Case Presentation
A 24-year-old previously healthy woman was brought to the hospital for acute altered mental status. One week prior to presentation, she had developed a sore throat, nausea, and vomiting. At that time, SARS-CoV-2 polymerase chain reaction and rapid streptococcal pharyngitis test results were both negative. On the day prior to presentation, the patient had developed an erythematous painful rash on her left arm. The following day she was noted to be agitated, combative, and having trouble communicating, prompting ED evaluation. In the ED, the patient was tachycardic to 108 beats/min and tachypneic to 30 breaths/min but normotensive and afebrile. Her initial workup was notable for leukocytosis with bandemia, acute liver injury with coagulopathy, and acute renal failure. She was intubated, transferred to our hospital, and admitted to the MICU. The patient’s medical history was notable for obesity and oral contraceptive use. She had no family history of autoimmune, rheumatologic, or hematologic disorders. She was a student and worked part time in retail. She had no recent travel or outdoor exposure. The patient’s family was unaware of any tobacco or drug use but did report that she drank socially.
Physical Examination Findings
The patient arrived in our ICU intubated and synchronous on volume assist-control mode with rate set at 30, tidal volume at 350 mL, positive end-expiratory pressure of 10 cm H20, and fractional inspiratory oxygen concentration at 100%. She was hypotensive (BP 100/53 mm Hg on norepinephrine of 5 μg/min) and mildly tachycardic at 103 beats/min. Her BMI was 36 kg/m2. General examination revealed a young woman, intubated, and sedated to Richmond Agitation Sedation Scale level 5; mildly tachycardic without murmurs or rubs; and soft abdomen. Ophthalmologic examination was normal without conjunctival injection. Dermatologic examination revealed confluent erythematous patches over the wrist (Fig 1 A), an erythematous region over the flexure surface of the left forearm, and a petechial rash on the back (Fig 1B). Gynecologic examination was unremarkable.
Figure 1.
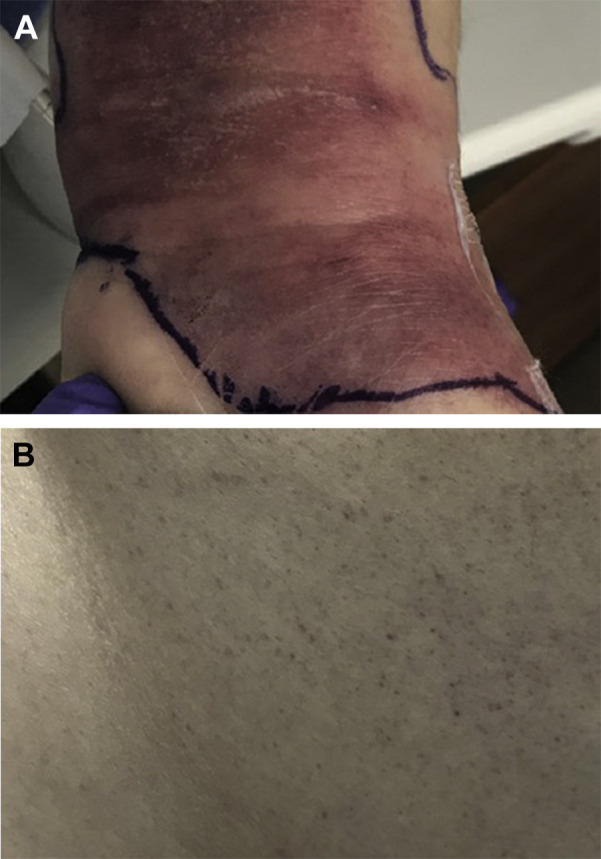
Initial dermatologic findings. A, Confluent erythematous rash over left wrist. B, Petechial rash over upper back.
