Abstract
Telomerase is the ribonucleoprotein complex responsible for the maintenance of the physical ends, or telomeres, of most eukaryotic chromosomes. In this study, telomerase activity has been identified in cell extracts from the nematode Ascaris suum. This parasitic nematode is particularly suited as a model system for the study of telomerase, because it shows the phenomenon of chromatin diminution, consisting of developmentally programmed chromosomal breakage, DNA elimination, and new telomere formation. In vitro, the A. suum telomerase is capable of efficiently recognizing and elongating nontelomeric primers with nematode-specific telomere repeats by using limited homology at the 3′ end of the DNA to anneal with the putative telomerase RNA template. The activity of this enzyme is developmentally regulated, and it correlates temporally with the phenomenon of chromatin diminution. It is up-regulated during the first two rounds of embryonic cell divisions, to reach a peak in 4-cell-stage embryos, when three presomatic blastomeres prepare for chromatin diminution. The activity remains high until the beginning of gastrulation, when the last of the presomatic cells undergoes chromatin diminution, and then constantly decreases during further development. In summary, our data strongly argue for a role of this enzyme in chromosome healing during the process of chromatin diminution.
Telomeres are specialized DNA-protein complexes at the ends of linear eukaryotic chromosomes that are essential for the maintenance of genome integrity. They protect the chromosome ends from fusion with each other and from degradation by exonucleases, prevent the activation of cell cycle checkpoints, and counter the terminal DNA loss that occurs when linear DNA is replicated by conventional DNA polymerases (7, 41, 49, 55, 62, 70). Synthesis and maintenance of telomeric DNA is primarily mediated by telomerase, a specialized RNA-dependent DNA polymerase. Telomerase is a ribonucleoprotein complex: it contains a reverse transcriptase catalytic subunit and associated protein subunits, as well as an intrinsic RNA molecule in which a short sequence serves as the template for the synthesis of the G-rich strand of telomeric DNA (reviewed in reference 53). Widespread among eukaryotes, telomerase activity has been identified in ciliates (23, 64, 76), yeast (11, 36, 37), Plasmodium falciparum (8), mammals (45, 60), amphibians (38), sponges (32), and higher plants (16, 18, 28). In single-cell eukaryotes, telomerase is constitutively active, and the maintenance of the telomere length is essential for the proliferative growth of the vegetative cells (for a review, see reference 22). In humans, on the other hand, telomerase activity is regulated during development and is involved in conferring long-term proliferation capacity on regenerative tissues, such as blood stem cells (9, 13, 29), and the germ line (31, 74).
In addition to maintaining preexisting telomeres, telomerase can catalyze the synthesis of telomeric repeats directly onto nontelomeric DNA. This has been observed during chromosome healing, a process by which a broken chromosome is stabilized by the formation of a new telomere (41). Chromosome healing may occur spontaneously after an accidental or an artificially induced chromosomal breakage or as a developmentally programmed event (reviewed in reference 43). Spontaneous chromosome healing is a random and rare process which is common to many eukaryotes and has been demonstrated to occur in plants, protozoans, yeast, nematodes, and vertebrates (33, 41, 57, 63, 69, 71–73). The DNA sequences at the sites of new telomere addition in the different eukaryotes analyzed so far support a telomerase-mediated healing mechanism (33, 50, 63, 72, 73). In metazoans, spontaneous chromosome healing may be developmentally controlled and tissue specific, such that healing can happen only when telomerase is available (2, 18, 26, 46), although telomerase-independent processes are also capable of capping broken chromosome ends (for a review, see reference 5).
Developmentally programmed chromosome breakage and healing has been observed during the life cycles of several eukaryotic species. During macronuclear formation in ciliates, massive genome rearrangements leading to the precise elimination of micronucleus-specific sequences culminate in the efficient formation of new telomeres on chromosomes specifically fragmented under developmental control (for a review, see reference 58). Point mutations in the template region of the telomerase RNA lead to the addition of altered telomeric DNA repeats directly onto chromosomal breakage sites in vivo (75), thus demonstrating that telomerase accounts for de novo telomere formation (reviewed in reference 6). Although telomerase is expressed at all developmental stages, the levels of telomerase activity, telomerase RNA, and telomerase reverse transcriptase peak when germ line micronuclei are converted into somatic macronuclei following conjugation (1, 10, 59).
Another interesting example of developmentally programmed chromosome healing occurs during the process of chromatin diminution in the early embryo of the parasitic nematode Ascaris suum. In all presomatic cells between the third and fifth cleavage divisions (44), somatic differentiation of this organism is marked by the programmed loss of about a quarter of the genomic DNA. The eliminated DNA consists largely of highly repetitive sequences but also includes single-copy genes (for a review, see reference 47). Chromatin diminution is directed by a complex molecular mechanism that initiates DNA cleavage within specific chromosome breakage regions (CBRs), several kilobases in length. At present, the molecular mechanism for recognition of the different CBRs is completely unknown, since they show no sequence homology with each other and lack any discernible cis-acting DNA signal specifying the positions of breakage. The ends of the reduced somatic chromosomes are healed by the de novo addition of several kilobases of the nematode telomeric hexamer TTAGGC. Within all CBRs that have been analyzed so far, the germ line chromosomes before breakage lack any preexisting internal telomeric repeats (30, 48). The presence of 1 to 6 bp identical to the telomeric repeats at the new telomere junctions, however, suggests that the 3′ ends of the broken chromosomes may have provided limited pairing with the putative telomerase RNA template of an A. suum telomerase (30, 48). Together with results obtained from other organisms, these molecular data argue for telomerase-mediated healing rather than for recombinational events.
In this study we demonstrate for the first time the presence of telomerase activity in nematodes. By using cell extracts, we developed a nematode telomeric repeat amplification protocol (nTRAP) PCR-based telomerase assay. We report on the identification of A. suum telomerase activity in vitro that is able to add telomeric repeats onto nontelomeric DNA. This telomerase activity shows a peak in early embryonic stages of A. suum undergoing chromatin diminution. Our results are consistent with earlier in vivo observations and provide in vitro evidence that a telomerase activity with minimal sequence specificity is involved in chromosome healing during the process of chromatin diminution.
MATERIALS AND METHODS
Preparation of A. suum cell extracts.
