Abstract
The high mortality rate from ovarian cancer is due to the asymptomatic nature of the course of the disease, which leads to the diagnosis of ovarian cancer in later stages. The sodium-dependent phosphate transporter NaPi2b encoded by SLC34A2 gene is expressed in 80–90% of epithelial ovarian cancers and used as a target for therapeutic antibodies XMT-1536, and XMT-1592, which are derived from MX35 antibodies and used in clinical trials for the treatment of ovarian and lung cancers. In this work, we aimed to evaluate NaPi2b as a molecular marker for diagnostics and predicting the course and outcome of ovarian cancer disease.
Quantitative analysis of SLC34A2 gene expression in ovarian tumor tissue was performed at the level of transcription and translation using real-time PCR, droplet digital PCR and Western blot analysis respectively. Statistical analysis was performed taking into account various clinicopathological characteristics of the ovarian cancer patients, including the stage of the disease, the tumor grade, the applying of neoadjuvant chemotherapy and the presence of ascites. In this work, we demonstrated that the expression of the human NaPi2b (hNaPi2b) transporter is downregulated in the tumors of patients receiving neoadjuvant therapy and during the development of disease. The data suggest that the level of expression of the SLC34A2 gene can serve as a potential marker for the monitoring and predicting responses to neoadjuvant and targeted therapy in patients with ovarian cancer.
Keywords: Ovarian cancer, Molecular marker, Predictive marker, Neoadjuvant therapy, NaPi2b, SLC34A2
Highlights
-
•
The NaPi2b targeting antibodies currently undergoing clinical developments for treatment of ovary and lung cancers.
-
•
The expression of phosphate transporter NaPi2b is downregulated in ovarian tumor cells of patients after neoadjuvant therapy.
-
•
The NaPi2b can serve as a potential marker for the monitoring of the use of neoadjuvant therapy in ovarian cancer patients.
-
•
The results should be considered in combinatorial treatments of ovarian cancer patients by therapeutic anti-NaPi2b antibodies.
1. Introduction
Ovarian cancer is one of the most common cancers in women. It causes 152,000 deaths worldwide every year [1]. Ovarian cancer is diagnosed as stage III-C or stage IV in more than 80% of patients and more than 80% of advanced tumors are high grade [2]. Cytoreductive surgery followed by intravenous chemotherapy based on platinum and taxanes is the standard treatment regimen for ovarian cancer [3]. However, relapses occur in about 80% of late-stage patients within 3 years [2]. Frequent relapses and high mortality in patients with ovarian cancer at the late stages make the problem of the effectiveness of ovarian cancer treatment urgent, which requires new approaches for the diagnosis and therapy of this disease. Early diagnostics using large-scale screening programs and personalized drug selection based on the tumor molecular profile remain the greatest needs in the fight against this deadly disease.
Currently, CA125 (carbohydrate antigen 125) is a well-known marker are used to monitor the course and outcome of ovarian cancer. The level of CA125 correlates with the stage of the disease and increases at the later stages. However due to its insufficient specificity this marker is not always detected at the early stages [4]. Other markers including HE4 (Human epididymis protein 4), CA72-4 (carbohydrate antigen 72–4), CA15-3 (carbohydrate antigen 15–3), CEA (carcinoid embryonic antigen), CA19-9 (carbohydrate antigen 19–9), and others are overexpressed in ovarian cancer and also don't exhibit a sufficient sensitivity and specificity for the diagnosis and prognosis of ovarian cancer [[5], [6], [7], [8]]. Therefore, the identification and characterization of new molecular markers for diagnosis and more accurate prediction of sensitivity to chemotherapy, including targeted chemotherapy, as well as the course and outcome of the disease, is an urgent task. The sodium-dependent phosphate transporter NaPi2b was identified as a target for MX35 monoclonal antibodies using a modified SEREX technology [9,10]. NaPi2b is overexpressed in 90% of epithelial ovarian cancer and other human malignancies including thyroid, lung, breast, and other cancers [[11], [12], [13]]. Currently NaPi2b is a target for therapeutic antibodies XMT-1536 and XMT-1592 which are in clinical trials for the treatment of ovarian and lung cancers [14,15]. NaPi2b plays an important role in maintaining human phosphate metabolism and its impairment may be important for the occurrence of certain pathologies, including hyperphosphatemia [[16], [17], [18], [19], [20]]. High concentration of Pi in the tumor microenvironment compared to normal tissues was identified as a marker for tumor progression [21]. High inorganic phosphorus content requirement for the malignant cells may underline contribution of phosphate transporters to tumor metabolism and their selection as potentially important pharmaceutical targets for controlling phosphate levels [22]. Although SLC34A2 is frequently expressed in ovarian cancer cell lines much less information is available for primary tumors [13]. It was also reported that NaPi2b may be a useful marker of lung cancer [23] however currently, there is no data on the possibility of using this sodium-driven phosphate transporter NaPi2b as a prognostic and predictive marker of ovarian cancer. Due to cell surface protein abundance of NaPi2b, an evaluation of the expression profiles is important for understanding the functional significance of this transporter in tumorigenesis and deciding whether the expression profiles could serve tools for clinical applications. In this regard, this work aimed to analyze the expression of the NaPi2b transporter gene at the level of transcription and translation in ovarian cancer tumors taking into account the clinicopathological characteristics of the disease, including patient survival.
