Case Presentation
A 24-year-old man, never smoker, with no medical or surgical history, not currently on medications, presented to the ED with a second episode of gross hemoptysis, 4 months after an initial episode that had not previously been evaluated. He described the current episode of hemoptysis as “enough to fill the sink”; however, he did not further quantify. He has no history of recurrent epistaxis, hematemesis, or other evidence of clotting disorder. He denied any fevers, chills, night sweats, or recent travel. He denied any sick contacts and has no history of TB exposure or risk factors. The patient denied any shortness of breath, wheezing, or chest pain. He had no lower extremity pain or swelling. He routinely exercises and generally lives a healthy lifestyle. He is a health care worker who has not routinely worked with patients infected with SARS-CoV-2, although he received his second (of two) COVID-19 vaccines 4 days before presentation.
Physical Examination Findings
On presentation, vital signs were normal without supplemental oxygen. Although the patient was anxious, he was otherwise well appearing. Physical examination was unrevealing, lungs were clear to auscultation in all fields without wheezing or rhonchi, and no dullness to percussion or egophony was appreciated.
Diagnostic Studies
Initial chest radiograph was normal; however, a subsequent contrasted chest CT demonstrated a 2.4-cm right hilar mass involving the right middle and lower lobe, with an endobronchial component extending from the distal bronchus intermedius causing complete right middle lobe obstruction and 85% obstruction of the proximal right lower lobe bronchi (Fig 1 ). No enlarged intrathoracic lymph nodes were appreciated; however, multiple prominent left axillary lymph nodes were appreciated with a maximum short axis diameter of 1.3 cm.
Figure 1.
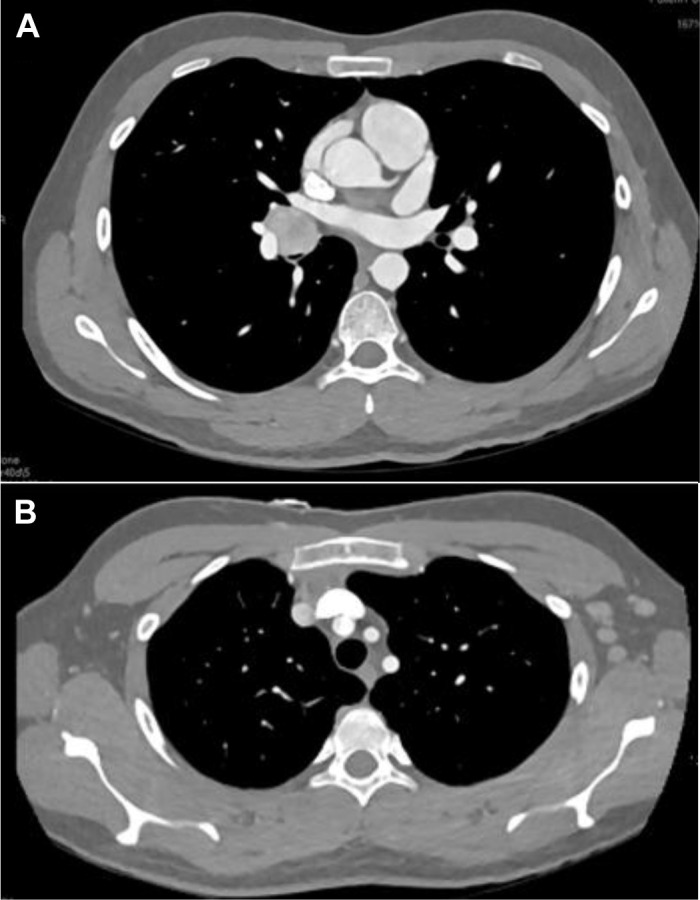
Representative CT scan slices from initial presentation demonstrating right hilar mass (A) and left axillary lymphadenopathy (B).
