Summary box.
The threat of new emerging infectious diseases demands improvements in infectious disease surveillance, which crucially depends on (real-time) data sharing and new technologies.
Infectious disease surveillance can be typified as global public good, and related important obstacles are financing and removing barriers for producing and sharing information.
Public financing and provision are important to enable cost-effective disease surveillance, since otherwise optimal levels for society are unlikely to be reached.
Additional investments in infectious disease surveillance are preferably based on sound economic evaluations considering the specific characteristics of infectious disease surveillance, however, a framework for cost-effectiveness analyses capturing the specific characteristics is yet non-existent.
Introduction
With the global increase in population density, urbanisation, and global travel and trade, the threat of widespread outbreaks of infectious diseases has increased relentlessly,1 as evidenced by recent examples of COVID-19 and Ebola. Further, although the most important causes of death shifted to non-communicable diseases, in some poorer parts of the world, communicable diseases remain the most important cause of death.2 Crucial in the prevention of and reaction to these threats is early detection, which demands an infectious disease surveillance system that can signal unusual events. How to set up and improve surveillance and how to prioritise investments are questions that need input from different scientific disciplines. Here, we focus on some economic considerations.
The benefits of improving disease surveillance
The best recognised purpose of disease surveillance is the (early) detection of epidemics and other health threats. New diagnostic tools such as unbiased and targeted next-generation sequencing (NGS) are being explored as options to improve surveillance as these allow to determine causes of unexplained disease outbreaks, trace and link sources of disease transmission, and facilitate a better understanding of how viruses and bacteria pass from animal to humans. With NGS, the same platforms and sometimes even the same protocols can be used for the analysis of viruses, bacteria, genes and parasites with the potential for cost saving through economies of scale (economies of scale occur when the costs per unit decrease when produced quantities increase, as total costs can be divided among more units), improving its affordability also for lower income countries.3 4 As most emerging infectious diseases are zoonotic, further improvements could come from incorporating concepts from ‘One Health’, integrating capacities across human and animal health sectors. In general, sharing data in a timely manner, both within and between sectors and countries, is expected to greatly enhance and accelerate the understanding of diseases and their patterning and reduce unit costs. Improved collaboration and data sharing could further help to reduce barriers for the adaption of new technologies through reducing current human capital constraints. For instance, limited access to the specific expertise is an important barrier for the adoption of technologies such as NGS for various countries.5 A last example of how surveillance could be improved is by limiting current workforce constraints. This was evidenced during the COVID-19 pandemic where, for instance, testing capacities were falling short at some points. Indeed, many of such improvements could also improve the effectiveness and efficiency of healthcare systems in general.6 7
Incentives for research and data sharing
Currently, there are limited incentives and a lack of appropriate infrastructure for data sharing, which requires a clear governance structure that ensures a balance between privacy and access and adheres to (inter)national ethical and legal requirements. Solutions have been proposed for many of the issues limiting timely sharing of data in genomics research.5 An example of an initiative to enhance data sharing is the model for improving disease surveillance set by the Collaborative Management Platform for detection and Analyses of (Re-)emerging and foodborne outbreaks (COMPARE) and its sequel Versatile Emerging infectious disease Observator (VEO) (https://www.compare-europe.eu; https://www.compare-europe.eu/VEO). The project aims to develop a global platform for sharing and analysing NGS data that are customisable so groups of users can share data rapidly when needed while retaining ownership.3
Financing disease surveillance and the ‘global public good’ concept
Hossain and colleagues8 found that low-income and middle-income countries spend an annual median of $0.04 per capita on vaccine-preventable disease surveillance. The Organization for Economic Cooperation and Development provides estimates of spending on epidemiological surveillance and risk and disease control programmes for 2018 ranging from 0.002% (Luxembourg) to 0.9% (Switzerland) of total healthcare expenditures. Recent research on the willingness to pay for surveillance in the EU found that, on average, people are willing to spend €264/year, which roughly translates into 5% of total health spending.9 Based on this, it could be argued that within the EU currently too little is spent on disease surveillance.