Adult A. suum organisms were collected in a slaughterhouse from the intestinal lumen of a pig and maintained overnight at 37°C in saline solution (4). Mature females were dissected, and the fertilized eggs from the first vaginal third of each uterus were isolated and stored at 4°C in 0.1 N H2SO4 (65). Synchronous development of the embryonated eggs was initiated in bulk by incubation of the egg suspension in an aerated rotatory shaker at 30°C (14). Samples of the culture corresponding to 3 to 7 ml of packed eggs (10,000 to 15,000 eggs/μl) were removed at the following times during embryonic development for the preparation of stage-specific extracts: days 1 (1-cell stage), 2 (2-cell), 3 (4-cell), 4 (8-cell), 5 (blastula), 7 (gastrula), 8 (late-gastrula), 9 (early-L1), 13 (L1 motile), and 20 (L2). The chitinous layer of the eggshell was removed by incubation of the eggs for 1 h in 0.4 M KOH–1.35% NaClO, several washes in cold MilliQ water, and a final wash in ice-cold homogenization buffer A (39), modified as follows: 20 mM Tris acetate (pH 8)–1.5 mM MgCl2–10 mM potassium glutamate–1 mM dithiothreitol–10 U of RNasin (Promega)/ml–100 μM phenylmethylsulfonyl fluoride (PMSF)–0.06 volume of protease inhibitor cocktail (31) (consisting of 7 μg of pepstatin/ml, 1.7 μg of aprotonin/ml, 1 μg of leupeptin/ml, 10 μg of E-64/ml, 10 μg of chymostatin/ml, and 12.5 μg of antipain/ml [Boehringer Mannheim]). A. suum whole-cell S100 extracts were prepared at 4°C essentially as previously described (27), with the following modifications: 0.1-mm-diameter glass beads were added to the Dounce homogenizer for the disruption of larval stages, the initial homogenate was brought to a concentration of 100 mM potassium glutamate by the addition of 0.1 volume of 1 M potassium glutamate, and after separation by centrifugation, the supernatant was dialyzed against 20 mM Tris acetate (pH 8)–100 mM potassium glutamate–1 mM dithiothreitol–0.2 mM EDTA–10% glycerol. For embryonic stages, 1 μl of S100 extract represents 10,000 to 40,000 embryos. Tissue- or cell-specific extracts were made as described above from the ovary, oviduct, and intestinal tract. Spermatids were collected from males by centrifugation from the pseudocoelomic fluid (66). The protein concentrations of the extracts were determined by the Bradford assay and the bicinchoninic acid assay (Pierce) with bovine serum albumin as a standard and were typically 5 to 15 mg/ml.
Control extracts were pretreated by heat inactivation for 10 min at 68°C or protease digestion with 1 μg of proteinase K (Boehringer Mannheim)/μl or RNase digestion with 10 to 100 ng of DNase-free RNase (Boehringer Mannheim)/μl for 20 min at 30°C. The untreated extract was incubated in parallel with water as a mock digestion. Telomerase protection against RNase A or against proteinase K was performed by the previous addition of 6 U of RNasin RNase inhibitor (Promega)/μl or 1 mM PMSF (Boehringer Mannheim), respectively. Dilution of the extracts was performed in the dialysis buffer to obtain the optimal concentration that does not inhibit or saturate the nTRAP assay.
The nTRAP assay.
To assay for telomerase, the sensitive PCR-based TRAP (31) was adapted to specifically detect nematode telomerase activity. In a typical nTRAP assay, telomerase was allowed to extend 0.1 μg of gel-purified oligonucleotide primer (for primer sequences, see Table 1) at 25°C for 30 min with 2 μl of extract at the optimal protein concentration in 47 μl of a buffer described in reference 31, containing 2 U of Taq DNA polymerase (Gibco-BRL), 10 mU of RNasin (Promega), and potassium glutamate instead of potassium chloride. After the telomerase extension step, RNase A was added to the tubes where it was missing. The reaction was stopped for 1.5 min at 95°C, and the reaction mixture was transferred to a PCR tube previously filled with 0.1 μg of downstream primer and 5 μCi of [α-32P]dATP (10 μCi/μl; 800 Ci/mmol). When the extract was treated with proteinase K prior to the reaction, Taq DNA polymerase was added after the denaturation step. The PCR mixture was subjected to 31 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 45 s, followed by a final extension step at 72°C for 1.5 min. Amplification of the telomerase products was monitored by incorporation of [α-32P]dATP into the PCR products. A 10-μl aliquot of the PCR mixtures was mixed with 5 μl of formamide loading dye mix (95% deionized formamide, 20 mM EDTA, 0.05% xylene cyanol FF, 0.05% bromophenol blue). Reaction products were denatured at 95°C for 3 min and chilled on ice before separation on a 12% polyacrylamide–7 M urea sequencing gel, which was exposed without drying to X-ray film (Fuji) for 1 day to 1 week at −70°C. nTRAP products were cloned in the pGEM-T vector (Promega) according to the recommendations of the manufacturer, and plasmid DNA was purified through spin columns (Qiagen) and sequenced by the Sanger dideoxy chain termination method using Sequenase 2.0 (U.S. Biochemical).
TABLE 1.
Pairs of oligonucleotide primers used in the nTRAP assay
| Primer | Sequencea
|
Size (nt) | |
|---|---|---|---|
| Upstream | Downstream | ||
| Modified TSb | |||
| nTS-CTT | 5′-AATCCGTCGAGCAGACTT-3′ | -------Telomerase extension------→ | 18 |
| nCX | ←-----PCR amplification----- | 3′-AATCCGTATCCGTATCCGTATCCG-5′ | 24 |
| Telomere addition site | |||
| me8-TTA | 5′-TTTGGTTATTTTATCTAAATTTTA-3′ | 24 | |
| TCP-23 | 3′-GAATCCGAATCCTTAAGCTATAG-5′ | 23 | |
| Terminal telomeric permutations of nTS | |||
| nTS-GCT | 5′-AATCCGTCGAGCAGAGCT-3′ | 18 | |
| nTS-CTT | 5′-AATCCGTCGAGCAGACTT-3′ | 18 | |
| nTS-TTA | 5′-AATCCGTCGAGCAGATTA-3′ | 18 | |
| nTS-TAG | 5′-AATCCGTCGAGCAGATAG-3′ | 18 | |
| TCP-CC | 3′-CCGAATGCTCTGCTTAAGCTATAG-5′ | 24 | |
Upstream primers (i.e., telomerase substrate) are shown in the 5′-to-3′ orientation, and their 3′-terminal telomeric nucleotides are boldfaced. Downstream primers are shown in the 3′-to-5′ orientation, and those of their nucleotides that are complementary to the sequence synthesized by telomerase are boldfaced. The 3′-terminal bases of the upstream primers that may induce primer dimer formation with the downstream primers are underlined (see the text).
TS, telomerase substrate.
For every primer pair used in the nTRAP assay, the oligonucleotides were designed by using MacVector 6.5 (Oxford Molecular Ltd.) such that primer interaction was minimal. In order to prevent staggered annealing of the downstream primer (34), the TCPs (telomere complementary primers) contained fewer than two telomeric repeats and were anchored at their 5′ ends with nontelomeric sequences. To serve as DNA size markers, gel-purified oligonucleotides were either 5′ phosphorylated with [γ-32P]ATP by using T4 polynucleotide kinase (Boehringer Mannheim) or 3′ elongated by 1 nucleotide with [α-32P]ddATP by using terminal deoxynucleotidyl transferase (Boehringer Mannheim) according to the manufacturer’s instructions.
