Abstract
Background
The prevalence of abnormal cardiovascular magnetic resonance (CMR) findings in recovered coronavirus disease 2019 (COVID-19) patients is unclear. This study aimed to investigate the prevalence of abnormal CMR findings in recovered COVID-19 patients.
Methods
A systematic literature search was performed to identify studies that report the prevalence of abnormal CMR findings in recovered COVID-19 patients. The number of patients with abnormal CMR findings and diagnosis of myocarditis on CMR (based on the Lake Louise criteria) and each abnormal CMR parameter were extracted. Subgroup analyses were performed according to patient characteristics (athletes vs. non-athletes and normal vs. undetermined cardiac enzyme levels). The pooled prevalence and 95% confidence interval (CI) of each CMR finding were calculated. Study heterogeneity was assessed, and meta-regression analysis was performed to investigate factors associated with heterogeneity.
Results
In total, 890 patients from 16 studies were included in the analysis. The pooled prevalence of one or more abnormal CMR findings in recovered COVID-19 patients was 46.4% (95% CI 43.2%–49.7%). The pooled prevalence of myocarditis and late gadolinium enhancement (LGE) was 14.0% (95% CI 11.6%–16.8%) and 20.5% (95% CI 17.7%–23.6%), respectively. Further, heterogeneity was observed (I2 > 50%, p < 0.1). In the subgroup analysis, the pooled prevalence of abnormal CMR findings and myocarditis was higher in non-athletes than in athletes (62.5% vs. 17.1% and 23.9% vs. 2.5%, respectively). Similarly, the pooled prevalence of abnormal CMR findings and LGE was higher in the undetermined than in the normal cardiac enzyme level subgroup (59.4% vs. 35.9% and 45.5% vs. 8.3%, respectively). Being an athlete was a significant independent factor related to heterogeneity in multivariate meta-regression analysis (p < 0.05).
Conclusions
Nearly half of recovered COVID-19 patients exhibited one or more abnormal CMR findings. Athletes and patients with normal cardiac enzyme levels showed a lower prevalence of abnormal CMR findings than non-athletes and patients with undetermined cardiac enzyme levels.
Trial registration The study protocol was registered in the PROSPERO database (registration number: CRD42020225234).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12968-021-00792-7.
Keywords: Cardiac magnetic resonance imaging, Magnetic resonance imaging, Coronavirus disease 2019
Background
The spread of coronavirus disease 2019 (COVID-19) was rapid, and COVID-19 was quickly designated as a pandemic since the first identified case in December 2019 in Wuhan, China [1]. As of July 7, 2021, more than 184 million people have been diagnosed with COVID-19 and nearly 4 million have died of the infection [2]. Although COVID-19 is primarily a respiratory disease, cardiovascular complications have been reported [3, 4] and are associated with higher mortality and risk of severe COVID-19 [5, 6]. Cardiac involvement in COVID-19 can manifest as myocarditis, heart failure, acute coronary syndrome, or arrhythmias [4, 7]. Among these, myocarditis has clinical significance because myocardial inflammation can result in permanent myocardial damage and contribute to the development of arrhythmia or chronic heart failure [7, 8].
Cardiovascular magnetic resonance (CMR) is used to diagnose cardiovascular complications of COVID-19, such as acute myocarditis, using the recently updated Lake Louise criteria [9]. Individual reports and one systematic review of CMR findings in COVID-19 patients have been published to date; however, most focused on patients in the active disease stage [10]. Notably, recent data indicated that the prevalence of abnormal CMR findings, such as myocardial edema and late gadolinium enhancement (LGE), in recovered COVID-19 patients is substantial [11–22]; however, their prevalence is highly variable. Although the clinical significance of abnormal CMR findings in recovered COVID-19 patients is not yet fully understood, determining the prevalence of such findings in certain subgroups of patients would benefit clinical decision-making. For example, the presence of myocardial scars after myocarditis can lead to sudden cardiac death, especially in athletes. Consequently, the prevalence of abnormal CMR findings in athletes who have recovered from COVID-19 affects their return to play [23–25].