2. Materials and methods
2.1. Design of the experiment
The experiments were performed using surgery samples from patients with ovarian cancer from the Republican Clinical Oncological Dispensary in Kazan (Kazan, Russian Federation). The tumor samples were obtained through the consent of the Local Ethics Committees. 48 tumor samples were obtained and the register of clinicopathological characteristics was created (see Table 1 in Supplementary material).
The SLC34A2 gene expression was determined at the level of transcription by real-time PCR and droplet digital PCR as well as the level of translation by Western blot analysis by taking into account clinicopathological characteristics such as the application neoadjuvant therapy, the stage, and the tumor grade, the presence of ascites in the abdominal cavity of patients and the overall and disease-free survival of patients. Real-time PCR, droplet digital PCR, and Western blot data were used then for correlation analysis, comparative analysis, and assessment of overall and disease-free survival.
2.2. Isolation of total RNA from the ovarian tumor tissue and synthesis of cDNA
Isolation of total RNA from tumor tissue was carried out with Trizol reagent according to manufacturer's instructions (Thermo Fisher Scientific, USA). 1 μg of total RNA (260/280 ratio ≥1.9) was used for the synthesis of cDNA which was performed using a commercial kit (Thermo Scientific, USA) according to the manufacturer's instructions. Total RNA and cDNA samples were stored at −70 °C.
2.3. Real-time multiplex PCR
The relative expression of SLC34A2 gene at the transcriptional level was defined using real-time PCR. The expression level of β-Actin gene (ACTB) was used as a reference. The samples were amplified on real-time CFX96 Touch device (BioRad, USA) according to the protocol of the manufacturer of the reagents (Thermo Scientific, USA). The relative expression was calculated in Microsoft Excel (Microsoft Corporation, USA) using the formula: RE = E-ΔCt (RE - relative expression; E − amplification efficiency (E = 2.0); ΔCt - the difference in threshold cycle between the target and reference genes) [24].
2.4. Droplet digital PCR
Droplet digital PCR was used to determine the absolute expression of SLC34A2 gene at the level of transcription [25]. The MyCycler thermal cycler (Bio-Rad, USA) was used to amplify the samples according to the manufacturer's protocol. The plate with amplified samples was placed in QX200 reader (Bio-Rad, USA) to read the results of droplet digital PCR.
2.5. Isolation a soluble fraction of proteins from ovarian tumor tissue cancer and Western blot
Isolation of the soluble fraction of proteins from ovarian tumor tissue was performed using RIPA buffer according to the manufacturer's protocol (Thermo Fisher Scientific, USA). Protein fractions were stored at −20 °C. Western blot was performed according to the protocols of the antibody manufacturers using primary rabbit antibodies against the N-terminal domain of NaPi2b protein (Cell Signaling, USA), and mouse antibodies against β-Actin (Thermo Scientific, USA) were used as a loading control. The expression of SLC34A2 gene of the NaPi2b transporter at the level of translation was determined by image analysis using the ImageLab software (Bio-Rad, USA).