Quantitation of nTRAP assays.
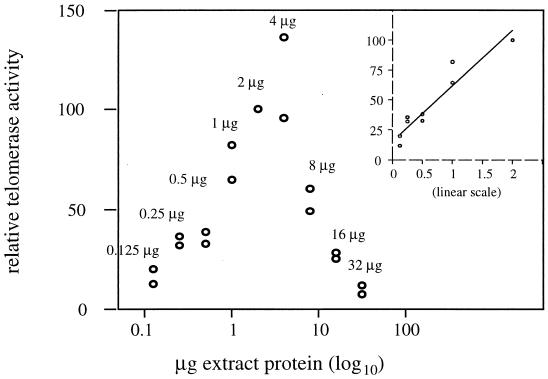
A twofold dilution series of the stage-specific extracts was tested in the nTRAP assay for 26, 31, or 36 PCR cycles to determine the conditions that give a linear response between the nTRAP signal and the amount of extract protein in the assay. nTRAP products were separated by polyacrylamide gel electrophoresis, and the signal intensity of a column covering the entire nTRAP ladder was quantitated from each lane with a Molecular Imager (Bio-Rad) for 4 to 24 h. The nTRAP signal was corrected for the signal from the reaction without extract and was expressed as a percentage of the activity observed with 2 μg of extract protein. The best results were obtained with 26 PCR cycles. Under these conditions, a linear regression analysis determined a linear relationship between the amount of protein per reaction and the telomerase level with high probability (P < 0.0001) (StatView 4.5, Abacus Concepts Inc.) within a range of 125 ng to 2 μg of extract protein (see Fig. 4 inset). For the schematic representation, a logarithmic scale covering a protein range from 125 ng to 32 μg was used for the x axis (Fig. 4). For the comparative analysis, each extract was normalized to a total protein concentration of 0.5 μg/μl by dilution in the dialysis buffer and the telomerase products were amplified for 26 cycles. For each extract, the signal from the nTRAP products, present in the entire lane starting from the first repeat, was measured, and the signal from the respective RNase A-treated reaction was subtracted as background. This specific telomerase activity is given as a percentage of the activity observed with the 4-cell-stage extract. For each extract, several reactions were performed to ensure the reproducibility of telomerase recovery in the extracts, of nTRAP assays, and of gel loading. The means and standard deviations, given with a 95% confidence level (i.e., the vast majority of the values fall within 2 standard deviations of the mean), were calculated from three to five independent reactions with one to three separate extracts of the different stages (one extract for spermatids and 2-cell, blastula, late-gastrula, and motile L1 stages; two extracts for oogonia, oocytes, intestines, and 1-cell, 8-cell, gastrula, and early-L1 stages; and three extracts for 4-cell and L2 stages). The means and standard deviations of relative telomerase activity are reported on a graph for each developmental stage relative to the normalized protein amount of the extract (see Fig. 5).
FIG. 4.
Quantitation of nTRAP products obtained with A. suum 4-cell extracts. The relationship between nTRAP products and the amount of extract protein present in the assay was determined from two independent titration experiments using the primer pair me8-TTA and TCP-23 (see Table 1) and 26 PCR cycles. Protein concentrations resulting from a twofold serial dilution ranged from 0.125 to 32 μg per reaction. The nTRAP products were resolved on 12% polyacrylamide gels similar to the gel in Fig. 1. The amount of telomerase activity for each dilution was measured from the entire lane and is expressed as a percentage of the activity observed with 2 μg of extract protein. The x axis represents a logarithmic scale of the amount of extract protein in micrograms. (Inset) The linearity of the nTRAP between 0.125 and 2 μg of extract protein is demonstrated by a straight line in the graph, which represents a linear regression on a decimal x axis (see Materials and Methods).
FIG. 5.
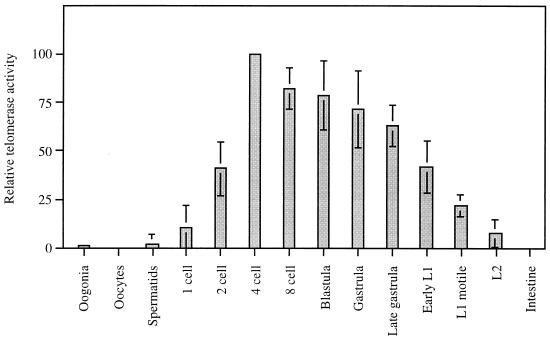
Comparison of relative telomerase activities in different A. suum developmental stages and tissues. The relative specific telomerase activity is expressed as a percentage of the maximal activity observed with the 4-cell-stage extracts. The signal intensities of nTRAP products from the entire lane were measured on polyacrylamide gels, similar to that presented in Fig. 1, and are presented for each specific extract in a bar chart relative to the normalized protein amount per assay. Telomerase activities are presented as means and standard deviations (positive and negative error bars at the 95% confidence level), calculated from three to five independent reactions, including one to three separate extracts of the different stages.
RESULTS
Detection of telomerase activity in A. suum 4-cell embryos.
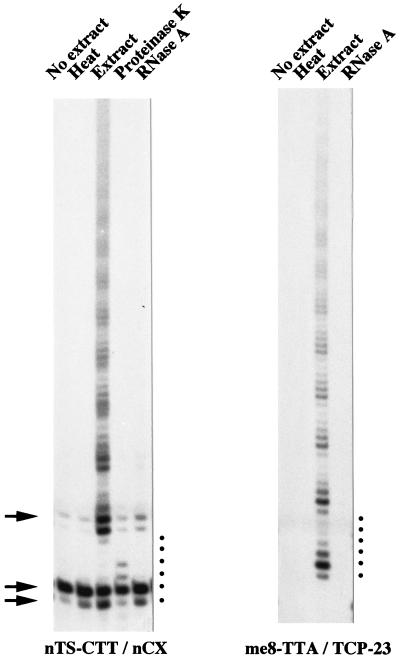
Since the conventional telomerase assay originally developed for a Tetrahymena sp. (24) failed to give a specific A. suum telomerase assay (data not shown), we have adapted the much more sensitive and telomerase-specific PCR-based human TRAP assay (31) for our extracts. In our nTRAP assays, we used nontelomeric oligonucleotide primers that were unlikely to self-associate and serve as substrates for DNA polymerase. At their 3′ ends, they contained 3 nucleotides corresponding to the nematode telomeric repeat TTAGGC (Table 1). The oligonucleotides were incubated in the presence of nucleotides and A. suum S100 whole-cell extract for extension with telomeric repeats by telomerase. In a second step, the telomerase extension products were PCR amplified with radiolabelled nucleotides and a telomere complementary oligonucleotide, as a downstream primer. The PCR products were separated on a sequencing gel and visualized by autoradiography. For our experiments, we used two different primer pairs. The first pair (nTS [nematode telomerase substrate]-CTT and nCX) was a modified version of the human TRAP upstream and downstream primers (31). The 3′ terminus of the nTS-CTT primer carried the half-telomeric repeat CTT, and the PCR downstream primer nCX contained mismatches in the nematode telomere complementary repeats to reduce primer interaction (Table 1). In the second primer pair, the nontelomeric primer me8-TTA was derived from a spontaneous de novo telomere addition site found in the Caenorhabditis elegans me8 mutation, a terminal deletion of the X chromosome (72). me8-TTA was used in conjunction with the downstream primer TCP-23 (Table 1). Both telomerase primers were efficiently elongated in S100 extracts prepared from A. suum 4-cell-stage embryos, resulting in typical TRAP ladders with 6-base periodicity upon PCR amplification (Fig. 1). No PCR products were formed in the absence of either upstream or downstream oligonucleotides (data not shown). Omission of the extract in the reaction abolished the accumulation of telomeric repeats (Fig. 1). However, the formation of primer dimers persisted in the form of extract-independent PCR products and was used as a positive PCR control (Fig. 1). Heat inactivation of the extract prior to the nTRAP assay demonstrated that the extract did not contain nucleic acids that interfered with the PCR (Fig. 1). Furthermore, the activity-induced ladder formation was sensitive to RNase A (100 ng/μl) and proteinase K (Fig. 1), attributes typical of telomerase activity (24). The addition of RNasin or PMSF before pretreatment of the extract with RNase A or proteinase K protected the activity and thus confirmed the sensitivity to RNase and protease, respectively (data not shown). Cloning and sequencing of me8-TTA nTRAP elongation products confirmed that they resulted from the addition of the telomeric permutation (GGCTTA)n directly after and in phase with the half-telomeric 3′-terminal bases of the primer (data not shown).