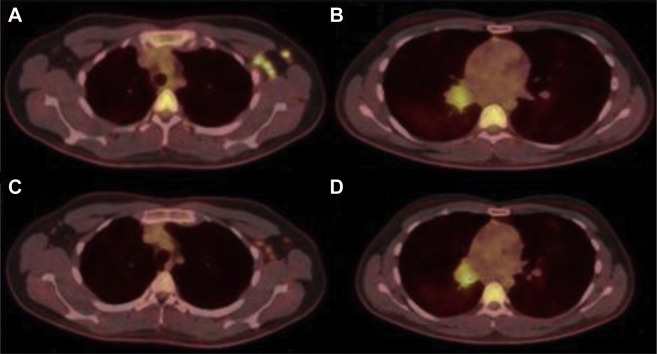
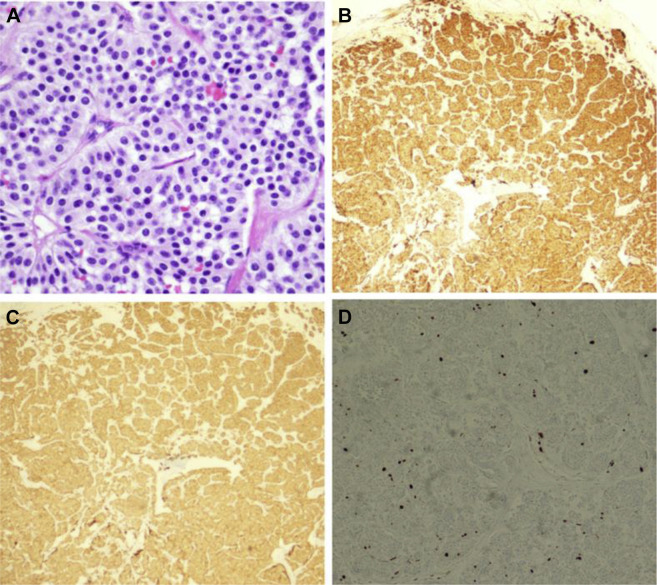
A subsequent fludeoxyglucose F 18 Injection (18F-FDG) PET-CT was performed, which demonstrated hypermetabolic activity of the right bronchial mass, with a maximum standardized uptake value (SUVmax) of 4.7, as well as multiple hypermetabolic left axillary lymph nodes with an SUVmax of 12.8 (Fig 2 ). The patient subsequently underwent rigid bronchoscopy with tumor debulking (using a combination of forceps, argon plasma coagulation, and cryoprobe) and tissue sampling. Pathologic analysis of the mass demonstrated nests of tumor cells with granular, eosinophilic cytoplasm, round monotonous nuclei, inconspicuous nucleoli, and coarse “salt and pepper” chromatin. Chromogranin and synaptophysin immunohisotchemical stains showed diffuse, strong cytoplasmic staining with Ki-67 immunohisotchemical stain showing a low proliferative index (Fig 3 ).
Figure 2.
Initial 18F-FDG PET-CT demonstrating significant avidity within the left axillary lymphadenopathy (A) and right hilar mass (B). Repeat study 4 weeks after initial image demonstrating significant reduction in avidity of left axillary lymphadenopathy(C) with stable findings in right hilar mass (D). 18F-FDG = fludeoxyglucose F 18 Injection.
Figure 3.
Pathologic analysis of the patient’s endobronchial mass. Hematoxylin and eosin stain (original magnification 400×) showing nests of tumor cells separated by delicate fibrovascular septae. The cells have granular, eosinophilic cytoplasm and round, monotonous nuclei with inconspicuous nucleoli, and coarse “salt and pepper” chromatin (A), Chromogranin immunohistochemical stain showing diffuse, strong cytoplasmic staining (B), synaptophysin immunohistochemical stain showing diffuse, strong cytoplasmic staining (C), and Ki-67 immunohistochemical stain demonstration of low proliferative index.
What is the pulmonary mass? What is the cause of the cause of the extrathoracic lymphadenopathy?
Diagnosis: Reactive lymphadenopathy caused by COVID-19 vaccination in the setting of typical bronchial carcinoid (low-grade)
Discussion
Typical bronchial carcinoid is a rare, primary lung cancer representing 1% to 2% of all lung malignancies. Histologically it is typified by nests of tumor cells with insular architecture and “salt and pepper” chromatin. Strong cytoplasmic staining with chromogranin and synaptophysin immunohistochemical stains support neuroendocrine differentiation. Typical carcinoid will show a low proliferative index on Ki-67 immunohistochemical staining, as seen in the patient. Bronchial carcinoid is generally a well-differentiated and slowly growing tumor, with 87% of cases presenting in the absence of lymph node metastasis. When patients with bronchial carcinoid do have radiographically identified lymphadenopathy, roughly 10% of cases will have hilar lymph node involvement, and fewer than 3% will have mediastinal lymph node involvement. Extrathoracic lymph node metastasis is largely unreported in the literature. In the patient with a radiographically concerning endobronchial mass, metastatic disease was considered; however, given the diagnosis of typical (low-grade) carcinoid and lack of ipsilateral hilar or mediastinal lymphadenopathy, we discussed evaluation for a second primary malignancy vs additional nonmalignant causes. These concerns were supported by the radiology report accompanying the patient’s imaging stating that “the left axillary lymphadenopathy is unlikely to be consistent with metastatic disease from the right-sided bronchial mass and is concerning for a secondary malignant process.” After discussion of the patient at our institution’s tumor board, short-interval follow-up imaging of the lymphadenopathy was recommended before pursuing excisional biopsy. Surgical resection of the carcinoid tumor was recommended. Repeat 18F-FDG PET-CT completed 4 weeks after the initial study showed significant reduction in both size and FDG uptake within the axillary lymph nodes, with an SUVmax of 4.6, compared with 12.8 on the initial imaging (Fig 2). Given the location and proximity to the patient’s SARS-CoV-2 vaccination in the ipsilateral deltoid, unlikely metastatic pattern, low risk for secondary malignancy, and diminished FDG avidity on repeat scan, a presumptive diagnosis of post-vaccination reactive adenopathy was made.