2.6. Statistical analysis
The obtained data were statistically analyzed using the TIBCO Statistica v.13.3 software (TIBCO Software Inc., USA). The Spearman's rank correlation coefficient was used for the correlation analysis of SLC34A2 gene expression at the level of transcription and translation in tumors of patients with ovarian cancer. The Mann-Whitney U test was used to analyze the SLC34A2 gene expression taking into account the tumor grade, the presence of neoadjuvant chemotherapy, and the presence of ascites in patients with ovarian cancer. The Kruskal-Wallis test was used to analyze the SLC34A2 gene expression at the level of transcription and translation taking into account the stage of the disease in patients with ovarian cancer. The Kaplan-Meier curves were used to analyze the overall and disease-free survival of patients with ovarian cancer.
3. Results
The SLC34A2 gene expression analysis at the level of transcription and translation in tumor samples of patients with ovarian cancer.
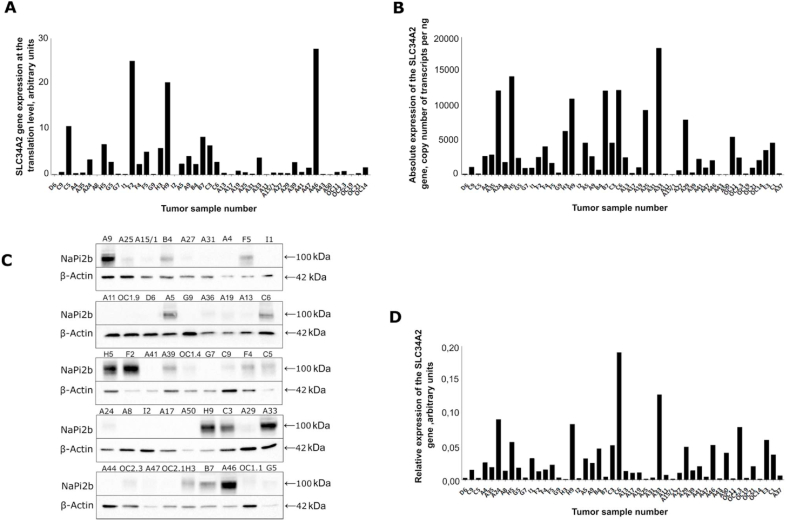
Total RNA and protein fractions were isolated from 48 tumor samples of patients with ovarian cancer and cDNA was synthesized. The relative expression of SLC34A2 gene was determined using real-time PCR, and the absolute expression of SLC34A2 gene was determined using droplet digital PCR. The SLC34A2 gene expression profile is presented as a histogram in Fig. 1.
Fig. 1.
The SLC34A2 gene expression at the level of transcription and translation in ovarian tumors. (A) The relative expression of SLC34A2 gene. (B) The absolute expression of SLC34A2 gene. (C) Western blot of SLC34A2 expression: A9, A25, A15/1, B4, A27, A31, A4, F5, I1, A11, OC1.9, D6, A5, G9, A36, A19, A13, C6, H5, F2, A41, A39, OC1. 4, G7, C9, F4, C5, A24, A8, I2, A17, A50, H9, C3, A29, A33, A44, OC2.3, A47, OC2.1, H3, B7, A46, OC1.1, G5 – tumor sample number. (D) Normalized expression of SLC34A2 gene at the level of translation in ovarian cancers.
The relative expression of SLC34A2 gene varies from 0.001 to 0.2 arbitrary units (Fig. 1A), and the absolute expression of SLC34A2 varies from 28 to 18400 gene transcripts per ng of RNA (Fig. 1B). Therefore, we have shown that the SLC34A2 gene expression at the level of transcription, determined using two different methods, ranges greatly in different tumors of patients with ovarian cancer.
To study the SLC34A2 gene expression at the level of translation, protein fractions from the same tumors were isolated and Western blot was performed. Fig. 1C shows the specific band with a molecular weight of about 100 kDa, corresponding NaPi2b (SLC34A2 gene) protein glycosylated form [18]. The relative expression of SLC34A2 gene at the level of translation, normalized to β-Actin, is shown in Fig. 1D. The Fig. 1C and D shows that the SLC34A2 gene expression at the level of translation in ovarian cancers also varies greatly.
