Abstract
Purpose
To assess the three‐dimensional outer retina thickness and choroid in eyes with white matter lesions (WMLs) using swept‐source optical coherence tomography (SS‐OCT).
Methods
Participants without dementia and stroke with cerebral WMLs were enrolled in our study. Optical coherence tomography (OCT) and OCT angiography (OCTA) were used to image and evaluate the outer retinal layer, choroidal structure, and perfusion of the choriocapillaris, microvessels of the choroid, respectively. Measurement of the outer retinal thickness, choroidal thickness and perfusion of the choriocapillaris was done by the SS‐OCT tool.
Results
Thirty‐one eyes from 16 WMLs and 40 eyes from 20 healthy controls were included in the data analyses. Outer retinal thickness was significantly reduced (P < .001) in WMLs participants when compared to healthy controls. Choroidal thickness was also significantly reduced (P < .001) in WMLs participants when compared to healthy controls. Choriocapillaris perfusion was significantly reduced (P = .002) in WMLs when compared to healthy controls. A significant correlation (Rho = .392, P = .032) was seen between the outer retinal thickness and choriocapillaris perfusion in WMLs participants.
Conclusions
Assessing retinal thickness and choroidal changes with the SS‐OCTA as a proxy for WML could prove to be a potentially valuable tool for early detection of cognitive decline and other neurodegenerative diseases.
Keywords: Choriocapillaris, choroidal thickness, swept‐source optical coherence tomography, white matter lesion
Short abstract
We aimed to assess the three‐dimensional outer retina thickness and choroid in eyes with white matter lesions (WMLs) using swept‐source optical coherence tomography (SS‐OCT). Assessing retinal thickness and choroidal changes with the SS‐OCTA, as a proxy for WML could prove to be a potentially valuable tool for early detection of cognitive decline and other neurodegenerative diseases.
1. INTRODUCTION
Recent years have seen a large increase in life expectancy due in part to a substantial decline in death rates in aging individuals. With increased long life comes an augmented peril of experiencing maladies, which increase in prevalence with increasing age such as Alzheimer's disease, the commonest cause of dementia (Prince et al., 2015). The projected rise in the number of the aging population has fueled clinical practitioners and researchers to plan for the care of the rising number with dementia and develop biomarkers to help in the early detection of these aging disorders; early detection will be pivotal in its prevention and facilitate therapeutic measures as well.
White matter lesions (WMLs), a common radiologic finding in the aging population, have been reported to be the predictable substrate for dementia and other cerebral small vessel diseases such as ischemic stroke (Peng et al., 2020). WMLs have been reported to be a product of ischemic impediments associated with the microcirculation of the brain and appear hyperintense on magnetic resonance imaging (MRI) scans (Wong, 2002). The presence of WMLs on radiologic scans increases the risk of cognitive decline and dementia (Miyao et al., 1992). Besides, recent reports (Levit et al., 2020; Sorond et al., 2020) have shown the importance of white matter integrity in the development of age‐related cognitive decline, and that neurovascular unit dysregulation and executive dysfunction often appear together WMLs. Thus, timely and effective approaches become critical as therapies are more likely to be successful in the early phase of the disease rather than the late phase.
Ophthalmological impairment has been reported to occur before the apparent clinical manifestation of most cerebral neurodegenerative disease (Colligris et al., 2018); these ophthalmological abnormalities have been reported to be associated with the visual cortex of the brain indicating the association between the eye and the brain (Ong et al., 2015). Moreover, reports have shown that similar mechanisms of cortical degeneration also occur in the retina and optic nerve (Ong et al., 2015). Recent imaging reports have suggested that dysfunction of the cerebral microcirculation may contribute to the WMLs disease cascade (Ong et al., 2015); it has been suggested that decreased blood flow occurs in WML which leads to neurotoxicity (Wang et al., 2016). In vivo reports using the optical coherence tomography (OCT) have shown significant thinning of the choroid in patients with dementia and WMLs (Iyigundogdu et al., 2017; Tak et al., 2017); as such, the choroid has been suggested to be a novel early and non‐invasive microvascular indicator in the eye for neuro‐axonal death in the brain. Nonetheless, very little is known about the retinal choroid; thus, the clinical use of the choroid as an indicator for neurodegeneration remains questionable given the inconsistent OCT reports. Besides, previous reports focused on the choroidal thickness with less attention on the choriocapillaris (microvasculature of the choroid).