An important characteristic of surveillance and interventions around infectious diseases is externalities, outcomes beyond the scope of those pursuing the activity. (That is, preventing the spread in one country may also prevent the spread to other countries. Although externalities might be an opportunity for collaboration, the risk is that actors consider only their own costs and benefits, leading to underprovision of surveillance activities). Public goods are a special case where externalities exist and goods are non-rivalrous and non-excludable. Infectious disease surveillance is sometimes described as a (global) public good (eg, people cannot be excluded from benefiting from a reduction in risk of infectious disease when its incidence is reduced (non-excludability), and one person benefiting from this reduction does not prevent anyone else from benefiting as well (non-rivalry)).10 Although the purity of the public good characteristics can be questioned,11 the concept can be useful to promote and justify public interference and funding. Non-excludability and thereby inability to demand payment eliminates commercial incentives for producing public goods in the private market. Consequently, the market fails to provide quantities optimal for society: an argument for governmental interference and financing. As no global government exists, global public goods are often financed by international organisations, national governments or transnational corporations.11 Such organisation of governmental and donor financing is a complex task, which requires attention for, among others, constraints related to fragmented financing within and across sectors and governmental levels.12
Investments in surveillance in developing countries are often initiated as development aid. From the global public good perspective, however, one could argue that financing by more developed countries is actually in those countries’ own interest. (One of the difficulties, however, is that countries typically have different priority diseases and thereby different surveillance priorities due to the various threats to different population groups. Where rich countries may fear the importation of new viruses, poor countries suffer from common infections which give rise to diarrhoea and respiratory diseases. Improved affordability of newer catch-all tools that can provide diagnosis for most common diseases and rule out emerging diseases would reduce these differences and stimulate international collaboration.) A recent example is the Access to COVID-19 Tools Accelerator (https://www.who.int/initiatives/act-accelerator), a global collaboration to produce and provide equitable access to tests, treatments and vaccines against COVID-19, which initially aids poorer countries though eventually helps to protect the entire world. Morton and colleagues addressed this perspective in a model based on which could be decided how development aid in global health should be spent.13 However, absence of hard evidence on cost-effectiveness of disease surveillance complicates the use of such analytical models.
The cost-effectiveness of disease surveillance
Methods of economic evaluation have been applied in cost-effectiveness of clinical interventions with a clear link between costs of an intervention and health benefits of the target patient group.14 Such evaluations are more complex in case of surveillance systems. In general, surveillance results in health improvements only when combined with other programmes as effective policy responses require a well-functioning health system and intragovernmental coordination. In case of healthcare emergencies, outbreaks further not only influence human health, health spending and labour market participation but also general security, animal health, and have broader disruptive effects on international trade and tourism. Furthermore, behavioural responses with respect to policies play a crucial role, making outcomes less predictable.15 Some issues regarding cost estimation are that costs from detected cases (including false positives) should be considered and that costs for the development of incentive structures for data sharing and facilitation of data sharing can be difficult to quantify and attribute to separate surveillance systems and activities. The latter relates to seeking an optimal balance between funding disease-specific (vertical) interventions and (horizontal) health system strengthening. Models were built (eg, previous works7 16) to address this, which take into account that investments in health systems will increase benefits of other programmes.
Another bottleneck is the absence of a methodological framework capturing the unique characteristics of surveillance. Earlier estimates of costs of surveillance and response activities were mainly restricted to predefined preparedness or response activities for endemic diseases and single pathogen models (see box 1 for an example).
Box 1. The economic evaluation of PulseNet.