Diagnostic Studies
Results of the patient’s hematologic and biochemical testing showed elevated inflammatory markers (WBC 44.9 × 103/μL with 44% polymorphonuclear leukocytes and 50% bands, erythrocyte sedimentation rate 83 mm/h, C-reactive protein 267 mg/L, and D-dimer 3,803 ng/mL), microcytic anemia with thrombocytopenia (hemoglobin 11.3 g/dL, mean corpuscular volume 76.9 fL, and platelets 106 × 103/μL), metabolic disarray with acute renal failure (sodium 111 mM, bicarbonate < 10 mM, venous pH 7.07, BUN 106 mg/dL, and creatinine 10 mg/dL), acute liver injury (albumin 2.4 g/dL, total bilirubin 9.1 mg/dL, aspartate transaminase 646 U/L, alanine transaminase 285 U/L, alkaline phosphatase 192 U/L, and international normalized ratio 3.6), elevated lipase levels (759 U/L), central hypothyroidism (thyroid-stimulating hormone 0.13 mIU/L and free thyroxine 0.64 ng/dL), and elevated creatine kinase (2,223 U/L). Urinalysis revealed large hematuria (20 RBC/high-power field), pyuria (20 WBC/high-power field, trace leukocyte esterase, and positive for bacteria), proteinuria (300 mg/dL), large bilirubinuria, and trace ketonuria. Her urine toxicology screen was unremarkable, and the urine pregnancy test result was negative. Serology studies for vasculitides and tickborne or viral infections (Table 1 ) were unremarkable.
Table 1.
Serologic Testing for Various Autoimmune and Infectious Etiologies
| Serologic Test | Result | Reference Range |
|---|---|---|
| Antinuclear antibody screen | Negative | Negative |
| ANCA | < 1:20 | < 1:20 |
| Myeloperoxidase Ab IgG | 0 | 0-19 AU/mL |
| Glomerular basement membrane IgG | 0 | 0-19 AU/mL |
| Lyme IgG Ab | < 0.04 | < 0.20 |
| Lyme IgM Ab | 0.00 | < 0.12 |
| RMSF IgG | < 1:64 | < 1:64 |
| RMSF IgM | < 1:64 | < 1:64 |
| Babesia IgG | < 1:16 | < 1:16 |
| Babesia IgM | < 1:20 | < 1:20 |
| Anaplasma phagocytophilum IgG | < 1:80 | < 1:80 |
| Aphagocytophilum IgM | < 1:16 | < 1:16 |
| HIV Ab/Ag screen | Nonreactive | Nonreactive |
| Hepatitis A IgM | Negative | Negative |
| Hepatitis B surface antigen | Negative | Negative |
| Hepatitis B surface Ab | 165.92 | < 7.99 mIU/mL |
| Hepatitis B core Ab | Negative | Negative |
| Hepatitis C Ab | Negative | Negative |
| Hepatitis E Ab IgG | Negative | Negative |
Ab = antibody; ANCA = antineutrophil cytoplasmic antibodies; RMSF = Rocky Mountain spotted fever.
Diffuse patchy airspace opacities were noted on the patient’s chest radiograph (Fig 2 ). CT scan of the abdomen and pelvis was notable for diffuse nonspecific gallbladder wall edema but no other evidence of acute abdominal pathology. CT imaging of the left forearm was notable for diffuse mild subcutaneous edema and mild intermuscular edema (Fig 3 ).
Figure 2.
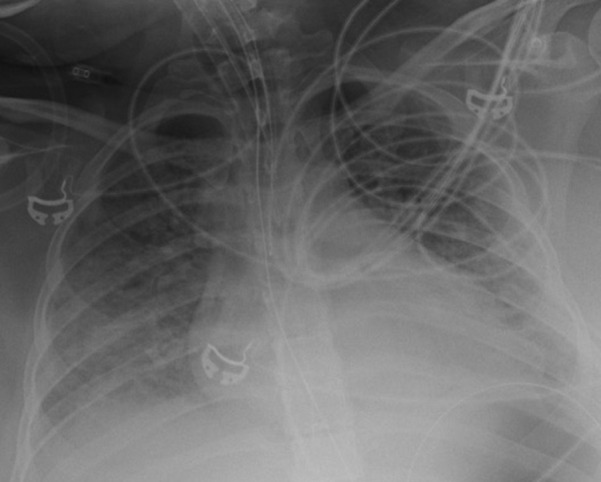
Chest radiograph. Portable film showing diffuse bilateral pulmonary infiltrates.
Figure 3.