The choroid has been reported to be affected in many retinal diseases (Mcleod et al., 2009) and neurodegenerative diseases such as dementia (Gharbiya et al., 2014). Alterations in choroidal homeostasis have been reported to occur in the aging population (Jirarattanasopa et al., 2012), even though it has not been reported comprehensively. Animal models of AD have shown significant alterations in the choriocapillaris (Asanad et al., 2019; Podoleanu & Rosen, 2008); moreover, atrophy of the choriocapillaris has been reported to occur in normal aging (Podoleanu & Rosen, 2008; Spaide, 2009). Since WMLs are substrates for both dementia and stroke (the most common neurodegenerative disease in the aging population), in vivo assessment of the choroidal structure and choriocapillaris may provide useful information on the possible pathological changes that occur in WML and give clues to changes that occur before the obvious neurodegenerative diseases.
Our current study aimed to quantitatively analyze the choroidal structure, choriocapillaris, and outer retina using the swept‐source optical coherence tomography (SS‐OCT) in subjects with WMLs. We also aimed to provide a more comprehensive understanding of the retinal choroid as a potential indicator for neurodegeneration in the brain.
2. METHODS
This observational cross‐sectional study was approved by the Ethics Board of West China Hospital and followed the Declaration of Helsinki. All participants enrolled in this study provided informed written consent before participating in this study.
Twenty participants who were free from dementia and stroke were enrolled from the Neurology Outpatient Department from West China Hospital, Sichuan Province, China. The inclusion criteria of our WMH participants were as follows: (1) participants aged 55 or more; (2) Chinese speaking; (3) adequate sensorimotor and language competency for cognitive assessment examination; (4) Fazekas score ≤ 3; (5) provided a signed written informed consent. Exclusion criteria were as follows: (1) history of neurodegenerative diseases or psychological disease which affects cognitive function; (2) indication of dementia from medical records; (3) presence of brain tumors, cerebromalacia on magnetic resonance imaging (MRI).
2.1. Retinal and choroidal image acquisition
A commercial SS‐OCT tool (VG200; SVision Imaging, Limited, Luoyang, China) (Daruich et al., 2017; Liegl & Ulbig, 2014) which contained a stainless steel laser was used to image the retina and choroid of each participant. With a scan rate of 200,000 A‐scans per second, the SS‐OCT tool had a central wavelength of 1050 nm (full width of 990–1100 nm). The OCT tool had an axial resolution of 5 μm in tissue and a lateral resolution of 15 μm at the retinal surface. Retinal thickness and choroidal thickness using the OCT and perfusion of the choriocapillaris using OCT angiography (OCTA); images were acquired with a high‐resolution raster scan protocol of 384 × 384 B scans which covered a 3 × 3 mm area centered on the fovea (Figure 1a). The retinal and choroidal parameters were automatically segmented by the OCT tool. The outer retina was defined as the thickness from the base of the outer plexiform layer (OPL) to the base of the retinal pigment epithelium (RPE) as shown in Figure 1b. Choroidal thickness was measured from the base of the RPE – Bruch membrane to the choroidoscleral junction (Figure 1b). The choroidal microvessels, choriocapillaris, were defined as the microvessels within the Bruch's membrane and the upper boundary of the stroma as shown in Figure 1c. Images with signal quality less than 6 were excluded (Lim et al., 2018). Other exclusion criteria were participants with ophthalmological diseases which could affect the retinal and choroidal structure such as diabetic retinopathy, age macular degenerative disease, central serous chorioretinopathy, glaucoma, cataracts, retinal pigment epithelial detachment, and participants with refractive errors more than +5.0 or −5 diopters.
FIGURE 1.