Therefore, the purpose of this study was to investigate the prevalence of abnormal CMR findings in recovered COVID-19 patients through meta-analysis.
Methods
Our methods followed the recommendations of the preferred reporting items for systematic reviews and meta-analyses statement [26], and the study protocol was registered in the PROSPERO database (registration number: CRD42020225234).
Literature search
Two cardiothoracic radiologists with 5 and 8 years of experience, in performing meta-analyses designed the search strategy in consensus. Each individual independently performed systematic searches of PubMed, EMBASE, the Cochrane library, SSRN, and MedRxiv/BioRxiv on March 3, 2021, to identify studies published since 2020. The search terms are listed in Additional file 1: Appendix S1.
Study selection
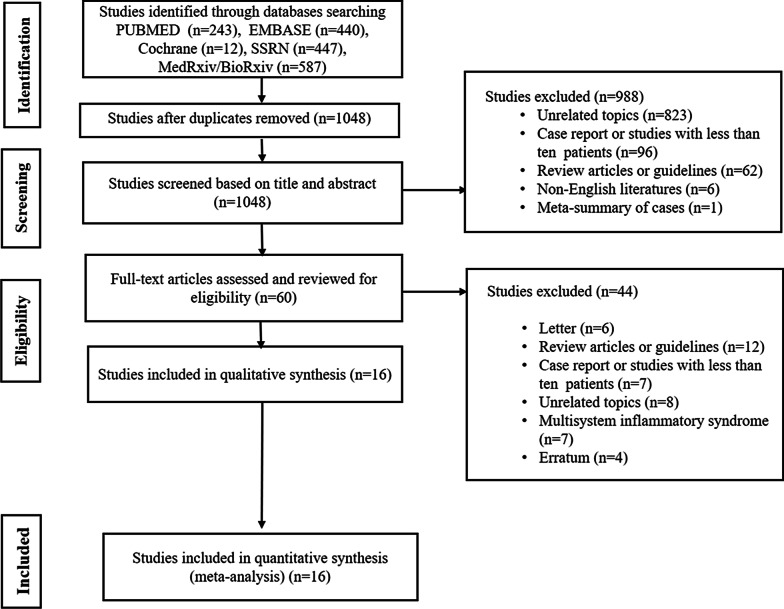
Two investigators independently reviewed the retrieved articles. A flowchart summarizing the literature search process is shown in Fig. 1. To determine the study eligibility, the full text of articles was evaluated for inclusion using the following criteria: (1) type of study, i.e., randomized controlled studies, prospective or retrospective cohort studies, and case–control studies with more than 10 patients; (2) study population, i.e., patients who recovered from COVID-19 and underwent CMR after recovery; and (3) primary outcome, i.e., the prevalence of abnormal CMR findings. Abnormal CMR findings included the presence of ventricular systolic dysfunction on cine imaging, the presence of myocardial or pericardial late gadolinium enhancement (LGE), abnormal signal intensity on T2-weighted (T2w) imaging, elevated native T1 or T2 values on the mapping sequence, a diagnosis of myocarditis based on the updated Lake Louise criteria, and the presence of pericardial effusion [9].
Fig. 1.
Flowchart of the literature review process
In contrast, a study was excluded if the study population was restricted to COVID-19 patients with multisystem inflammatory syndrome or reported CMR findings during the acute stage of COVID-19.
Data extraction
Two investigators independently extracted data with disagreements resolved by consensus. The extracted parameters included the following: (a) article information and patient characteristics; (b) CMR protocol, i.e., CMR scanner type (1.5 or 3 T) and obtained CMR sequences including cine, parametric mapping (T1 and T2), LGE, and T2w; and (c) CMR findings, i.e., the number of patients with normal and abnormal CMR findings, abnormal cine findings (ventricular systolic dysfunction), elevated parametric mapping (native T1 and T2) and extracellular volume (ECV) values, presence of LGE (myocardial or pericardial), myocardial segments with abnormal T2 or LGE areas, myocardial LGE patterns (non-ischemic, ischemic, or dual) that fulfilled the diagnostic criteria for myocarditis on CMR based on the Lake Louise criteria [9], and presence of pericardial effusion. LGE at the right ventricular (RV) insertion points in the interventricular septum was not considered to indicate LGE presence because it is a common non-specific finding in athletes [27].