3.1. Correlation analysis of SLC34A2 gene expression determined by different methods
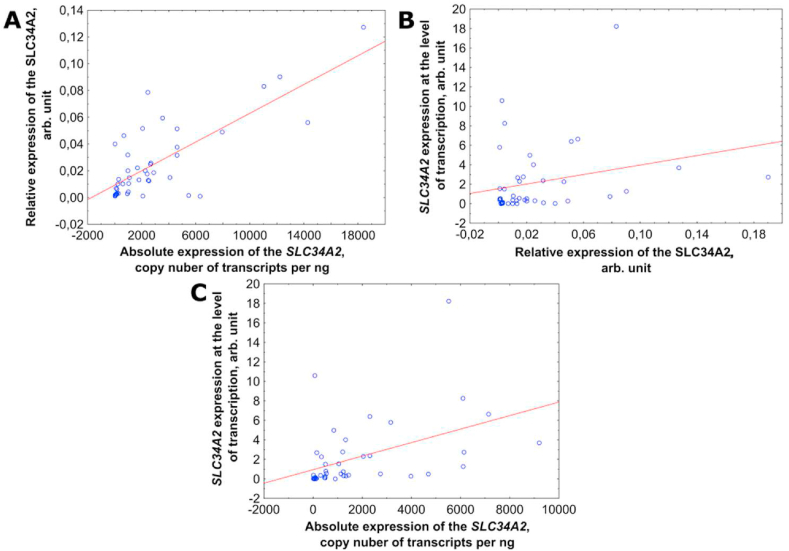
Correlation analysis was used to compare the SLC34A2 gene expression determined by three different methods including real-time PCR, droplet digital PCR, and Western-Blot. Since the distribution of expression values differed from normal, the correlation coefficient was calculated using Spearman's test. Scatter plots of SLC34A2 gene expression values determined by different methods are shown in Fig. 2.
Fig. 2.
Scatter plots of SLC34A2 gene expression values determined by three methods. (A) The relative expression of SLC34A2 gene vs the absolute expression of SLC34A2 gene. (B) The SLC34A2 gene expression at the level of translation vs the relative expression of SLC34A2 gene. (C) The SLC34A2 gene expression at the level of translation vs the absolute expression of SLC34A2 gene.
The correlation was revealed between the values of SLC34A2 gene expression at the level of transcription and translation, determined by different methods (p-value <0.05). The Spearman correlation coefficient Rs showed a statistical significance mean correlation between the absolute and the relative gene expression of SLC34A2 gene (Rs = 0.55). The correlation was shown including between the absolute gene expression and the gene expression at the translational level of SLC34A2 (Rs = 0.62). A weak correlation (Rs = 0.32) was conferred between the relative expression and the expression at the translational level of SLC34A2.
Thus, we identified a relationship between the absolute and the relative gene expression of SLC34A2 gene, which allowed us to conclude that the data obtained using real-time PCR and droplet digital PCR are reproducible and consistent with each other.
The SLC34A2 gene expression analysis at the level of transcription and translation with considering the clinicopathological characteristics of patients with ovarian cancer.
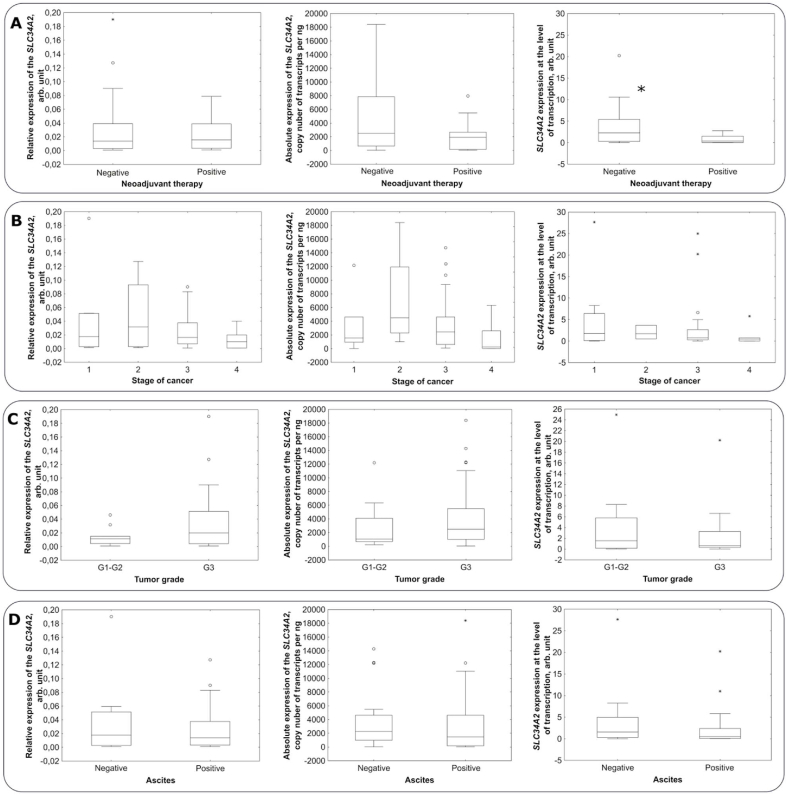
The Mann-Whitney U test was used to study the SLC34A2 gene expression at the level of transcription and translation taking into accounts the presence of neoadjuvant chemotherapy in the treatment regimen for patients with ovarian cancer. Box plots comparing the SLC34A2 gene expression at the level of transcription (real-time PCR, droplet digital PCR) and translation (Western blot) for groups of patients who had received or not neoadjuvant chemotherapy are presented in Fig. 3A.