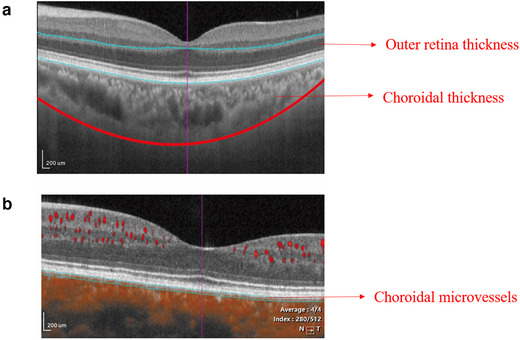
Representation of the face image of the outer retina, choroidal thickness, and choriocapillaris. (a) Imaging of the 3 × 3 mm area centered on the fovea. (b) The outer retinal thickness was from the base of the outer plexiform layer to the base of the retinal pigment epithelium (the layer between the blue lines). The choroidal thickness was defined as the base of the RPE – Bruch membrane to the choroidoscleral junction (the lower blue line to the red line). (c) The central cross‐section with focal flows in an SS‐OCT sample image. The choriocapillaris was defined as the microvessels within the Bruch's membrane and the upper boundary of the stroma (between the two blue lines)
Retinal and choroidal thickness with choriocapillaris perfusion were evaluated according to the Early Treatment Diabetic Retinopathy Study (ETDRS) map. Perfusion of the choriocapillaris was defined as the total length of perfused choriocapillaris per unit area in square millimeters (mm2). The global outer retina thickness, global choroidal thickness, and perfusion of choriocapillaris reflect the mean value of these three parameters within a scanning area of 3 × 3 mm which were used for our data analyses.
2.2. Statistical analyses
Data were expressed as mean values (standard deviations) for normally distributed parameters. Shapiro‐Wilk test was used to test the normality of our data. The parameters from the SS‐OCT tool were compared using generalized estimating equation (GEE) models to account for inter‐eye dependencies while adjusting for risk factors. Pearson correlation was used to evaluate the correlation between clinical variables and SS‐OCT parameters. P values less than .05 were considered statistically significant. Our data analyses were done with IBM Statistical Package for Social Sciences Statistics (SPSS version 24).
3. RESULTS
Thirty‐one eyes from 16 WMLs and 40 eyes from 20 healthy controls were included in our data analyses. Four WMLs participants were excluded because they could not cooperate with the SS‐OCT imaging while one eye of a WML participant was excluded due to low signal quality (SQ < 6). Demographics and clinical information are displayed in Table 1. The graphical representation of the outer retinal thickness and choroidal structure is shown in Figure 2. WML participants showed colder colors in the outer retina thickness and choroidal structure when compared with healthy controls as seen in Figure 2.
TABLE 1.
Demographics and clinical information
| WML | HC | P value | |
|---|---|---|---|
| Number | 16 | 20 | |
| Number of eyes | 31 | 40 | |
| Age, years | 59.31 ± 5.90 | 55.84 ± 3.72 | .053 |
| Gender (F) | 5 | 10 | .437 |
| Body mass index, BMI | 23.83 ± 2.04 | 23.65 ± 3.19 | .872 |
| Educational background | |||
| Illiteracy | 1 | 1 | |
| Elementary education | 6 | 5 | |
| Middle school or higher | 9 | 14 | |
| Hypertension, number (%) | 4 (25) | 0 | |
| Diabetes mellitus, number (%) | 1 (6.25) | 0 | |
| Hyperlipidemia | 2 | 0 | |
| Smoking | 3 | 2 | |
| Alcohol | 5 | 4 | |
| Radiological findings | |||
| Presence of lacunes (%) | 2 (12.5) | ||
| Presence of microbleeds | 1 (6.25) | ||
| Mean Fazekas score | |||
| Periventricular | 1.5 (0.71) | ||
| Deep | 1.4 (0.70) | ||
| Ophthalmological examination | |||
| Axial length, mm | 23.05 ± 1.26 | 22.89 ± 1.23 | .624 |
| IOP, mmHg | 13.72 ± 1.05 | 14.01 ± 2.11 | .583 |
| Visual acuity, Snellen chart | 0.84 ± 0.23 | 0.99 ± 0.24 | .002 |
| Visual acuity, LogMAR | 0.09 ± 0.13 | −0.01 ± 0.12 | .004 |
FIGURE 2.