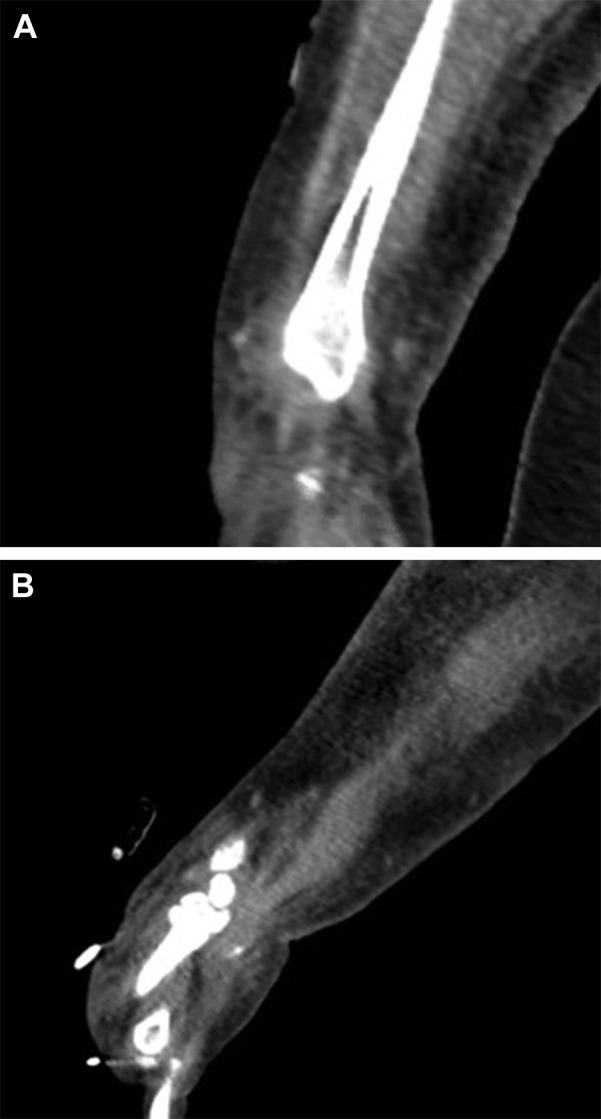
CT scan of the left forearm. Diffuse mild subcutaneous and mild intramuscular edema seen on coronal (A) and sagittal (B) cuts of the left forearm.
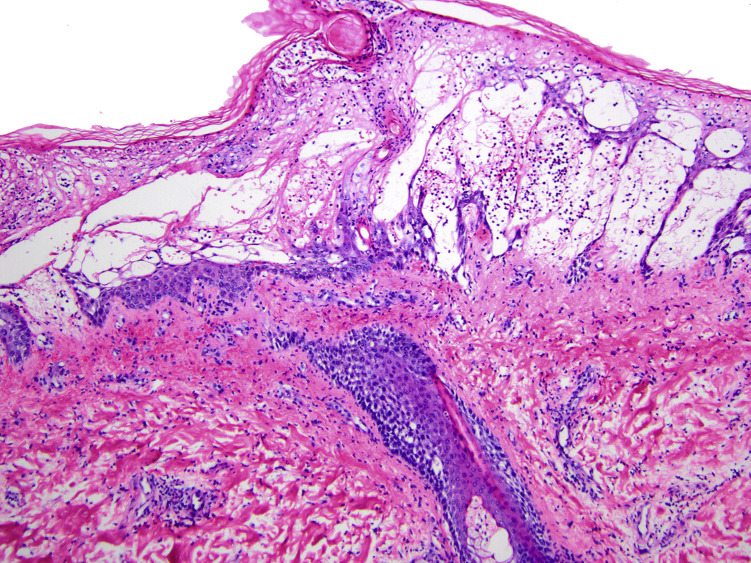
Punch biopsy of the patient’s left forearm revealed diffuse epidermal edema with sparse mixed cellular infiltrate, papillary dermal edema, and purpura most consistent with cellulitis/erysipelas (Fig 4 ). Periodic acid-Schiff stain was negative for fungal organisms.
Figure 4.