Fig. 3.
Box plots comparing the SLC34A2 gene expression at the level of transcription and translation with considering the clinicopathological characteristics. (A) Box plots comparing the SLC34A2 gene expression at the level of transcription and translation with consideration the receipt of neoadjuvant chemotherapy (p = 0.87; 0.13; 0.05). (B) Box plots comparing the SLC34A2 gene expression at the level of transcription and translation taking into account the stage of the disease (p = 0.7; 0.13, 0.33). (С) Box plots comparing the SLC34A2 gene expression at the level of transcription and translation taking into account the tumor grade (p = 0.2; 0.4; 0.73). (D) Box plots comparing the SLC34A2 gene expression at the level of transcription and translation taking into account the presence of ascites fluid in patients (p = 0.71; 0.31; 0.28).
In the group of patients who had received neoadjuvant chemotherapy, the SLC34A2 gene expression at the level of translation is lower than in patients who had not received neoadjuvant therapy. Neoadjuvant therapy was predominantly carried out according to the TCb regimen, which included the drugs carboplatin and paclitaxel. The decreased expression level of the phosphate transporter NaPi2b in patients who had received neoadjuvant therapy can serve as a marker for monitoring the course of the disease and an indicator of the appointment of targeted therapy against the NaPi2b transporter, including monoclonal antibodies, which are in clinical trials for the treatment of ovarian cancer [11]. Notably, no such changes were revealed at the level of transcription, which may be related to the peculiarities of regulation of the phosphate transporter NaPi2b at the level of translation.
The Kruskal-Wallis test for multiple comparisons of independent samples was used to compare the SLC34A2 gene expression at the level of transcription and translation in 4 groups with different disease stages. Box plots comparing the SLC34A2 gene expression at the level of transcription and translation by groups of patients with different disease stages are shown in Fig. 3B. There were no significant differences between the groups of patients with different stages of ovarian cancer disease. Nevertheless, the trend towards a decrease in the SLC34A2 gene expression at the level of transcription with an increase in the stage of the disease from the second to the fourth was revealed in patients with ovarian cancer. The trend towards a decrease in the SLC34A2 gene expression with an increase in the stage of the disease from the third to the fourth was revealed at the level of translation. Both trends will be verified further in clinical studies with larger number of samples. A possible regulatory mechanisms underlining these effect still remain to be clarified also.
The Mann-Whitney U test was used to compare the SLC34A2 expression at the level of transcription and translation taking into account the tumor grade. Corresponding box plots are shown in Fig. 3C. There were no statistically significant differences between the SLC34A2 gene expression at the level of transcription and translation in groups of patients with different tumor grades.
The Mann-Whitney U test was applied to study the SLC34A2 gene expression at the level of transcription and translation by taking into accounts the presence of ascites fluid in patients with ovarian cancer. Box plots comparing the SLC34A2 gene expression at the level of transcription and translation in groups of patients with ovarian cancer with and without ascites fluid in the abdominal cavity are shown in Fig. 3D. There were no statistically significant differences between the SLC34A2 gene expression at the level of transcription and translation in the groups of patients with and without ascites.