PulseNet is a surveillance system designed to identify and facilitate investigation of foodborne illness outbreaks. This molecular subtyping network of public health and food regulatory agency laboratories provides stakeholders information to improve decision-making and provides powerful incentives for the industry. It furthermore enhances the focus of regulatory agencies and limits the impact of outbreaks. The health and economic impacts associated with PulseNet were studied in an economic evaluation.20 Effectiveness was measured as a reduction of reported illness due to improved information, enhanced accountability of the industry and more rapid recalls. Economic costs comprised programmes costs and medical costs and productivity costs averted due to reduced illness. Based on data collected between 1994 and 2009, it was estimated that the system reduced the number of illnesses from Salmonella by 266 522, from Escherichia coli (E. coli) by 9489, and from Listeria monocytogenes by 56. This reduced medical and productivity costs by $507 million. Direct effects from improved recalls additionally reduced illnesses from E. coli by 2819 and Salmonella by 16 994, which further reduced costs with $37 million. Annual costs for PulseNet to public health agencies were $7.3 million, representing a more than fivefold return on investment.
Uncertainty is an important characteristic of infectious diseases that should be considered in evaluations, for instance, by using the ‘value of information’ and ‘real option’ concepts. Value of information focuses on uncertainty surrounding decisions and the role additional information can play in reducing uncertainty.17 In box 2, we explain this concept using an example. The example shows how information can aid policy makers to optimise outcomes, which we partly illustrate using a decision tree (figure 1) and simplified sensitivity analysis (figure 2), though also reveals other practical difficulties as how to get the industry to cooperate and how to divide the burden and consequences. Pooling of risks through insurance could be an option here. Although incentives for prevention and control in the market will be limited when it is known that potential losses will be reimbursed, such moral hazard is inherent to insurance and options to limit this, such as the deductible, can be applied. The real option approach focuses on the dynamic character of uncertainty by valuing the option to postpone an investment provided that some initial investment is made to ensure such options are available.18 This is important in the case of disease surveillance as appropriate timing of information provision and other actions is crucial.
Box 2. Value of information and the closure of mink farms.
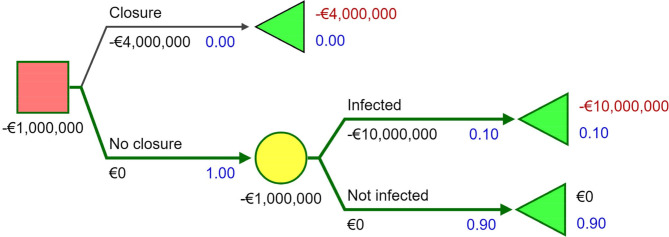
We demonstrate the concept of value of information using a recent example of with COVID-19-infected farmed minks in the Netherlands.21 We perform a simplified analysis for a policy maker facing the decision of whether to close the approximately 125 mink farms.* Immediate permanent closure of a mink farm is assumed to cost approximately €4 million per farm, based on earlier research.22 Not closing a farm while infected is assumed to cost €10 million,† a combination of the monetary value of lost life-years after transmission to humans and economic consequences in the industry. It is assumed that 10%† of the farms is likely to be infected. With no further information available the expected value of not closing a farm would be -€1 million (10%*-€10 million). With the expected value of closing a farm of -€4 million, the expected value of not closing the farm would be higher (-€1 million> -€4 million) and that option would be economically preferred. This is presented in the decision tree in figure 1. ‡ For 125 farms, this decision would lead to an expected loss of €125 million.
Now, suppose sequencing and sequence analysis can be introduced to trace the origin and transmission of the infection. With this information and monitoring, it is possible to detect new cases quickly and monitor actual statuses in the farms. This would enable a policy maker to close only farms that are infected or where there is high risk of infection. In that situation, only 10% of the farms will be closed with an expected loss of €50 million. The value of perfect information is the expected value with perfect information (-€50 million) minus the expected value with only probabilistic information (-€125 million), which is €75 million. A policy maker would be willing to pay a maximum of €75 million for sequencing and sequence analysis in this scenario.
In this example, we made assumptions on (among others) the values of input parameters. In real life decision-making, however, there is often uncertainty about the value of these parameters, which can be demonstrated through various types of sensitivity analyses. In figure 2, we show simple example of a sensitivity analysis, where we present the expected value of perfect information (and thereby investment in sequencing) for different values of the probability of infection, costs of closing a farm and infection in an open farm, where we use earlier presented numbers for the base case and the highest and lowest values as presented in the figure.†
*We assume closed farms will not reopen since permanent closure of mink farms is planned for 2024.