Subgroup analysis
Subgroups were stratified according to (a) whether a patient group was limited to athletes and (b) levels of cardiac enzymes (troponin I or high-sensitivity troponin T) when CMR was performed. Studies wherein the cardiac enzyme data were not extractable were assigned to the “undetermined cardiac enzyme level” subgroup. An analysis of an “elevated cardiac enzyme level” subgroup could not be performed, because there were only seven patients in three studies who had elevated cardiac enzyme levels and extractable CMR findings [11, 28, 29].
Quality assessment
Two investigators independently performed quality assessments of the selected studies using the Newcastle–Ottawa Quality Scale [30]: for each question within the Selection and Exposure/Outcome categories, the maximum score is 1, and for the Comparability category, the top score is 2. A study with a total score of 6 or higher was considered of “high quality.”
Statistical analysis
The pooled prevalence and 95% confidence interval (CI) of each CMR finding were estimated using a generalized linear mixed model. The heterogeneity between studies was assessed using chi-square-based Q statistics and I2 statistics [31, 32], and significant heterogeneity was defined as a P-value of < 0.1 or an I2 value of > 50%. Subgroup analysis of the prevalence of CMR findings was performed for the “athlete” versus (vs.) “non-athlete” subgroups and the “normal cardiac enzyme level” vs. “undetermined cardiac enzyme level” subgroups. Meta-regression analysis was performed for major CMR parameters to investigate their contribution to a study’s heterogeneity, using the covariates “athlete” and “undetermined cardiac enzyme level.” Variables with P-values of < 0.2 in the univariable meta-regression analysis were included in the multivariable analysis. A P-value of < 0.05 was considered to indicate a statistically significant difference in the multivariable analysis. Publication biases were drawn as funnel plots and evaluated using the Egger test [33]. The analysis was performed using R (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria) with the “metafor” and “meta” packages [34, 35].
Results
Study characteristics
Following the literature search, 890 patients from 16 studies were included in this meta-analysis [11–14, 16–22, 28, 29, 36, 37]. Tables 1 and 2 summarize the study characteristics and CMR protocols of the included studies, respectively. A greater percentage of the included studies were conducted retrospectively (62.5%) at a single institution (93.8%). Most studies (81.3%) obtained cine, parametric mapping (native T1 and T2), and LGE sequences [11–14, 16–19, 21, 22, 28, 36, 37]. Similarly, nine studies obtained T2w sequences [11, 12, 16, 17, 20, 21, 28, 29, 36], and one study obtained a non-contrast-enhanced CMR without an LGE sequence [17].
Table 1.
Study characteristics
| First author (year) | Journal | Study design | Study sites (countries) | Patient description | Study period | Population (n) | Reason for exclusion (n) | Number of patients including in the analysis | Age (years) | Sex (n, male/female) | Diagnosis of COVID-19 by RT-PCR | Other tests for cardiac evaluation | Presence of cardiac symptoms at the time of CMR | Cardiac enzyme level at the time of CMR | Population restricted to athletes | CMR field strength | CMR scan time | CMR sequences |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ng et al. (2020) | JACC Cardiovasc Imaging | Retrospective, single-center, observational | Hong Kong | Recovered COVID-19 patients | NA | 16 | Ischemic etiology (1) | 15 | Median 68 (IQR: 53–69) | 9/7 | Yes | Troponin, CRP | Various (5/16) | Undetermined | No | 1.5 T (GE) | Median 56 days after recovery | Cine, Mapping (T1 and T2), LGE |