3.2. Characteristics of disease-free and overall survival in patients with ovarian cancer with consideration the SLC34A2 gene expression
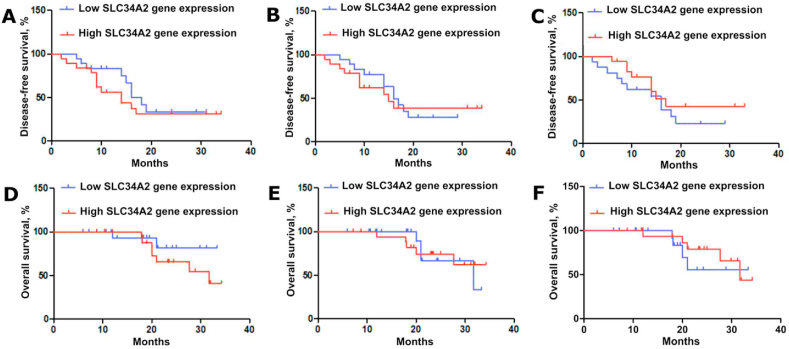
The Kaplan-Meier curves were used to analyze overall and disease-free survival in patients with ovarian cancer with consideration the SLC34A2 gene expression at the level of transcription and translation. Tumor samples were divided into two groups with low and high SLC34A2 gene expression. High expression of SLC34A2 gene includes expression values above the median, and low expression includes expression values below the median. Disease-free survival curves for patients with ovarian cancer are shown in Fig. 4A–C.
Fig. 4.
Kaplan-Meier survival curves taking into account the SLC34A2 gene expression at the level of transcription and translation. Kaplan-Meier disease-free survival curves: (A) The relative expression of SLC34A2 gene (p = 0.42); (B) The absolute expression of SLC34A2 gene (p = 0.58); (C) The SLC34A2 gene expression at the level of translation (p = 0.42). Kaplan-Meier overall survival curves: (D) The relative expression of SLC34A2 gene (p = 0.2); (E) The absolute expression of SLC34A2 gene (p = 0.43); (F) – The SLC34A2 gene expression at the level of translation (p = 0.2).
There were no differences in disease-free survival in patients with different expression of SLC34A2 gene both at the level of transcription and translation.
Overall survival curves for patients with ovarian cancer are shown in Fig. 4D–F. There were no differences in overall survival in patients with different expressions of SLC34A2 gene both at the level of transcription and translation.
4. Discussion
In the study, we first showed that the expression of the NaPi2b transporter is reduced in tumors of patients who received neoadjuvant chemotherapy. This fact can be critical for ovarian cancer patients who will receive neoadjuvant therapy with targeted drugs directed against the NaPi2b transporter. Currently, NaPi2b-specific therapeutic monoclonal antibodies XMT-1536 (trade name UpRi) (NCT03319628) and XMT-1592 (NCT04396340) are successfully undergoing clinical trials for the treatment of ovarian and non-small cell lung cancers, demonstrating safety and clinical efficacy. XMT-1536 and XMT-1592 are humanized auristatin F (AF-HPA) conjugated antibodies created on the dolaflexin and dolasynthen technology platforms respectively [15,16].
We found no relationships in the survival rate of ovarian cancer patients, the tumor grade, and presence of ascites with the expression level of the NaPi2b transporter gene. The tendency of NaPi2b transporter gene expression decrease during the increase in the disease stage was revealed, which may be associated with a decrease of differentiation extent of tumor cells at later stages. As we have shown previously, the expression of the NaPi2b transporter is observed to a greater extent in differentiated ovarian cancers [11,14].
We demonstrated that the NaPi2b protein abundance is lower in tumor ovarian cells of patients who had received neoadjuvant therapy. This fact suggests that patients with ovarian carcinoma who had received neoadjuvant therapy will be insensitive to therapy with monoclonal antibodies specific for NaPi2b and therapeutic antibodies XMT-1536 and XMT-1592 may not be effective when neoadjuvant chemotherapy is administered concurrently.
It is interesting to note that we observed a decrease in NaPi2b expression in tumors of patients who had received a neoadjuvant chemotherapy only at the protein level. This fact should be investigated further due to importance of understanding of the mechanism of regulation of phosphate transport and possibility of clinical application of NaPI2b as cell surface marker for prescribed targeted therapy in patients with ovarian carcinoma. Whether regulation at level of translation involves an internalization of transporter exhibiting potential “moonlighting” properties is unknown and subject of current investigations.
In summary, this study suggest that the level of expression of the sodium-dependent phosphate NaPi2b transporter gene can serve as a potential marker for the monitoring and predicting responses to neoadjuvant and targeted therapy in patients with ovarian cancer.