Punch biopsy specimen of the left forearm. Hematoxylin and eosin stain at low power reveals diffuse epidermal and dermal edema, purpura, and sparse cellular infiltrate, including some neutrophils. There was no evidence of vasculitis or thrombotic vasculopathy.
What is the diagnosis and causal organism?
Diagnosis: Streptococcal toxic shock syndrome Causal organism: Group A Streptococcus (Streptococcus pyogenes)
Discussion
Differential diagnosis for acute altered mental status with multiorgan failure in a young, previously healthy adult is broad and includes infectious etiologies such as meningitis, COVID-19, gram-negative sepsis, typhoid, Rocky Mountain spotted fever, leptospirosis, and toxic shock syndrome (TSS). Noninfectious causes to consider include thyroid storm, Kawasaki disease or other vasculitides, severe necrotizing pancreatitis, and heat stroke. Initial antibiotic choice should include broad coverage for gram-positive, gram-negative, and anaerobic organisms, as well as coverage for rickettsial infections with a tetracycline and antitoxin control with a bacteriostatic antibiotic.
TSS is a syndrome of multiorgan failure caused by bacterial exotoxin release and subsequent diffuse capillary leak. Most commonly, TSS is caused either by Staphylococcus aureus or Streptococcus pyogenes (group A streptococcus [GAS]). However, there have been cases of Mycoplasma and Yersinia species also causing a TSS-like syndrome.
Patients with TSS often present with a flu-like prodrome in the days prior to presentation, including sore throat, GI distress, and sometimes a rash. Patients with streptococcal TSS may present with a subtle-appearing rash with occult underlying soft tissue necrosis. Streptococcal TSS can cause a severe systemic illness that may seem out of proportion to the initial rash severity, making careful dermatologic examination critical to early diagnosis and treatment. Dermatopathologic findings are variable and cannot be used to confirm or rule out TSS but may be useful in ruling out vasculitis or invasive infection.
As TSS progresses, patients become critically ill and typically seek emergency medical treatment. Presentation of TSS includes hypotension and evidence of systemic end-organ involvement, including kidney and liver injury. Staphylococcal TSS definitionally presents with a desquamating rash and fever, which are sometimes but not always present in streptococcal TSS. In addition, staphylococcal TSS is more likely to present with mucus membrane involvement, whereas streptococcal TSS is more likely to present with ARDS, invasive soft tissue infection, and bacteremia (Table 2 ).
Table 2.
Case Definition for Streptococcal Toxic Shock Syndrome, as First Defined by the Working Group on Severe Streptococcal Infections
| I. Isolation of group A streptococci (Streptococcus pyogenes) |
| A. From a normally sterile site (eg, blood, cerebrospinal fluid, pleural or peritoneal fluid, tissue biopsy, surgical wound) |
| B. From a nonsterile site (eg, throat, sputum, vagina, superficial skin lesion) |
| II. Clinical signs of severity |
| A. Hypotension: systolic BP ≤ 90 mm Hg in adults or less than the fifth percentile for age in children and |
B. Two or more of the following signs:
|
| Definite case: an illness fulfilling criteria IA and II (A and B) |
| Probable case: an illness fulfilling criteria IB and II (A and B), if no other etiology for the illness can be defined |
The severe systemic symptoms seen in streptococcal TSS are mediated by two different groups of toxins secreted by GAS, superantigens SpeA and SpeC, and the M protein. In a normally functioning immune system, foreign antigens are phagocytosed and processed by monocytes, then presented via the major histocompatibility complex II to T cells. If the unique complex is recognized, that particular T-cell population will be activated, allowing for selective activation of only 0.01% of the total T-cell population. In contrast, superantigens such as SpeA and SpeC bind directly to the Vβ segment of the T-cell receptor, thus indiscriminately activating large populations of T cells. Those T cells activate macrophages that secrete large amounts of pro-inflammatory cytokines, leading to increased vascular permeability and activation of the complement cascade. GAS also secretes M protein, which in addition to activating T cells forms intravascular complexes with fibrinogen. These complexes activate neutrophils, which in turn release reactive oxygen species and enzymes, further exacerbating tissue damage, vascular permeability, and end-organ damage. The consumption of fibrinogen by the M proteins can also lead to disseminated intravascular coagulation.