FIG. 1.
A. suum telomerase activity with nontelomeric primers. An nTRAP assay was conducted with the primer pair nTS-CTT and nCX and the primer pair me8-TTA and TCP-23 (see Table 1 for primer sequences) in a cell extract from A. suum 4-cell-stage embryos. The nTRAP products were resolved on a 12% polyacrylamide gel and subjected to autoradiography. Both primers were efficiently used as substrates (Extract). The primer dimers resulting from the interaction of the nTS-CTT and TCP-CC primers are indicated by arrows. The periodicity of the banding profile is marked by six dots on the right of each panel. No elongation was seen if the extract was omitted from the assay (No extract), inactivated by heat prior to the reaction (Heat), or pretreated with proteinase K or RNase A. The proteinase K experiment gave the same result for the me8-TTA primer, but it is not shown here.
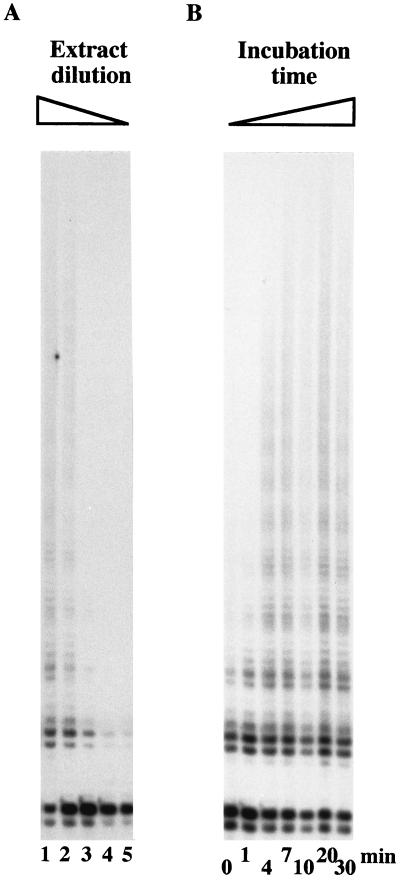
To titrate the telomerase activity, the nTS-CTT primer was incubated in the nTRAP reaction with a 10-fold dilution series of the A. suum 4-cell-stage extract under the same conditions used for the PCR amplification of the telomerase products (Fig. 2A). Serial dilution of the extract revealed that the formation of nTRAP products was roughly proportional to the amount of A. suum extract in the reaction mixture (10 μg to 100 ng of protein per assay). The detection limit of telomerase activity was between 100 and 10 ng of protein per assay, equivalent to approximately 200 to 20 4-cell embryos per reaction (Fig. 2A, lanes 3 and 4). Conversely, by increasing the incubation time of the telomerase extension step with the undiluted extract from 0 to 30 min, a maximum number of detectable repeats was added after 20 min (Fig. 2B). Since the reaction rate is relatively high, a few repeats were already produced at the “zero” time point, during the short time when the reaction mixture was warmed up from the preincubation at 4°C to the denaturation step preceding the PCR (Fig. 2B). Thus, the first time point in our experiment may be closer to an initial burst reaction than to a real zero time point. A net increase of telomerase products is seen already after 4 min of incubation (Fig. 2B). Based on the quantitation of the entire nTRAP ladder in our results and in a temperature range from 15 to 37°C, we found the nTRAP assay to be optimal with 2 μg of protein extract for an extension step of 30 min between 23 and 27°C followed by amplification with 31 PCR cycles. Furthermore, the experiments presented in Fig. 2 confirmed that the elongation of the nTS-CTT oligonucleotide primer by telomeric repeats is completed by the activity of the A. suum extract and does not arise from a PCR artifact. In summary, our data demonstrate the presence of a heat-labile ribonucleoprotein activity in A. suum cell extracts from 4-cell-stage embryos that elongates nontelomeric oligonucleotide primers with hexameric repeats of the nematode telomere sequence. We propose that it represents A. suum telomerase activity.
FIG. 2.
Titration and kinetics of the A. suum telomerase reaction. Decreasing amounts of extract or increasing times of incubation were used in the telomerase extension step with the nTS-CTT primer. Otherwise, the conditions for the nTRAP assay were kept constant. (A) A tenfold dilution series of the A. suum 4-cell-stage extract in a protein range from 10 μg to 1 ng was incubated for 10 min. (B) Ten micrograms of protein from an A. suum extract was incubated for 0 (initial burst reaction; see the text), 1, 4, 7, 10, 20, or 30 min. The reaction products were resolved on a 12% polyacrylamide gel and subjected to autoradiography.
Telomerase elongation of nontelomeric primers depends on their 3′ ends.