Ethical approval
Research Involving Human Participants. The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent, and the Ethics Committee of Kazan Federal University approved the study protocol.
Informed consent
Written informed consents were obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
Russian Foundation for Basic Research, Russia [project no. 19-34-90173]; Russian Science Foundation, Russia [project no. 20-14-00166]; Kazan Federal University Strategic Academic Leadership Program, Russia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.101104.
Contributor Information
A.K. Nurgalieva, Email: alsina97@mail.ru.
V.E. Popov, Email: vepopov1@gmail.com.
V.S. Skripova, Email: vsk190@gmail.com.
L.F. Bulatova, Email: minigulovalf@gmail.com.
D.V. Savenkova, Email: darina.sava1@gmail.com.
R.A. Vlasenkova, Email: r.mukhamadeeva@yandex.ru.
S.Z. Safina, Email: ksafin@mail.ru.
E. Zh Shakirova, Email: shakirovaej@mail.ru.
V.V. Filonenko, Email: filonenko@imbg.org.ua.
M.V. Bogdanov, Email: mikhail.v.bogdanov@uth.tmc.edu.
R.G. Kiyamova, Email: kiyamova@mail.ru.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hirst J., Crow J., Godwin A. Ovarian cancer genetics: subtypes and risk factors. Ovarian Cancer Pathog. Treat. 2018 doi: 10.5772/intechopen.72705. [DOI] [Google Scholar]
- 2.Bookman M.A. Can we predict who lives long with ovarian cancer? Cancer. 2019;125(Suppl 24):4578–4581. doi: 10.1002/cncr.32474. [DOI] [PubMed] [Google Scholar]
- 3.Aebi S., Castiglione M., Group E.G.W. Newly and relapsed epithelial ovarian carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20(Suppl 4):21–23. doi: 10.1093/annonc/mdp117. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M., Cheng S., Jin Y., Zhao Y., Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim. Biophys. Acta Rev. Canc. 2021;1875:188503. doi: 10.1016/j.bbcan.2021.188503. [DOI] [PubMed] [Google Scholar]
- 5.Yedema C., Massuger L., Hilgers J., Servaas J., Poels L., Thomas C., Kenemans P. Pre‐operative discrimination between benign and malignant ovarian tumors using a combination of CA125 and CA15. 3 serum assays. Int. J. Canc. 1988;41:61–67. doi: 10.1002/ijc.2910410813. [DOI] [PubMed] [Google Scholar]
- 6.Negishi Y., Iwabuchi H., Sakunaga H., Sakamoto M., Okabe K., Sato H., Asano G. Serum and tissue measurements of CA72-4 in ovarian cancer patients. Gynecol. Oncol. 1993;48:148–154. doi: 10.1006/gyno.1993.1026. [DOI] [PubMed] [Google Scholar]
- 7.Gadducci A., Cosio S., Carpi A., Nicolini A., Genazzani A.R. Serum tumor markers in the management of ovarian, endometrial and cervical cancer. Biomed. Pharmacother. 2004;58:24–38. doi: 10.1016/j.biopha.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Dowdy S., Tipton T., Podratz K., Lu W.G., Xie X., Jiang S.W. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev. Mol. Diagn. 2009;9:555–566. doi: 10.1586/erm.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiyamova R., Gryshkova V., Usenko V., Khozaenko Y., Gurtovyy V., Yin B., Ritter G., Old L., Gout I., Filonenko V. Identification of phosphate transporter NaPi2b as MX35 cancer antigen by modified SEREX approach. Біополімери і клітина. 2008;24:218–224. [Google Scholar]
- 10.Yin B.W., Kiyamova R., Chua R., Caballero O.L., Gout I., Gryshkova V., Bhaskaran N., Souchelnytskyi S., Hellman U., Filonenko V., et al. Monoclonal antibody MX35 detects the membrane transporter NaPi2b (SLC34A2) in human carcinomas. Canc. Immun. 2008;8:3. [PMC free article] [PubMed] [Google Scholar]
- 11.Shyian M., Gryshkova V., Kostianets O., Gorshkov V., Gogolev Y., Goncharuk I., Nespryadko S., Vorobjova L., Filonenko V., Kiyamova R. Quantitative analysis of SLC34A2 expression in different types of ovarian tumors. Exp. Oncol. 2011;33:94–98. [PubMed] [Google Scholar]