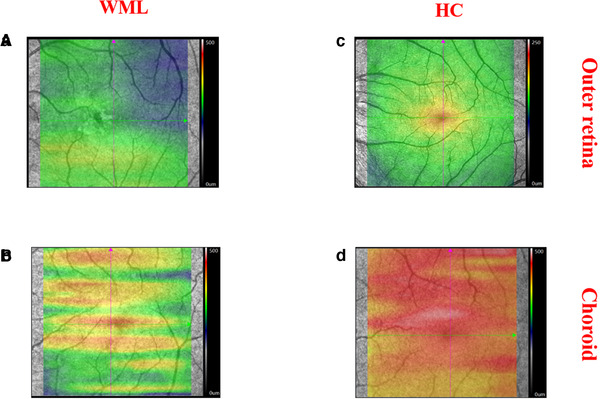
Graphical depiction of the outer retinal thickness and choroidal structure (thickness and choriocapillaris). Bright colors represent areas with significant thickness while dull colors represent significant thinning of the layer. (a) The outer retinal thickness of WML participants. (b) The outer retinal thickness of HC. (c) The thickness of the choroidal structure in WML patients. (d) The thickness of the choroidal structure in HC
3.1. Comparison of macula and choroid between the two groups
Outer retinal thickness was significantly reduced (P < .001, Table 2) in WMLs participants (166.80 ± 6.52 μm) when compared to healthy controls (181.07 ± 11.96 μm). Choroidal thickness was also significantly reduced (P < .001, Table 2) in WMLs participants (282.19 ± 92.16 μm) when compared to healthy controls (383.44 ± 73.20 μm).
TABLE 2.
Comparison of SS‐OCT parameters between WMLs and healthy controls
| WML | HC | P value | |
|---|---|---|---|
| Outer retina, μm | 166.80 ± 6.52 | 181.07 ± 11.96 | <.001 |
| Choroidal thickness, μm | 282.19 ± 92.16 | 383.44 ± 73.20 | <.001 |
| CC, mm2 | 0.84 ± 0.08 | 0.90 ± 0.03 | .002 |
| Signal quality | 7.97 ± 0.66 | 9.55 ± 0.50 | <.001 |
Generalized estimating equations (GEE) were used to compare the differences in the outer retina, choroidal thickness, and CVI between WML and HC while adjusting for risk factors, signal quality of the angiograms, axial length and inter eye dependencies.
Abbreviations: CVI, choroidal; HC, healthy controls; VI, vascular index; WML, white matter lesions.
Perfusion of the choriocapillaris was significantly reduced (P = .008, Table 2) in WMLs (.84 ± .08) when compared to healthy controls (.90 ± .03).
A significant correlation (Rho = .392, P = .032) was seen between the outer retinal thickness and perfusion of the choriocapillaris in WMLs participants.
4. DISCUSSION
The retinal choroid is a highly vascularized structure of the posterior eye, and similar to any microvessels in the body, it is likely to undergo structural and functional changes with increasing age. This may involve a reduction of its microvascular perfusion leading to the reduction of perfusion to the outer retinal thickness which may ultimately affect the visual function of an individual.
The primary function of the choroid (choriocapillaris) is to oxygenate the choroid, outer retina, some portions of the inner retina (inner and outer nuclear layers) and has a significant effect on the optic nerve; thus the choroid supplies oxygen to a part of the inner retina (neurosensory portion) and foveal avascular zone (Laviers & Zambarakji, 2014). Consequently, microvascular changes in the choroid can therefore affect the structural and microvascular changes in the inner retina as previously reported on individuals with WMLs (Peng et al., 2020).