| Huang et al. (2020) | JACC Cardiovasc Imaging | Retrospective, single-center, observational | China | Recovered COVID-19 patients | NA | 26 | None | 26 | Median 38 (IQR: 32–45) | 10/16 | Yes | hs-troponin I assay | Yes (26) | Normal (26) | No | 3 T (Skyra, Siemens) | Median 47 days (IQR: 36–58) after symptom onset | Cine, T2WI, mapping (T1 and T2), LGE |
| Rajpal et al. (2020) | JAMA Cardiol | Prospective, single-center, observational | U.S | Athletes recovered from COVID-19 | Between June 2020 and August 2020 | 26 | None | 26 | Mean 19.5 (SD: 1.5) | 16/10 | Yes | ECG, troponin I assay, echocardiography | Various (12/26) | Normal (26) | Yes | 1.5 T (Magnetom Sola, Siemens) | 11–53 days after recommended quarantine | Cine, mapping (T1 and T2), LGE, ECV |
| Knight et al. (2020) | Circulation | Retrospective, single-center, observational | England | Recovered COVID-19 | Until April 2020 | 51 | Acute coronary syndromes (6) pulmonary emboli (12), or known cardiac pathology (7) | 29 | Mean (SD) 64 (9) | 24/5 | Yes | NR | Yes (29) | Undetermined | No | 1.5 T (Avanto Aera;Siemens) | Mean 46 days after symptom onset | Cine, Mapping (T1 and T2), LGE, Adenosine stress perfusion |
| Puntmann et al. (2020) | JAMA Cardiol | Prospective, single-center, observational | Germany | Recovered COVID-19 patients | Between April 2020 and June 2020 | 100 | None | 100 | Mean 49 (SD: 14) | 53/47 | Yes | Hs-troponin T assay | Various (36/100) | Undetermined | No | 3 T (Skyra, Siemens) | Median 71 (IQR 64–92) after COVID-19 diagnosis | Cine, Mapping (T1 and T2) LGE |
| Eiros et al. (2020) | MedRxiv | Retrospective, single-center observational | Spain | Recovered COVID-19 patients (health care workers) | Between May 25, 2020 and June 12, 2020 | 142 | Claustrophobia (1),history of hypertrophic myocardiopathy (1), inherited immune deficiency (1) | 139 | Median 52 (IQR: 41–57) | 39/100 | 103 diagnosed by RT-PCR, 36 by serology | ECG; NT-pro-BNP and hs-troponin T assays | Various (91/139) | Normal (138), elevated (1) | No | 1.5 T (Achiva, Philips) | Median 10.4 (IQR: 9.3–11.0) weeks after symptom onsetb | Cine, T2WI Mapping (T1 and T2), LGE |
| Vago et al. (2020) | JACC Cardiovasc Imaging | Retrospective, single-center observational | Hungary | Athletes recovered from COVID-19 | NA | 12 | None | 12 | Median 23 (IQR: 20–23) | 2/10 | Yes | CRP, NT-pro-BNP, and hs-troponin T assays | Yes (12) | Normal (11), undetermined (1) | Yes | 1.5 T (Magnetom, Aera, Siemens) | Median 17 (IQR: 17–19) days after positive PCR in 10 female athletes, 67 and 90 days in 2 male athletes | Cine, T2WI, mapping (T1 and T2), LGE |
| Brito et al. (2020) | JACC Cardiovasc Imaging | Retrospective, single-center observational | U.S | Student athletes recovered from COVID-19 | By August 2020 | 54 | Claustrophobia (1), no CMR (5) | 48 | Median 19 (range 19–21)a | 46/8a | PCR or antibody test | Echocardiography, troponin I assay, ECG | Various (37/48) | Undetermined | Yes | 1.5 T (Magnetom, Aera; Siemens) | Median 27 days (range 22–33 days) from diagnosis of COVID-19 | Cine, T2WI, mapping (T1 and T2), LGE |
| Clark et al. (2021) | Circulation | Retrospective, single-center, observational | U.S | Athletes recovered from COVID-19 | Since August 2020 | 22 | None | 22 | Median 20 | 9/11 | Yes | ECG, troponin I assay, echocardiography | NR | Normal (18) | Yes | 1.5 T (Avanto fit, Siemens) | Median 52 days after COVID-19 diagnosis | Cine, mapping (T1 and T2), LGE, ECV |