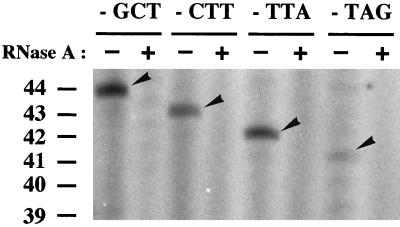
To analyze the interaction of A. suum telomerase with the 3′ end of the nontelomeric DNA substrate, we designed a set of four different nTS primers that had the same length and sequence but differed at their 3′ ends by telomeric permutations of 3 bases (Table 1). By using TCP-CC as the downstream primer (Table 1), the nTRAP assay with our 4-cell-stage extract produced a prominent band; this band was specific for each of the permutated nTS primers and corresponded to the first repeat added by the A. suum telomerase in the reaction (Fig. 3). The bands did not appear in reactions lacking the extract (data not shown) and were sensitive to low levels (10 ng/μl) of RNase A (Fig. 3). The nTRAP products were offset by 1 bp from one another, demonstrating that they were specific to the 3′-end half-telomeric permutations of the nTS primer. This is consistent with the interpretation that the free 3′ end of substrate DNA base pairs with the putative A. suum telomerase RNA template and that the de novo-synthesized telomere repeats are in phase with the terminal telomere sequences. Thus, our findings are in agreement with the telomerase elongation model proposed earlier (25).
FIG. 3.
Primer recognition by the A. suum telomerase. Four different nTS primers, identical in size and 5′ sequence but differing at their 3′ termini by 3 base permutations of the A. suum telomeric repeat, were assayed in 4-cell extracts with (+) or without (−) RNase A pretreatment. Elongation products of the permutated primers were amplified with the anchored downstream primer TCP-CC (see Table 1 for primer sizes and sequences), resolved on a 12% polyacrylamide gel, and subjected to autoradiography. The smallest nTRAP products of each nTS permutated primer, corresponding to the first repeat added by telomerase, are presented. Telomerase-specific bands (arrows) are sensitive to RNase pretreatment of the extract. The sizes of the nTRAP products are indicated.
Telomerase activity peaks in early embryos undergoing chromatin diminution.
If telomerase is involved in the elimination process in A. suum, we would expect telomerase activity to be high in early embryonic stages. To test this hypothesis, we compared in vitro telomerase activity from cultured A. suum embryos at different developmental stages. Embryonic stages were monitored under the microscope before extract preparation. As described previously (14), the viability (>95%), i.e., the ability to undergo mitosis, and the synchrony (>75%) of the embryos in culture were very high. After 1 day of incubation, embryogenesis was initiated, and the first rounds of zygotic division were completed at 24-h intervals. The 1- and 2-cell extracts represented prediminution stages. The 4-cell extracts consisted of about 86% 4- to 5-cell embryos, 12% 2- to 3-cell embryos, and <2% undeveloped embryos. In 4-cell-stage embryos, three somatic founder cells prepare for and execute chromatin diminution within the next cleavage round of embryogenesis (20, 54). The 8-cell and blastula (16 to 32 cells) extracts were prepared from slightly less synchronized embryos but covered a period in embryogenesis during which the two last presomatic cells undergo chromatin diminution, at the beginning of gastrulation. Gastrula extracts were made from postdiminution and cell-proliferative stages, in which the two primordial germ cells do not divide until the end of embryogenesis. Late-gastrula extracts were made largely from elongating embryos. The early-L1 extracts represented morphogenetic stages ranging from the tadpole-shaped embryo to the early vermiform first-stage larvae (L1). The extracts of L1 motile stages were established exclusively from well-formed, actively moving L1 larvae, and the L2 extracts were prepared from infectious second-stage larvae (L2). We also prepared extracts from dissected tissue: somatic extracts from the intestines of adult worms and germ line extracts from spermatids, developing oocytes, and oogonia (which, however, contained some ovarian tissue).
The quality of the extracts was estimated by visualization of the proteins on a denaturing polyacrylamide-sodium dodecyl sulfate gel stained with Coomassie blue. The protein concentrations and banding patterns of all the extracts were similar and revealed no specific protein degradation (data not shown). The total volume of the embryo remains constant, and the eggshell remains impermeable, through embryogenesis to the hatching L2 larval stage. Moreover, the embryonic content of proteins, nonprotein nitrogens (15), and ribosomes (52) does not change during this period of development, suggesting that the total protein content per embryo remains constant. Therefore, telomerase activity could be compared relative to a normalized total protein content per nTRAP assay.
In order to evaluate whether the levels of telomerase activity of the different extracts could be compared in a semiquantitative manner, we have estimated the linearity of the nTRAP assay with the me8-TTA and TCP-23 primer pair. We titrated A. suum 4-cell extracts in nTRAP assays for 26, 31, and 36 PCR cycles in a twofold dilution series in a protein range of 125 ng to 32 μg per assay, representing 2.5 × 102 to 6.4 × 104 4-cell embryos. When 26 PCR cycles were used, the nTRAP signal was proportional to the amount of A. suum extract present in the reaction mixture, from 125 ng to 2 μg of protein per assay (Fig. 4). Concentrations of extract protein higher than 4 μg per reaction inhibited the PCR amplification, an effect previously described in the human TRAP assay (9, 56). Based on these results, we determined the telomerase activities from different developmental stages by using 1 μg of protein per assay and 26 PCR cycles for each extract (Fig. 5). The intensities of the resulting RNase-sensitive signals were expressed as the percentage of 4-cell embryo extracts which always produced the highest level of telomerase activity (Fig. 5). A control experiment with 2 μg of protein per reaction gave identical results (data not shown), thus confirming that under these conditions the nTRAP assay was not inhibited or saturated with the different extracts.
As shown in Fig. 5, relative levels of specific telomerase activity were detected throughout embryogenesis in a regulated manner. During the first two rounds of embryonic division, telomerase activity increased 10-fold and reached a maximum level in 4-cell-stage embryos, when three of the four blastomeres are ready to undergo chromatin diminution. During gastrulation, morphogenesis, and larval development, telomerase activity progressively decreased. Telomerase activity was not detected in extracts prepared from the adult intestinal tracts. In germ line extracts from oocytes and spermatids, and surprisingly also from oogonia, relative specific telomerase levels were reproducibly low or undetectable. Mixing experiments excluded the presence of developmentally regulated telomerase inhibitors in those extracts that show low or no telomerase activity (data not shown).
DISCUSSION
The adaptation of the PCR-based telomerase assay (31) to A. suum cell extracts (nTRAP) has enabled us to identify telomerase activity in a nematode. Our results show that A. suum telomerase from 4-cell-stage extracts is capable of efficiently elongating different nontelomeric primers with nematode-specific telomere repeats. Three bases of permutated telomeric sequences located at the 3′ ends of nontelomeric nTS primers were recognized specifically by A. suum telomerase, suggesting that they anneal with the putative RNA template and are elongated by the addition of the next base in the telomere repeat. The results described here are, therefore, fully in agreement with the telomere elongation-translocation model proposed by Greider and Blackburn (25). Furthermore, the genomic DNA sequences of virtually all of a large number of analyzed chromosome breakpoints (>80), which have been healed in vivo during the process of chromatin diminution, contain at their chromosome-telomere junction 1 to 6 nucleotides that correspond to and are in frame with the newly added telomeric repeats (30, 48). Thus, in vivo, even one single base may be sufficient to pair efficiently with the RNA template and to initiate the synthesis of telomeric repeats. Since all 4 bases are represented in the putative RNA template of A. suum telomerase, any free 3′ end of DNA can potentially be healed. Telomere addition with minimal 3′-terminal sequence requirements has also been found during spontaneous chromosomal healing in humans (19, 35, 73) and Plasmodium (40, 57, 63) and has been suggested to occur in the free-living nematode C. elegans (72). In vitro, human and Plasmodium telomerases were able to elongate oligonucleotide primers covering the sites of new telomere addition with as few as 2 nucleotides complementary to the RNA template (8, 46).