Perfusion of the choriocapillaris reflects the total length of perfused microvasculature in the analyzed region. With the improvement in the imaging software of retinal imaging tools, the posterior segment of the eye (specifically the choroidal structure and microvessels) can now be visualized non‐invasively. It has been reported that the perfusion of the choriocapillaris is a reliable benchmark for the quantification of microvessels in the choroid (Agrawal et al., 2016); nonetheless, reports on the choroidal microvasculature on WMLs are currently scant. Previous reports (De Jong et al., 2008; Hilal et al., 2014; Ikram et al., 2006) using fundus camera imaging have shown that participants with WMLs have significantly altered microvascular network in the retina (narrower retinal venules and sparser and more tortuous retinal vessels) when compared with age‐ and sex‐matched controls. A previous report using the OCTA showed that WMLs have significantly reduced capillary density around the optic nerve head and deep capillary density when compared with healthy controls (Peng et al., 2020). Our current report used the SS‐OCT to evaluate the three‐dimensional map of the choroid to demonstrate the alterations that occur in the choroid of white matter lesions. White matter microstructural damage and mitochondrial dysfunction have been suggested to cause impairment of neurons and glial cells which may lead to vascular alterations (Mancuso et al., 2006; Reeve et al., 2018). Moreover, inflammatory cascades have been suggested as the cause of neuroaxonal damage and vascular dysfunction of the neurovascular choroid (Marchesi, 2011; Wherrett, 2003). Since the retinal choroid has been suggested to reflect the choroidal plexus with the cerebral microcirculation, we suggest that these microvascular changes in the choroid may reflect the cerebral changes (microstructural changes, neurodegeneration, and cerebral microvascular changes) that occur during the disease cascade. Furthermore, irregularities in the choroidal blood flow may cause ischemia in the optic nerve head and retinal ganglion cells blood circulation and may explain the damage to the structures in WMLs in our previous report (Peng et al., 2020). To the best of our knowledge, this is the first report on the microvascular choroidal changes in WMLs; nonetheless, future studies may be needed.
Iyigundogdu et al. (2017) found a significant reduction in the choroidal thickness of WML subjects with migraine when compared with healthy controls. However, Tak et al. (2017) did not report a significant difference in the choroidal thickness between WMLs participants and healthy controls. Our current report showed a significant reduction in the choroidal thickness of WML participants when compared to healthy controls. Aging, one of the most important risk factors associated with WML (De Leeuw, 2001; Jin et al., 2020), has been reported to cause accumulation of lipids and atherosclerosis (Head et al., 2017; Wang & Bennett, 2012) which creates an ischemic setting alarming the structure of the choroid resulting in its thinning as seen in our report. Choroidal thinning in WMLs may be associated with hypoperfusion due to the irregularities in the choriocapillaris as seen in our results or the atrophic changes in the choroid as aforementioned (Iyigundogdu et al., 2017; Tak et al., 2017). On the other hand, choroidal thinning may be the result of the decreased metabolic activity associated with atrophying retinal ganglion cells as shown in the previous reports (Qu et al., 2019; Tak et al., 2017). Unlike the previous reports, our study enrolled WMLs participants without migraine but WMLs of vascular origin. Our study suggests that choroidal structural thinning in WMLs may be associated with aging and the microvascular dysfunction in the disease cascade.
Our study assessed the thickness of the outer retina in WML participants. A few reports have analyzed the outer retinal thickness of the retina in some neurodegenerative diseases such as Parkinson's disease (Garcia‐Martin et al., 2014; Roth et al., 2014; Spund et al., 2013) and Alzheimer's disease (Uchida et al., 2018); however, the results of these reports have been inconsistent. These discrepancies in the reports aforementioned may be due to different clinical phases of the disease, different races, and the OCT tool or algorithm used in the study. The segmentation algorithm across OCT systems varies and this must be considered, especially if conventional segmentation algorithms are used. Our reports defined the outer retina as the thickness from the base of the outer plexiform layer (OPL) to the base of the retinal pigment epithelium (RPE). Our result showed significantly reduced outer retina in WMLs participants when compared with healthy controls. The outer retina mainly contains photoreceptor cells, which play a significant role in neurotransmission. The significant reduction in the outer retinal thickness in WML may be ascribed to trans synaptic neuronal loss of the ONL due to the decreased synaptic contribution from the photoreceptor cells. Contrary to this, we also showed a significant correlation between the perfusion of the choriocapillaris and outer retina layer thickness. Because the outer retina layers mainly receive their oxygen and metabolism from the choroid (Laviers & Zambarakji, 2014; Margolis & Spaide, 2009), the changes of the outer retinal layer thickness may be a reflection of the reduced oxygenation from the choroid. Conversely, since the outer retina contains photoreceptors which help in vision, the thinning of these layers may affect the vision of WML participants.