†These numbers are hypothetical and generated solely to illustrate the principle of value of information in the context of infectious diseases.
‡Decision tree was prepared with freeware Silver decisions from the website (http://silverdecisions.pl/SilverDecisions.html?lang=en).
Figure 1.
Decision tree.
Figure 2.
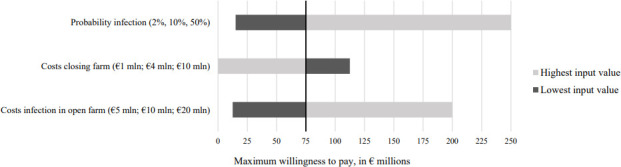
Sensitivity analysis.
Other issues in the context of economic evaluations of surveillance are the choice of the appropriate perspective and decision rules. (Two main perspectives used are the healthcare perspective (where only costs and benefits, the latter typically quantified in quality-adjusted life years (QALYs), within the healthcare sector are considered relevant) and the societal perspective (where all costs and benefits in society are considered).) A societal perspective appears to be most appropriate, recognising the broad impact of disease surveillance and facilitating comparisons across interventions (both in different sectors). Obtaining the value or decision rules for investments in surveillance is complex. In terms of the value of health, a recent review indicated an average value of a QALY of around €75 000.19 This figure only captures the value of health gains, and furthermore requires health gains to be specified in QALYs to be useful. The aforementioned study covering several countries within the EU into the valuation of an improved surveillance system indicated an average willingness to pay of around €22/month per household.9 Figures like this suggest that truly effective surveillance systems may well offer value for money from a societal perspective, given the broad range of benefits they potentially offer.
Conclusion
The threat of new emerging infectious diseases creates a need to continuously improve disease surveillance systems to prevent and control outbreaks. The key mechanism by which surveillance creates value is by producing information. Generally, the cost of sharing information is much less than the cost of producing it, and so from an economic point of view, it is desirable to share information as widely as possible. Unfortunately, incentive structures to ensure the production of information typically hinder sharing—as once information is shared, it is harder for the generator to capture the value for himself. Reward and regulatory mechanisms are critical to the creation and diffusion of innovative technologies for surveillance, and for the optimal use of the information which these products will generate. Economists have experience with such mechanisms in other settings and could contribute to the design of such mechanisms for surveillance technologies such as NGS. Considering substantial positive externalities of disease surveillance, market failure and consequently underprovision are likely. Hence, we argue for public interference in surveillance. The extent of such public involvement is an important area for further research; however, public financing and provision in itself is presumably required to enable cost-effective disease surveillance, since otherwise optimal levels for society are unlikely to be reached. Higher prevalence and burden of infectious diseases in low-income and middle-income countries, combined with less financial resources available for disease surveillance in these countries, furthermore demand financial support by higher-income countries. Additionally, considering current spending on surveillance, there is potential for substantial additional investments. For optimal use of scarce resources, further investments are preferably based on sound economic evaluations. Assessing cost-effectiveness of disease surveillance not only requires estimates of costs and effectiveness, which are difficult to estimate, but also a different analytical framework, which is yet non-existent. We highlighted methods, which, together with current practice of economic evaluation of individual interventions, could form the basis of such a framework.
Acknowledgments
We thank R.S. Sikkema for comments that greatly improved the manuscript.
Footnotes
Handling editor: Seye Abimbola
Contributors: LdV was primarily responsible for writing the manuscript, in cooperation with MK, AM and PvB. All authors read, edited and approved the final manuscript.
Funding: The contributions of MK and PvB to this manuscript are funded by the European Commission under the Horizon 2020 research and innovation programme (Grant agreement No. 643476) and part of the COMPARE project (http://www.compare-europe.eu/).
Disclaimer: The funding source had no involvement in the study.