Treatment for staphylococcal TSS includes bactericidal agents such as nafcillin or oxacillin, or, if methicillin-resistance is suspected, vancomycin. Streptococcal TSS can usually be treated with penicillin G, which is bactericidal against most strains of GAS. Treatment for both types of TSS should also include an “antitoxin” agent such as clindamycin; clindamycin and other lincosamides are bacteriostatic agents that inhibit protein synthesis at the 50S ribosome, thereby preventing further production of superantigens and M protein.
In addition to antibiotic treatment, IV immunoglobulin (IVIG) is recommended in the treatment of TSS. IVIG was shown to reduce mortality in recent studies of patients with TSS and is hypothesized to do so by enhancing bacterial recognition by neutrophils (opsonization) and by directly neutralizing the M protein and superantigen toxins. As with any severe infection, the treatment for TSS also includes source control. Any foreign objects in the body, such as nasal packing or menstrual hygiene products, should be identified and removed expediently. Radiographic testing should be performed to assess for severity of skin and soft tissue infection. CT scan is the preferred initial test, as it is most useful in identifying GAS within the soft tissues. Other radiographic findings may include abscess or inflammatory changes beneath the fascia. If soft tissue necrosis is present or suspected, clinicians should promptly seek expert surgical consultation. Conservative surgical management should be considered, as radical surgical excision or debridement of even severe soft tissue infections has not been shown to significantly improve outcomes.
Even with prompt and appropriate treatment, streptococcal TSS is associated with high mortality. Without the use of IVIG, mortality has been estimated at 30% to 80%; with the use of IVIG, mortality may decrease to as low as 10%. The presence or absence of bacteremia does not seem to affect mortality, further illustrating the role that toxins play in the pathophysiology of streptococcal TSS.
Clinical Course
The patient was started on renal replacement therapy, and broad-spectrum antibiotics with vancomycin, piperacillin-tazobactam, clindamycin, and doxycycline were initiated. Gram stain of the left arm wound culture showed gram-positive cocci in pairs in chains, and blood cultures grew group A S pyogenes, and thus the patient’s antibiotics were narrowed to penicillin and clindamycin. IVIG was given, and she underwent two surgical debridements of the left arm. She was extubated after 5 days and had full renal recovery after 6 days. After leaving the ICU, the patient’s course was complicated by pyomyositis and sternal osteomyelitis, for which she underwent chest wall incision and drainage, and was treated with a prolonged course of IV antibiotics. After 2 months in the hospital, the patient was transferred to an acute rehabilitation facility. Since then, she has returned home and is recovering well.
Clinical Pearls
-
1.
The differential diagnosis for rapid-onset multiorgan failure in a previously healthy or young person should include TSS.
-
2.
Streptococcal TSS can present with illness out of proportion to rash severity, and thus careful dermatologic examination should be used when searching for the source of infection.
-
3.
TSS should be managed with a combination of bactericidal and bacteriostatic/antitoxin antibiotics, IVIG, and source control.
-
4.
Early surgical consultation with conservative surgical management is encouraged, as radical surgical excision does not improve outcomes and may lead to excessive morbidity.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Other contributions: CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met. The authors thank David N. Silvers, MD, for his dermatopathology expertise and interpretation of this patient’s skin biopsy.
Suggested Readings
- Defining the group A streptococcal toxic shock syndrome Rationale and consensus definition. The Working Group on Severe Streptococcal Infections. JAMA. 1993;269(3):390–391. [PubMed] [Google Scholar]
- McCormick J.K., Yarwood J.M., Schlievert P.M. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- Herwald H., Cramer H., Mörgelin M., et al. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004;116(3):367–379. doi: 10.1016/s0092-8674(04)00057-1. [DOI] [PubMed] [Google Scholar]
- Lappin E., Ferguson A.J. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- Low D.E. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29(3):651–675. doi: 10.1016/j.ccc.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Linnér A., Darenberg J., Sjölin J., Henriques-Normark B., Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. 2014;59(6):851–857. doi: 10.1093/cid/ciu449. [DOI] [PubMed] [Google Scholar]