Lymphadenopathy is a nonspecific but very common finding on radiologic studies, with a broad differential that includes both malignant and benign causes. Axillary lymphadenopathy in the setting of recent vaccinations, including H1N1, MMR (measles, mumps, rubella), and smallpox, has been well described, although infrequently reported. The prevalence of axillary adenopathy in the setting of the SARS-CoV-2 vaccination, however, appears to be much more common, likely as a result of the extremely strong immunogenic response to the two-vaccine series. In a phase 3 trial of the Moderna vaccine, axillary adenopathy was reported in 19.8% of recipients. Presence of FDG avidity within axillary nodes has also been well described in previous vaccines such as H1N1, but most reports describe relatively low average SUVmax (typically <5.0). Although 18F-FDG PET-CT characteristics of post-SARS-CoV-2 vaccination associated lymphadenopathy are currently limited, in other case reports, the SUVmax appears to be much higher than seen after other vaccines, as was seen in this patient with an SUVmax of 12.8.
Despite the ongoing SARS-CoV-2 pandemic, patients will continue to require radiologic imaging for screening, diagnostic, staging, and surveillance purposes. As more people receive the SARS-CoV-2 vaccination, it is highly likely that ipsilateral, axillary lymphadenopathy will become a more common finding on these routine radiologic studies. Although most of the attention regarding vaccine-associated adenopathy has been within the breast cancer community, it is important that those who manage intrathoracic cancers are also aware of this phenomenon. Although lymph node sampling or excision is often imperative to properly stage malignancy, the characteristics and typical metastatic patterns of primary tumors must be balanced with the appearance of lymphatic involvement that could support vaccination as an alternative cause of lymphadenopathy rather than metastatic spread. Eliciting a thorough vaccination history will help clinicians and radiologists accurately interpret and evaluate enlarged or radiographically concerning lymph nodes. Unnecessary interventions must be avoided without inhibiting the rapid diagnosis and staging of possible malignancies.
Clinical Course
After rigid bronchoscopy, the patient was discharged with resolution of his presenting hemoptysis and had remained otherwise asymptomatic. His repeat 18F-FDG PET-CT was completed 4 weeks after his initial scan and showed significant reduction in avidity of his axillary lymphadenopathy, although it is important to note that he continues to have PET avid lymphadenopathy attributed to his SARS-CoV-2 vaccine greater than 1 month from the time of vaccination. He has subsequently followed up in the pulmonary clinic for preoperative evaluation and pulmonary function testing and was ultimately referred to cardiothoracic surgery for evaluation and treatment. Surgical bilobectomy was performed successfully without complications, and intraoperative regional lymph node sampling did not reveal evidence of metastasis. The patient is now recovering and appears to have an excellent prognosis.
Clinical Pearls
-
1.
Similar to previous vaccines, the SARS-CoV-2 vaccines currently available can result in significant lymphadenopathy.
-
2.
In patients with a recent history of SARS-CoV-2 vaccination (even greater than 1 month), unilateral axillary lymphadenopathy seen on imaging may be attributed to the vaccine.
-
3.
A thorough vaccine history must be included in all clinic and hospital patient encounters to prevent unnecessary interventions and procedures.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met. The authors thank Dr Jonathan Strain, MD, for his assistance with the pathology portion of this patient presentation.
Footnotes
DISCLAIMER: The authors are military service members or employees of the U.S. Government. This work was prepared as part of official duties. Title 17, U.S.C. §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C. §101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Suggested Reading
- de Andrade J.M., Marana H.R., Sarmento Filho J.M., Murta E.F., Velludo M.A., Bighetti S. Differential diagnosis of axillary masses. Tumori. 1996;82(6):596–599. doi: 10.1177/030089169608200617. [DOI] [PubMed] [Google Scholar]
- Gustafsson B.I., Kidd M., Chan A., Malfertheiner M.V., Modlin I.M. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- Studdiford J., Lamb K., Horvath K., Altshuler M., Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28(9):1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- Burger I.A., Husmann L., Hany T.F., Schmid D.T., Schaefer N.G. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36(10):848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- Dialani V., James D.F., Slanetz P.J. A practical approach to imaging the axilla. Insights Imaging. 2015;6:217–229. doi: 10.1007/s13244-014-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddey H.L., Riegel A.M. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94(11):896–903. [PubMed] [Google Scholar]
- Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention. www.cdc.gov/vaccines/ covid-19/info-by-product/pfizer/reactogenicity.html
- Edmonds C.E., Zuckerman S.P., Conant E.F. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination [Published online ahead of print February 5, 2021] AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- Mehta N., Sales R.M., Babagbemi K., et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]