In addition, Plasmodium telomerase can catalyze the synthesis of telomeric repeats directly onto nontelomeric DNA without any apparent annealing with the RNA template. In this case, telomere addition occurs predominantly with a unique permutation of the telomeric sequence (8). The ability of telomerase to initiate telomere synthesis, without proper annealing of the 3′ nucleotide, may also be important for ciliated protozoans during developmentally programmed new telomere addition (75). In vitro, telomere synthesis onto nontelomeric primers occurs by two activities of the ciliate telomerase enzyme: either by direct extension with a predominant permutation of the telomeric sequence (42, 68) or by cleavage-initiated extension (12, 21, 42). The in vivo studies with A. suum have shown no evidence for de novo telomere formation by such mechanisms. A. suum telomerase may not possess such properties due to its flexible sequence requirements.
In A. suum, the specific telomerase activity that synthesizes telomeres onto nontelomeric DNA is regulated during development. Only a small amount of activity was found in 1-cell embryos, while during the first two rounds of embryonic cell divisions, it was up-regulated to reach a maximum level in 4-cell-stage embryos, when three of the four blastomeres prepare for chromatin diminution. The activity remained relatively high throughout further embryonic development until the beginning of gastrulation, when the last of the presomatic cells undergoes chromatin diminution, and then constantly decreased. The temporal correlation of telomerase activity with chromatin diminution, rather than with the cell proliferation potential of the embryo, which is highest during gastrulation, provides strong indirect evidence that it is involved in the process of healing the broken chromosomes. Similarly, telomerase activity in ciliated protozoans was found to be more abundant during macronuclear formation, when massive de novo telomere addition occurs (1).
In intestinal cells of adult A. suum animals, our nTRAP assay did not detect telomerase activity. This was not surprising, since the development of nematodes is characterized by the phenomenon of eutely, i.e., the number and position of somatic cells in adult worms remains constant (67). Thus, somatic tissues contain no dividing cells and hence may not need telomerase activity. Mixing experiments of different extracts excluded the possibility that developmentally regulated telomerase inhibitors were present in larval or intestinal extracts that showed low levels of telomerase activity or none (data not shown). Furthermore, telomerase activity was absent from mature oocytes and spermatids, representing nonproliferating germ line cells.
Surprisingly, however, our nTRAP assay did not detect significant telomerase activity in extracts from oogonia either. It is unlikely that proliferating germ cells contain no telomerase and that telomere maintenance in these cells relies completely on different mechanisms, such as recombination or transposition (5). Furthermore, mixing of extracts from oogonia and 4-cell-stage embryos ruled out the existence of inhibitors of telomerase itself (originating from the somatic ovarian tissue that was contained in our oogonial preparations) or of the nTRAP assay (data not shown). It is possible that telomerase activity in oogonia is too low to be detected by the nTRAP assay but is still sufficient for telomere length maintenance in germ cells. During chromatin diminution, telomerase activity may be up-regulated. In ciliated protozoans, e.g., the expression of the telomerase RNA and reverse transcriptase genes increases greatly during conjugation, a time of new macronuclear telomere formation (1, 10, 59), and in human cells, expression of the reverse transcriptase catalytic subunit has been proposed to be required for telomerase activation (51). Since the nematode telomerase components are not yet cloned, this question could not be addressed at present. Another interesting hypothesis is that the ability to add telomeres onto broken chromosomes does not represent an intrinsic feature of A. suum telomerase but depends on developmentally regulated modifications. In this case, oogonia may have normal levels of telomerase, which, however, are unable to efficiently elongate nontelomeric primers in our nTRAP assays. According to this model, the specificity of A. suum telomerase is altered in cells undergoing chromatin diminution in order to permit efficient chromosome healing during this process. Early blastomeres may contain developmentally regulated cofactors that enable telomerase-DNA interaction by reducing efficiently the requirements for long stretches of Watson-Crick base-paired alignments on the RNA template. A factor-assisted change in the behavior of telomerase exists during the formation of the Euplotes macronucleus, where a developmentally regulated chromosome healing factor that collaborates with telomerase to initiate developmentally programmed de novo telomere formation was identified (3). In Tetrahymena, on the other hand, no difference in DNA processing has been found in preparations from vegetative and developing cells (68). In vivo, however, chromosomal breakage and telomere addition are tightly linked (17). Thus, during the formation of the macronucleus, Tetrahymena telomerase is likely to be part of a multisubunit complex that recognizes specific sequences on the DNA, catalyzes cleavage, and forms telomeres on the nascent ends. It is possible that A. suum telomerase is also part of a multifactorial “elimination complex” that efficiently directs telomere addition in vivo. Alternatively, specific DNA binding proteins could recognize the chromosomal breakage sites and recruit or activate telomerase to promote telomere formation in a reaction that is separate from DNA cleavage. Models in which yeast telomere binding proteins, if located internally, enhance telomere formation by increasing the activity of telomerase or attracting it have been proposed (33, 61).
Efficient healing of the truncated chromosomes during chromatin diminution is a critical event that ensures somatic chromosomal stability. Altogether, the telomerase data gained from our in vitro studies provide strong indirect evidence for a role of this enzyme during this process. Our data generally correlate well with biological data obtained earlier from A. suum and demonstrate that A. suum telomerase has the relaxed sequence requirements necessary to recognize the ends of broken chromosomes. The interesting question of how the level of telomerase activity and its specificity are regulated during A. suum development remains to be addressed and awaits purification or cloning of the telomerase components.
ACKNOWLEDGMENTS
We are grateful to Tim Nilsen and members of his laboratory for excellent instruction and advice on the biochemistry of A. suum and to Calvin Harley and Nam Kim for sharing details on the TRAP assay before publication. We thank Joachim Lingner, Karin Brunschwig, Vincent Bernard, and Nathalie Niederberger for helpful discussions, Monique Zetka and Francesca Palladino for critical reading of the manuscript, and Yolande Molleyres and Hubert Gachoud for technical assistance.
This work was supported by grants 31-001.91 and 31-40776.94 from the Swiss National Science Foundation.