| Malek et al. (2021) | J Magn Reson Imaging | Retrospective, single-center observational | Germany | Student athletes recovered from COVID-19 | Diagnosed COVID-19 between August and October 2020 | 26 | None | 26 | Median 19 (IQR 19–21) | 5/21 | Yes | ECG, CRP, hs-troponin I assay | NR | Normal (26) | Yes | 1.5 T (Magnetom Avanto Fit, Siemens) | Median 32 days (IQR 22–62 days) after diagnosis | Cine, T2WI, Mapping (T1 and T2), LGE |
| Li et al. (2021) | Radiology | Prospective, single-center, observational | China | Recovered COVID-19 patients | Between May and September 2020 | 78 | Due to discharge < 90 days (n = 5), abnormal cardiac enzyme (n = 3), abnormal ECG findings (n = 4), not underwent CMR (n = 16), history of cardiovascular disease or HTN (7), contrast allergy (1), image quality (2) | 40 | Mean 54 (SD: 12) | 24/160 | Yes | ECG, CRP, CK, CKMB Troponin I assays | No | Normal (40) | No | 3 T (Skyra, Siemens) | Mean 124 ± 17 days after discharge, Mean 158 ± 18 after admission | Cine, LGE, Strain |
| Starekova et al. (2021) | JAMA Cardiology | Retrospective, single-center observational | U.S | Athletes recovered from COVID-19 | Between January 1, 2020, and November 29, 2020 | 145 | none | 145 | Mean 20 (range: 17–23) | 108/37 | Yes | ECG, Troponin I, NT-proBNP, CRP, ESR assays and echocardiography | Various (1/145) | Normal (141), elevated (2) | Yes | 1.5 T or 3 T (GE) | Median 15 days after diagnosis | Cine, T2WI, Mapping (T1 and T2), LGE |
| Wang et al. (2021) | J Cardiovasc Magn Reson | Prospective, single-center, observational | China | Recovered COVID-19 patients | From May 8 to July 20, 2020 | 47 | History of cardiovascular disease (3) | 44 | Mean 47.6 (SD: 13.3) | 19/25 | Yes | NR | NR | Undetermined | No | 3 T (Ingenia, Philips) | Mean 102.5 ± 20.6 days after diagnosis | Cine, T2WI, T2 star map, LGE, strain |
| Pan et al. (2021) | J Magn Reson Imaging | Prospective, single-center observational | China | Recovered COVID-19 patients | Between March 2020 and April 2020 | 31 | History of cardiovascular disease, presence of cardiac symptoms, or elevated cardiac enzymes (10) | 21 | Median 36 (IQR: 31–47) | 10/11 | Yes | NR | No | Undetermined | No | 3 T (Signa, GE) | Median 46 day (IQR 43–50 days) | Cine, T2WI, Mapping (T1 and T2) |
| Zhou et al. (2021) | Plos one | Prospective, single-center, observational | Hong Kong | Recovered COVID-19 patients | Diagnosed up to April 2020 | 97 | No CMR (85) | 12 | Mean 46.5 (SD:18.6)a | 52/45a | Yes | ECG, Troponin I, NT-proBNP assay and echocardiography | NR | Normal (7), elevated (4) | No | NR | NR | Cine, T2WI, LGE |
| Kotecha et al. (2021) | Eur Heart J | Retrospective, Multicenter study | U.K | Recovered COVID-19 patients | Discharged up to 20 June 2020 | 820 | No CMR (672) | 148 | Mean 64 (SD:12) | 104/44 | Yes | NR | NR | Undetermined | No | 1.5 T (Magnetom, Aera, Siemens) | Median 56 days (IQR 30–88 days) after discharge | Cine, Mapping (T1 and T2), LGE, stress perfusion |
CMR cardiovascular magnetic resonance imaging, CRP C-reactive protein, ECG electrocardiography, ECV extracellular volume, hs-troponin T high-sensitivity troponin T, IQR interquartile range, LGE late gadolinium enhancement, NA not available, NR not reported, NT-pro-BNP N-terminal pro-natriuretic peptide, PCR polymerase chain reaction, RT-PCR real-time polymerase chain reaction, SD standard deviation, T2w T2-weighted imaging, US United States, WBC white blood count
aOnly provided value of the entire study population
bMedian 9.4 weeks (IQR: 8.1–10.0 weeks) and median 4.4 weeks (IQR: 3.6–5.0 weeks) after the positive RT-PCR test and diagnosed through antibodies testing, respectively
Table 2.