- 12.Kiyamova R., Shyian M., Lyzogubov V.V., Usenko V.S., Gout T., Filonenko V. Immunohistochemical analysis of NaPi2b protein (MX35 antigen) expression and subcellular localization in human normal and cancer tissues. Exp. Oncol. 2011;33:157–161. [PubMed] [Google Scholar]
- 13.Gryshkova V., Goncharuk I., Gurtovyy V., Khozhayenko Y., Nespryadko S., Vorobjova L., Usenko V., Gout I., Filonenko V., Kiyamova R. The study of phosphate transporter NAPI2B expression in different histological types of epithelial ovarian cancer. Exp. Oncol. 2009;31:37–42. [PubMed] [Google Scholar]
- 14.Bodyak N.D., Mosher R., Yurkovetskiy A.V., Yin M., Bu C., Conlon P.R., Demady D.R., DeVit M.J., Gumerov D.R., Gurijala V.R. The dolaflexin-based antibody–drug conjugate XMT-1536 targets the solid tumor lineage antigen SLC34A2/NaPi2b. Mol. Canc. Therapeut. 2021;20:896–905. doi: 10.1158/1535-7163.MCT-20-0183. [DOI] [PubMed] [Google Scholar]
- 15.Fessler S., Dirksen A., Collins S.D., Xu L., Lee W., Wang J., Eydelloth R., Ter-Ovanesyen E., Zurita J., Ditty E. XMT-1592, a site-specific Dolasynthen-based NaPi2b-targeted antibody-drug conjugate for the treatment of ovarian cancer and lung adenocarcinoma. AACR. 2020 https://www.mersana.com/wp-content/uploads/2020/06/MRSN_Dolasynthen-AACR-2020-Poster.pdf abstract # 7067. [Google Scholar]
- 16.Corut A., Senyigit A., Ugur S.A., Altin S., Ozcelik U., Calisir H., Yildirim Z., Gocmen A., Tolun A. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am. J. Hum. Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huqun Izumi S., Miyazawa H., Ishii K., Uchiyama B., Ishida T., Tanaka S., Tazawa R., Fukuyama S., Tanaka T., et al. Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am. J. Respir. Crit. Care Med. 2007;175:263–268. doi: 10.1164/rccm.200609-1274OC. [DOI] [PubMed] [Google Scholar]
- 18.Kiyamova R., Gryshkova V., Ovcharenko G., Lituyev D., Malyuchik S., Usenko V., Khozhayenko Y., Gurtovyy V., Yin B., Ritter G. Development of monoclonal antibodies specific for the human sodium-dependent phosphate co-transporter NaPi2b. Hybridoma. 2008;27:277–284. doi: 10.1089/hyb.2008.0015. [DOI] [PubMed] [Google Scholar]
- 19.Murer H., Foster I., Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Archiv. 2004;447:763–778. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo A., Negoro T., Seo T., Kitao Y., Shindo M., Segawa H., Miyamoto K. Inhibitory effect of JTP-59557, a new triazole derivative, on intestinal phosphate transport in vitro and in vivo. Eur. J. Pharmacol. 2005;517:111–119. doi: 10.1016/j.ejphar.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Bobko A.A., Eubank T.D., Driesschaert B., Dhimitruka I., Evans J., Mohammad R., Tchekneva E.E., Dikov M.M., Khramtsov V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017;7:41233. doi: 10.1038/srep41233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacerda-Abreu M.A., Russo-Abrahão T., Monteiro R.Q., Rumjanek F.D., Meyer-Fernandes J.R. Inorganic phosphate transporters in cancer: functions, molecular mechanisms and possible clinical applications. Biochim. Biophys. Acta Rev. Canc. 2018;1870:291–298. doi: 10.1016/j.bbcan.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M., Wang D.Y., Kamo T., Zhu Y., Tsujiuchi T., Konishi Y., Tanaka M., Sugimura H. Isolation and localization of type IIb Na/Pi cotransporter in the developing rat lung. Am. J. Pathol. 2000;157:21–27. doi: 10.1016/S0002-9440(10)64512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bio‐Rad . Bio-Rad Laboratories Inc; 2014. Droplet Digital™ PCR Applications Guide. [Google Scholar]
- 25.Bio‐Rad L. Bio-Rad Laboratories Inc; 2006. Real-time PCR Applications Guide. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.