REFERENCES
- 1.Avilion A A, Harrington L A, Greider C W. Tetrahymena telomerase RNA levels increase during macronuclear development. Dev Genet. 1992;13:80–86. doi: 10.1002/dvg.1020130113. [DOI] [PubMed] [Google Scholar]
- 2.Barnett M A, Buckle V J, Evans E P, Porter A C, Rout D, Smith A G, Brown W R. Telomere directed fragmentation of mammalian chromosomes. Nucleic Acids Res. 1993;21:27–36. doi: 10.1093/nar/21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bednenko J, Melek M, Greene E C, Shippen D E. Developmentally regulated initiation of DNA synthesis by telomerase: evidence for factor-assisted de novo telomere formation. EMBO J. 1997;16:2507–2518. doi: 10.1093/emboj/16.9.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett K L, Ward S. Neither a germ line-specific nor several somatically expressed genes are lost or rearranged during embryonic chromatin diminution in the nematode Ascaris lumbricoides var. suum. Dev Biol. 1986;118:141–147. doi: 10.1016/0012-1606(86)90081-3. [DOI] [PubMed] [Google Scholar]
- 5.Biessmann H, Mason J M. Telomere maintenance without telomerase. Chromosoma. 1997;106:63–69. doi: 10.1007/s004120050225. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn E H. Developmentally programmed healing of chromosomes. In: Blackburn E H, Greider C W, editors. Telomeres. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 193–218. [Google Scholar]
- 7.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 8.Bottius E, Bakhsis N, Scherf A. Plasmodium falciparum telomerase: de novo telomere addition to telomeric and nontelomeric sequences and role in chromosome healing. Mol Cell Biol. 1998;18:919–925. doi: 10.1128/mcb.18.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broccoli D, Young J W, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryan T M, Sperger J M, Chapman K B, Cech T R. Telomerase reverse transcriptase genes identified in Tetrahymena thermophila and Oxytricha trifallax. Proc Natl Acad Sci USA. 1998;95:8479–8484. doi: 10.1073/pnas.95.15.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn M, Blackburn E H. Telomerase in yeast. Science. 1995;269:396–400. doi: 10.1126/science.7618104. [DOI] [PubMed] [Google Scholar]
- 12.Collins K, Greider C W. Tetrahymena telomerase catalyzes nucleolytic cleavage and nonprocessive elongation. Genes Dev. 1993;7:1364–1376. doi: 10.1101/gad.7.7b.1364. [DOI] [PubMed] [Google Scholar]
- 13.Counter C M, Gupta J, Harley C B, Leber B, Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995;85:2315–2320. [PubMed] [Google Scholar]
- 14.Davis A H, Kidd G H, Carter C E. Chromosome diminution in Ascaris suum: two fold increase of nucleosomal histone to DNA ratios during development. Biochim Biophys Acta. 1979;565:315–325. doi: 10.1016/0005-2787(79)90208-9. [DOI] [PubMed] [Google Scholar]
- 15.Fairbairn D. The biochemistry of Ascaris. Exp Parasitol. 1957;6:491–554. doi: 10.1016/0014-4894(57)90037-1. [DOI] [PubMed] [Google Scholar]
- 16.Fajkus J, Kovarik A, Kralovics R. Telomerase activity in plant cells. FEBS Lett. 1996;391:307–309. doi: 10.1016/0014-5793(96)00757-0. [DOI] [PubMed] [Google Scholar]
- 17.Fan Q, Yao M. New telomere formation coupled with site-specific chromosome breakage in Tetrahymena thermophila. Mol Cell Biol. 1996;16:1267–1274. doi: 10.1128/mcb.16.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald M S, McKnight T D, Shippen D E. Characterization and developmental patterns of telomerase expression in plants. Proc Natl Acad Sci USA. 1996;93:14422–14427. doi: 10.1073/pnas.93.25.14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flint J, Craddock C F, Villegas A, Bentley D P, Williams H J, Galanello R, Cao A, Wood W G, Ayyub H, Higgs D R. Healing of broken human chromosomes by the addition of telomeric repeats. Am J Hum Genet. 1994;55:505–512. [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein P. Chromatin diminution in early embryogenesis of Ascaris umbricoides L. var. suum. J Morphol. 1977;152:141–152. doi: 10.1002/jmor.1051520202. [DOI] [PubMed] [Google Scholar]
- 21.Greene E C, Bednenko J, Shippen D E. Flexible positioning of the telomerase-associated nuclease leads to preferential elimination of nontelomeric DNA. Mol Cell Biol. 1998;18:1544–1552. doi: 10.1128/mcb.18.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greider C W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 23.Greider C W, Blackburn E H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 24.Greider C W, Blackburn E H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 25.Greider C W, Blackburn E H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 26.Hanish J P, Yanowitz J L, de Lange T. Stringent sequence requirements for the formation of human telomeres. Proc Natl Acad Sci USA. 1994;91:8861–8865. doi: 10.1073/pnas.91.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannon G J, Maroney P A, Denker J A, Nilsen T W. Trans-splicing of nematode pre-messenger RNA in vitro. Cell. 1990;61:1247–1255. doi: 10.1016/0092-8674(90)90689-c. [DOI] [PubMed] [Google Scholar]
- 28.Heller K, Kilian A, Piatyszek M A, Kleinhofs A. Telomerase activity in plant extracts. Mol Gen Genet. 1996;252:342–345. doi: 10.1007/BF02173780. [DOI] [PubMed] [Google Scholar]
- 29.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek M A, Shay J W, Ishioka S, Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 30.Jentsch, S., G. Khoudoli, C. Zenhäusern, H. Tobler, and F. Müller. Unpublished data.