Our study should be interpreted with caution as we enrolled participants that were quite young. For instance, the healthy controls were quite younger than the WMLs. Another major limitation of our study is the small sample size which lowers the eagerness and makes the findings difficult to interpret in the aging population. The study started with 20 WMLs participants and shrunk to 16 after the exclusion of un‐cooperated patients (2) and images that could not meet our imaging criteria (2). Nonetheless, this study can be repeated with larger sample size. Also, our study consisted of only Chinese individuals; thus, our data cannot be translated to other ethnic groups. Future studies with WML participants from different ethnic groups can be conducted. Another limitation of this study is the prospective nature of our study and the lack of volumetric MRI data to further support clinical MRI ratings.
Notwithstanding our limitations, our study showed that participants with WMLs have significantly reduced outer retinal thickness, reduced choroidal thickness, and significantly reduced choriocapillaris perfusion when compared with healthy controls. We also showed that the choriocapillaris perfusion is associated with the outer retinal changes in WMLs. To our knowledge, this is the first study comparing the in vivo anatomical changes of the choroid and outer retina in WMLs via the SS‐OCT. Assessing retinal thickness and choroidal changes with the SS‐OCTA as a proxy for WML could prove to be a potentially valuable tool for early detection of cognitive decline and other neurodegenerative diseases.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2240.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (81870937, 82071320), and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYG D18009).
Kwapong, W. R., Gao, Y., Yan, Y., Zhang, Y., Zhang, M., & Wu, B. (2021). Assessment of the outer retina and choroid in white matter lesions participants using swept‐source optical coherence tomography. Brain and Behavior, 11, e2240. 10.1002/brb3.2240
Funding information
National Natural Science Foundatio (81870937, 82071320); West China Hospital, Sichuan University (ZYG D18009)
William Robert Kwapong and Yuzhu Gao contributed equally to this project.
[Correction added on 30 August 2021, after first online publication: Peer review history statement has been added.]
Contributor Information
Ming Zhang, Email: mingzhangscu@163.com.
Bo Wu, Email: dr.bowu@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Agrawal, R., Gupta, P., Tan, K.‐A., Cheung, C. M. G., Wong, T.‐Y., & Cheng, C.‐Y. (2016). Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population‐based study. Science Reports‐UK, 6, 21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanad, S., Ross‐Cisneros, F., Barron, E., Nassisi, M., Sultan, W., Karanjia, R., & Sadun, A. A. (2019). The retinal choroid as an oculovascular biomarker for Alzheimer's Dementia: A histopathological study in severe disease. Alzheimer's and Dementia, 11, 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colligris, P., Perez De Lara, M. J., Colligris, B., & Pintor, J. (2018). Ocular manifestations of Alzheimer's and other neurodegenerative diseases: The prospect of the eye as a tool for the early diagnosis of Alzheimer's disease. Journal of Ophthalmology, 2018, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruich, A., Matet, A., & Behar‐Cohen, F. (2017). Central serous chorioretinopathy. Developments in Ophthalmology, 58, 27–38. [DOI] [PubMed] [Google Scholar]
- De Jong, F. J., Vernooij, M. W., Ikram, M. K, Ikram, M. A, Hofman, A., Krestin, G. P., Van Der Lugt, A., De Jong, P. T. V. M., & Breteler, M. M. B. (2008). Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters: The Rotterdam study. Ophthalmology, 115(5), 887–892. [DOI] [PubMed] [Google Scholar]
- De Leeuw, F.‐E. (2001). Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study—The Rotterdam scan study. Journal of Neurology, Neurosurgery, and Psychiatry, 70(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Martin, E., Larrosa, J. M., Polo, V., Satue, M., Marques, M. L., Alarcia, R., Seral, M., Fuertes, I., Otin, S., & Pablo, L. E. (2014). Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. American Journal of Ophthalmology, 157(2), 470–478.e2. [DOI] [PubMed] [Google Scholar]
- Gharbiya, M., Trebbastoni, A., Parisi, F., Manganiello, S., Cruciani, F., D'antonio, F., De Vico, U., Imbriano, L., Campanelli, A., & De Lena, C. (2014). Choroidal thinning as a new finding in Alzheimer's disease: Evidence from enhanced depth imaging spectral domain optical coherence tomography. Journal of Alzheimers Disease, 40(4), 907–917. [DOI] [PubMed] [Google Scholar]