Competing interests: MK and PvB report grants from the European Commission, during the conduct of the study. AM reports personal fees from the Office of Health Economics, personal fees from the Wellcome Trust, personal fees from AstraZeneca and non-financial support from EFPIA, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Smith KF, Goldberg M, Rosenthal S, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11:20140950. 10.1098/rsif.2014.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet 2017;390:1211–59. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alleweldt F, Kara S, OSINSKI A, et al. Developing a framework to assess the costeffectiveness of compare – a global platform for the exchange of sequence-based pathogen data. Rev Sci Tech 2017;36:311–22. 10.20506/rst.36.1.2631 [DOI] [PubMed] [Google Scholar]
- 4.Alleweldt F, Kara Şenda, Best K, et al. Economic evaluation of whole genome sequencing for pathogen identification and surveillance – results of case studies in Europe and the Americas 2016 to 2019. Euro Surveill 2021;26. 10.2807/1560-7917.ES.2021.26.9.1900606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarestrup FM, Koopmans MG. Sharing data for global infectious disease surveillance and outbreak detection. Trends Microbiol 2016;24:241–5. 10.1016/j.tim.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 6.van Baal P, Morton A, Severens JL. Health care input constraints and cost effectiveness analysis decision rules. Soc Sci Med 2018;200:59–64. 10.1016/j.socscimed.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton A, Thomas R, Smith PC. Decision rules for allocation of finances to health systems strengthening. J Health Econ 2016;49:97–108. 10.1016/j.jhealeco.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hossain A, Politi C, Mandalia N, et al. Expenditures on vaccine-preventable disease surveillance: analysis and evaluation of comprehensive multi-year plans (cMYPs) for immunization. Vaccine 2018;36:6850–7. 10.1016/j.vaccine.2018.07.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Himmler S, van Exel J, Perry-Duxbury M, et al. Willingness to pay for an early warning system for infectious diseases. Eur J Health Econ 2020;21:763–73. 10.1007/s10198-020-01171-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul I, Grunberg I, Stern MA. Global public goods. Oxford University Press, 1999. [Google Scholar]
- 11.Smith R, Beaglehole R, Woodward D, et al. Global public goods for health: health, economic and public health perspectives, 2003. http://books.google.fr/books?id=oR3vCQR_b6gC [Google Scholar]
- 12.Sparkes SP, Kutzin J, Earle AJ. Financing common goods for health: a country agenda. Health Systems & Reform 2019;5:322–33. 10.1080/23288604.2019.1659126 [DOI] [PubMed] [Google Scholar]
- 13.Morton A, Arulselvan A, Thomas R. Allocation rules for global donors. J Health Econ 2018;58:67–75. 10.1016/j.jhealeco.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes, 2005. Available: http://www.amazon.co.uk/Methods-Economic-Evaluation-Health-Programmes/dp/0198529457
- 15.Philipson T. Chapter 33 Economic epidemiology and infectious diseases. In: Handbook of health economics. Vol 1., 2000: 1761–99. [Google Scholar]
- 16.Hauck K, Morton A, Chalkidou K, et al. How can we evaluate the cost-effectiveness of health system strengthening? A typology and illustrations. Soc Sci Med 2019;220:141–9. 10.1016/j.socscimed.2018.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341–64. 10.1016/S0167-6296(98)00039-3 [DOI] [PubMed] [Google Scholar]
- 18.Dixit AK, Pindyck RS. Investment under uncertainty 2012.
- 19.Ryen L, Svensson M. The willingness to pay for a quality adjusted life year: a review of the empirical literature. Health Econ 2015;24:1289–301. 10.1002/hec.3085 [DOI] [PubMed] [Google Scholar]
- 20.Scharff RL, Besser J, Sharp DJ, et al. An economic evaluation of PulseNet: a network for foodborne disease surveillance. Am J Prev Med 2016;50:S66–73. [DOI] [PubMed] [Google Scholar]
- 21.Oreshkova N, Molenaar RJ, Vreman S, et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and may 2020. Eurosurveillance 2020;25. 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baltussen W, van der Veen H. In Dutch: “Sanering nertsenhouderij in Nederland: een actualisatie”. Wageningen, 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.