- 31.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 32.Koziol C, Borojevic R, Steffen R, Muller W E. Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: the telomerase activity in somatic cells. Mech Ageing Dev. 1998;100:107–120. doi: 10.1016/s0047-6374(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 33.Kramer K M, Haber J E. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 1993;7:2345–2356. doi: 10.1101/gad.7.12a.2345. [DOI] [PubMed] [Google Scholar]
- 34.Krupp G, Kuhne K, Tamm S, Klapper W, Heidorn K, Rott A, Parwaresch R. Molecular basis of artifacts in the detection of telomerase activity and a modified primer for a more robust ‘TRAP’ assay. Nucleic Acids Res. 1997;25:919–921. doi: 10.1093/nar/25.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamb J, Harris P C, Wilkie A O, Wood W G, Dauwerse J G, Higgs D R. De novo truncation of chromosome 16p and healing with (TTAGGG)n in the alpha-thalassemia/mental retardation syndrome (ATR-16) Am J Hum Genet. 1993;52:668–676. [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J J, Zakian V A. An in vitro assay for Saccharomyces telomerase requires EST1. Cell. 1995;81:1127–1135. doi: 10.1016/s0092-8674(05)80017-0. [DOI] [PubMed] [Google Scholar]
- 37.Lue N F, Peng Y. Identification and characterization of a telomerase activity from Schizosaccharomyces pombe. Nucleic Acids Res. 1997;25:4331–4337. doi: 10.1093/nar/25.21.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantell L L, Greider C W. Telomerase activity in germline and embryonic cells of Xenopus. EMBO J. 1994;13:3211–3217. doi: 10.1002/j.1460-2075.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maroney P A, Hannon G J, Nilsen T W. Transcription and cap trimethylation of a nematode spliced leader RNA in a cell-free system. Proc Natl Acad Sci USA. 1990;87:709–713. doi: 10.1073/pnas.87.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattei D, Scherf A. Subtelomeric chromosome instability in Plasmodium falciparum: short telomere-like sequence motifs found frequently at healed chromosome breakpoints. Mutat Res. 1994;324:115–120. doi: 10.1016/0165-7992(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 41.McClintock B. The stability of broken ends of chromosomes of Zea mays. Genetics. 1941;26:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melek M, Greene E C, Shippen D E. Processing of nontelomeric 3′ ends by telomerase: default template alignment and endonucleolytic cleavage. Mol Cell Biol. 1996;16:3437–3445. doi: 10.1128/mcb.16.7.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melek M, Shippen D E. Chromosome healing: spontaneous and programmed de novo telomere formation by telomerase. Bioessays. 1996;18:301–308. doi: 10.1002/bies.950180408. [DOI] [PubMed] [Google Scholar]
- 44.Meyer O. Celluläre Untersuchungen an Nematoden-Eiern. Jena Z Naturwiss. 1895;29:391–410. [Google Scholar]
- 45.Morin G B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 46.Morin G B. Recognition of a chromosome truncation site associated with alpha-thalassaemia by human telomerase. Nature. 1991;353:454–456. doi: 10.1038/353454a0. [DOI] [PubMed] [Google Scholar]
- 47.Müller F, Bernard V, Tobler H. Chromatin diminution in nematodes. Bioessays. 1996;18:133–138. doi: 10.1002/bies.950180209. [DOI] [PubMed] [Google Scholar]
- 48.Müller F, Wicky C, Spicher A, Tobler H. New telomere formation after developmentally regulated chromosomal breakage during the process of chromatin diminution in Ascaris lumbricoides. Cell. 1991;67:815–822. doi: 10.1016/0092-8674(91)90076-b. [DOI] [PubMed] [Google Scholar]
- 49.Müller H J. The remaking of chromosomes. Collecting Net. 1938;8:181–195. [Google Scholar]
- 50.Murray A W, Claus T E, Szostak J W. Characterization of two telomeric DNA processing reactions in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4642–4650. doi: 10.1128/mcb.8.11.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 52.Noll F, Bielka H. Zur Biochemie der Embryogenese von Ascaris. Acta Biol Med Germ. 1968;20:565–575. [PubMed] [Google Scholar]
- 53.Nugent C I, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998;12:1073–1085. doi: 10.1101/gad.12.8.1073. [DOI] [PubMed] [Google Scholar]
- 54.Oliver B C, Shen S S. Cytoplasmic control of chromosome diminution in Ascaris suum. J Exp Zool. 1986;239:41–55. [Google Scholar]
- 55.Olovnikov A. Principles of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201:1496–1499. [PubMed] [Google Scholar]
- 56.Piatyszek M A, Kim N W, Weinrich S L, Hiyama K, Hiyama E, Wright W E, Shay J W. Detection of telomerase activity in human cells and tumors by a telomeric repeat amplification protocol (TRAP) Methods Cell Sci. 1995;17:1–15. [Google Scholar]
- 57.Pologe L G, Ravetch J V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988;55:869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- 58.Prescott D M. The DNA of ciliated protozoa. Microbiol Rev. 1994;58:233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price C M, Adams A K, Vermeesch J R. Accumulation of telomerase RNA and telomere protein transcripts during telomere synthesis in Euplotes. J Eukaryot Microbiol. 1994;41:267–275. doi: 10.1111/j.1550-7408.1994.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 60.Prowse K R, Avilion A A, Greider C W. Identification of a nonprocessive telomerase activity from mouse cells. Proc Natl Acad Sci USA. 1993;90:1493–1497. doi: 10.1073/pnas.90.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ray A, Runge K W. The C terminus of the major yeast telomere binding protein Rap1p enhances telomere formation. Mol Cell Biol. 1998;18:1284–1295. doi: 10.1128/mcb.18.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandell L L, Zakian V A. Loss of a yeast telomere: arrest, recovery and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 63.Scherf A, Mattei D. Cloning and characterization of chromosome breakpoints of Plasmodium falciparum: breakage and new telomere formation occurs frequently and randomly in subtelomeric genes. Nucleic Acids Res. 1992;20:1491–1496. doi: 10.1093/nar/20.7.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shippen L D, Blackburn E H. Telomere terminal transferase activity from Euplotes crassus adds large numbers of TTTTGGGG repeats onto telomeric primers. Mol Cell Biol. 1989;9:2761–2764. doi: 10.1128/mcb.9.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spicher A, Etter A, Bernard V, Tobler H, Müller F. Extremely stable transcripts may compensate for the elimination of the gene fert-1 from all Ascaris lumbricoides somatic cells. Dev Biol. 1994;164:72–86. doi: 10.1006/dbio.1994.1181. [DOI] [PubMed] [Google Scholar]
- 66.Tobler H, Smith K D, Ursprung H. Molecular aspects of chromatin elimination in Ascaris lumbricoides. Dev Biol. 1972;27:190–203. doi: 10.1016/0012-1606(72)90097-8. [DOI] [PubMed] [Google Scholar]
- 67.von Ehrenstein G, Schierenberg E. Cell lineages and development of Caenorhabditis elegans and other nematodes. In: Zuckerman B M, editor. Nematodes as biological models. Vol. 1. New York, N.Y: Academic Press; 1980. pp. 1–71. [Google Scholar]
- 68.Wang H, Blackburn E H. De novo telomere addition by Tetrahymena telomerase in vitro. EMBO J. 1997;16:866–879. doi: 10.1093/emboj/16.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Lapitan N L V, Roder M, Tsuchyia T. Characterization of telomeres in Hordeum vulgare chromosomes by in situ hybridization. II. Healed broken chromosomes in telotrisomic 4L and acrotrisomic 4L4s lines. Genome. 1992;35:975–980. [Google Scholar]
- 70.Watson J D. Origin of concatameric T4 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 71.Werner J E, Kota R S, Gill B S. Distribution of telomeric repeats and their role in the healing of broken chromosome ends in wheat. Genome. 1992;35:844–848. [Google Scholar]
- 73.Wilkie A O, Lamb J, Harris P C, Finney R D, Higgs D R. A truncated human chromosome 16 associated with alpha-thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature. 1990;346:868–871. doi: 10.1038/346868a0. [DOI] [PubMed] [Google Scholar]
- 74.Wright W E, Piatyszek M A, Rainey W E, Byrd W, Shay J W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 75.Yu G L, Blackburn E H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991;67:823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- 76.Zahler A M, Prescott D M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988;16:6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]