient, dupilumab was effective despite a very long history of AA and failure of greater than one year of oral cyclosporine and topical Janus kinase inhibitor (tofacitinib). Of note, there have been case reports of dupilumab being associated with de-novo AA, an interesting finding given the drastic improvement of disease in our patient.10 Larger controlled clinical trials are needed to further investigate dupilumab's utility in the treatment of patients with all forms of AA.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Spano F., Donovan J.C. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61(9):751–755. [PMC free article] [PubMed] [Google Scholar]
- 2.Darrigade A.S., Legrand A., Andreu N. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol. 2018;179(2):534–536. doi: 10.1111/bjd.16711. [DOI] [PubMed] [Google Scholar]
- 3.Penzi L.R., Yasuda M., Manatis-Lornell A., Hagigeorges D., Senna M.M. Hair regrowth in a patient with long- standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154(11):1358–1360. doi: 10.1001/jamadermatol.2018.2976. [DOI] [PubMed] [Google Scholar]
- 4.Uchida H., Kamata M., Watanabe A. Dupilumab improved alopecia areata in a patient with atopic dermatitis: a case report. Acta Derm Venereol. 2019;99(7):675–676. doi: 10.2340/00015555-3183. [DOI] [PubMed] [Google Scholar]
- 5.Ludriksone L., Elsner P., Schliemann S. Simultaneous effectiveness of dupilumab in atopic dermatitis and alopecia areata in two patients. J Dtsch Dermatol Ges. 2019;17(12):1278–1280. doi: 10.1111/ddg.13990. [DOI] [PubMed] [Google Scholar]
- 6.Harada K., Irisawa R., Ito T., Uchiyama M., Tsuboi R. The effectiveness of dupilumab in patients with alopecia areata who have atopic dermatitis: a case series of seven patients. Br J Dermatol. 2020;183(2):396–397. doi: 10.1111/bjd.18976. [DOI] [PubMed] [Google Scholar]
- 7.Jagielska D., Redler S., Brockschmidt F.F. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132(9):2192–2197. doi: 10.1038/jid.2012.129. [DOI] [PubMed] [Google Scholar]
- 8.Suarez-Farinas M., Ungar B., Noda S. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–1287. doi: 10.1016/j.jaci.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Trüeb R.M., Dias M.F.R.G. Alopecia areata: a comprehensive review of pathogenesis and management. Clin Rev Allergy Immunol. 2018;54(1):68–87. doi: 10.1007/s12016-017-8620-9. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell K., Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4(2):143–144. doi: 10.1016/j.jdcr.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]