- Head, T., Daunert, S., & Goldschmidt‐Clermont, P. J. (2017). The aging risk and atherosclerosis: A fresh look at arterial homeostasis. Frontiers in Genetics, 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal, S., Ong, Yi‐T, Cheung, C. Y., Tan, C. S., Venketasubramanian, N., Niessen, W. J., Vrooman, H., Anuar, A. R., Chew, M., Chen, C., Wong, T. Y., & Ikram, M. K. (2014) Microvascular network alterations in retina of subjects with cerebral small vessel disease. Neuroscience Letter, 577, 95–100. [DOI] [PubMed] [Google Scholar]
- Ikram, M. K, De Jong, F. J., Van Dijk, E. J., Prins, N. D., Hofman, A., Breteler, M. M. B., & De Jong, P. T. V. M. (2006). Retinal vessel diameters and cerebral small vessel disease: The Rotterdam scan study. Brain, 129(Pt 1), 182–188. [DOI] [PubMed] [Google Scholar]
- Iyigundogdu, I., Derle, E., Asena, L., Kural, F., Kibaroglu, S., Ocal, R., Akkoyun, I., & Can, U. (2017). Relationship between white matter hyperintensities and retinal nerve fiber layer, choroid, and ganglion cell layer thickness in migraine patients. Cephalalgia, 38, 332–339. [DOI] [PubMed] [Google Scholar]
- Jin, H., Ding, Z., Lian, S., Zhao, Y., He, S., Zhou, L., Zhuoga, C., Wang, H., Xu, J., Du, A., Yan, G., & Sun, Y. (2020). Prevalence and risk factors of white matter lesions in Tibetan patients without acute stroke. Stroke; A Journal of Cerebral Circulation, 51(1):149–153. [DOI] [PubMed] [Google Scholar]
- Jirarattanasopa, P., Ooto, S., Nakata, I., Tsujikawa, A., Yamashiro, K., Oishi, A., & Yoshimura, N. (2012). Choroidal thickness, vascular hyperpermeability, and complement factor H in age‐related macular degeneration and polypoidal choroidal vasculopathy. Investigative Ophthalmology and Visual Science, 53(7):3663–3672. [DOI] [PubMed] [Google Scholar]
- Laviers, H., & Zambarakji, H. (2014). Enhanced depth imaging‐OCT of the choroid: A review of the current literature. Graefes Archive for Clinical and Experimental Ophthalmology, 252(12), 1871–1883. [DOI] [PubMed] [Google Scholar]
- Levit, A., Hachinski, V., & Whitehead, S. N. (2020). Neurovascular unit dysregulation, white matter disease, and executive dysfunction: The shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience, 42(2), 445–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegl, R., & Ulbig, M. (2014). Central serous chorioretinopathy. Ophthalmologica, 232, 65–76. [DOI] [PubMed] [Google Scholar]
- Lim, H. B., Kim, Y. W., Kim, Ju Mi, & Jo, Y. J., Kim, J. Y. (2018). The importance of signal strength in quantitative assessment of retinal vessel density using optical coherence tomography angiography. Scientific Reports, 8(1), 12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso, M., Siciliano, G., Filosto, M., & Murri, L. (2006). Mitochondrial dysfunction and Alzheimer's disease: New developments. Journal of Alzheimers Disease, 9(2), 111–117. [DOI] [PubMed] [Google Scholar]
- Marchesi, V. T. (2011). Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative‐induced inflammation and dysregulated amyloid metabolism: Implication for early detection and therapy. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 25, 5–13. [DOI] [PubMed] [Google Scholar]
- Margolis, R., Spaide, R. F. (2009). A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. American Journal of Ophthalmology, 147, 811–815. [DOI] [PubMed] [Google Scholar]
- Mcleod, D. S, Grebe, R., Bhutto, I., Merges, C., Baba, T., & Lutty, G. A. (2009). Relationship between RPE and choriocapillaris in age‐related macular degeneration. Investigative Ophthalmology and Visual Science, 50(10), 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyao, S., Takano, A., Teramoto, J., & Takahashi, A. (1992). Leukoaraiosis in relation to prognosis for patients with lacunar infarction. Stroke; A Journal of Cerebral Circulation, 23(10), 1434–1438. [DOI] [PubMed] [Google Scholar]
- Ong, Yi‐T, Hilal, S., Cheung, C. Y., Venketasubramanian, N., Niessen, W. J., Vrooman, H., Anuar, A. R., Chew, M., Chen, C., Wong, T. Y., & Ikram, M. K. (2015). Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neuroscience Letter, 584, 12–16. [DOI] [PubMed] [Google Scholar]
- Peng, C., Kwapong, W. R., Xu, S., Muse, F. M., Yan, J., Qu, M., Cao, Y., Miao, H., Zhen, Z., Wu, Bo, & Han, Z. (2020). Structural and microvascular changes in the macular are associated with severity of white matter lesions. Frontiers in Neurology, 11, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podoleanu, A. G., & Rosen, R. B. (2008). Combinations of techniques in imaging the retina with high resolution. Progress in Retinal and Eye Research, 27(4), 464–499. [DOI] [PubMed] [Google Scholar]
- Prince, M., Wimo, A., Guerchet, M., Ali, G.‐C., Wu, Y.‐T., & Prina, M. (2015). World Alzheimer report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends.
- Qu, M., Kwapong, W., Peng, C., Cao, Y., Lu, F., Shen, M., & Han, Z. (2019). Retinal sublayer defect is independently associated with the severity of hypertensive white matter hyperintensity. Brain Behavior, 10, e01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve, A. K., Grady, J. P., Cosgrave, E. M., Bennison, E., Chen, C., Hepplewhite, P. D., & Morris, C. M. (2018). Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson's disease. NPJ Parkinson's Disease, 4(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, N. M., Saidha, S., Zimmermann, H., Brandt, A. U., Isensee, J., Benkhellouf‐Rutkowska, A., Dornauer, M., Kühn, A. A., Müller, T., Calabresi, P. A., & Paul, F. (2014). Photoreceptor layer thinning in idiopathic Parkinson's disease. Movement Disorders, 29(9), 1163–1170. [DOI] [PubMed] [Google Scholar]
- Sorond, F. A., Whitehead, S., Arai, K., Arnold, D., Carmichael, S. T, De Carli, C., Duering, M., Fornage, M., Flores‐Obando, R. E., Graff‐Radford, J., Hamel, E., Hess, D. C., Ihara, M., Jensen, M. K., Markus, H. S., Montagne, A., Rosenberg, G., Shih, A. Y., Smith, E. E., …. Barone, F. (2020). Proceedings from the Albert Charitable Trust Inaugural Workshop on white matter and cognition in aging. Geroscience, 42(1), 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide, R. F. (2009) Age‐related choroidal atrophy. American Journal of Ophthalmology, 147(5):801–810. [DOI] [PubMed] [Google Scholar]
- Spund, B., Ding, Y., Liu, T., Selesnick, I., Glazman, S., Shrier, E. M., & Bodis‐Wollner, I. (2013). Remodeling of the fovea in Parkinson disease. Journal of Neural Transmission (Vienna), 120(5), 745–753. [DOI] [PubMed] [Google Scholar]
- Tak, A., Sengul, Y., & Bilak, S. (2017). Evaluation of white matter hyperintensities and retinal fiber layer, ganglion cell layer, and inner‐plexiform layer in migraine patients. Neurological Sciences, 39, 489–496. [DOI] [PubMed] [Google Scholar]
- Uchida, A., Pillai, J. A., Bermel, R., Bonner‐Jackson, A., Rae‐Grant, A., Fernandez, H., Bena, J., Jones, S. E., Leverenz, J. B., Srivastava, S. K., & Ehlers, J. P. (2018). Outer retinal assessment using spectral‐domain optical coherence tomography in patients with Alzheimer's and Parkinson's disease. Investigative Ophthalmology and Visual Science, 59(7), 2768–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. C., & Bennett, M. (2012). Aging and atherosclerosis: Mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circulation Research, 111(2), 245–259. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Liu, G., Hong, D., Chen, F., Ji, X., & Cao, G. (2016). White matter injury in ischemic stroke. Progress in Neurobiology, 141, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherrett, J. (2003). Cerebrovascular pathologies in Alzheimer disease. Geriatrics and Aging, 6, 55–57. [Google Scholar]
- Wong, T. Y. (2002). Cerebral white matter lesions, retinopathy, and incident clinical stroke. Jama, 288(1), 67–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