Cardiovascular magnetic resonance findings of the included studies
| First author (year) | CMR abnormality, n (%) | Fulfilled diagnostic criteria of myocarditis on CMRa (n) | Cine abnormality (n) | T1 mapping abnormality (n) | T2w abnormality (n) | T2 mapping abnormality (n) | T2 segment | T2 abnormality (T2w or T2 map) | ECV abnormality (n) | Myocardial LGE (n) | Pericardial LGE (n) | Total LGE | LGE segment | LGE pattern | Increased T2 value without LGE (n) | LGE without T2 elevation (n) | Pericardial effusion (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ng et al. (2020) | 9 (66.7%) | 4 | NR | 5 | NA | 5 | Global | 5 | NR | 3 | NR | 3 | NR | Non-ischemic (3)a | 2 | 1 | 0 |
| Huang et al. (2020) | 15 (57.7%) | 7 | NR | NR | 14 | NR | NR | 14 | NR | 8 | 0 | 8 | Inferior or lateral at the mid and basal segments | Focal linear subepicardial and patchy mesocardial | 7 | 1 | 7 |
| Rajpal et al. (2020) | 13 (50%) | 4 | 1 | 0 | NA | 4 | Mid-inferoseptal (3) mid-anteroseptal (2), basal inferoseptal (1) | 4 | 1 | 12 | 0 | 12 | Septal (19), inferior or lateral (5) at the mid and basal segments | Patchy (6), linear (3), epicardial (1), RV insertion (2) | 0 | 8 | 2 |
| Knight et al. (2020) | 20 (69%) | NR | 2 | NR | NA | 0 | NR | 0 | NA | 20 | 0 | 20 | NR | Non-ischemic (11), ischemic (5), dual (4) | NR | NR | 2 |
| Puntmann et al. (2020) | 78 (78%) | NR | NR | 73 | NA | 60 | NR | 60 | NR | 32 | 22 | NR | NR | Nonischemic (20), ischemic (12), pericardial (22) | NR | NR | 20 |
| Eiros et al. (2020) | 104 (75%) | 51 | 7 | 58 | 6 | 6 | NR | NR | 52 | 10 | 0 | 10 | NR | NR | NR | NR | 42 |
| Vago et al. (2020) | 0 (0%) | 0 | 0 | 0 | 0 | 0 | NR | 0 | NA | 0 | 0 | 0 | NR | NR | 0 | 0 | NR |
| Brito et al. (2020) | 26 (54.2%) | 0 | 1 | 9 | 0 | 0 | NR | 0 | NA | 1 | 19 | NR | Lateral | Pericardial (19), myocardial (1) | 0 | 1 | 28 |
| Clark et al. (2021) | 4 (6.8%) | 2 | 0 | NA | NA | 1 | Mid septum | NR | NA | 3 | 1 | 4 | NR | NR | NA | NA | NA |
| Malek et al. (2021) | 7 (26.9%) | 0 | 2 | 0 | 3 | 1 | NR | 4 | 0 | 1 | 0 | 1 | Inferolateral segment | Mid wall | 4 | 1 | 2 |
| Li et al. (2021) | 24 (60%) | NR | NA | NA | NR | NA | NR | NA | 24 | 1 | 0 | 1 | Mid-inferior segment | NR | NR | NR | NA |
| Starekova et al. (2021) | 4 (2.8%) | 2 | NA | 2/141 | 2 | 1/102 | Apical inferolateral, and basal inferior segment | 2 | NR | 4 | 1 | 4 | Apical inferolateral, and basal inferior segment | Mid myocardial and subepicardial (1), epicardial (1), mid myocardial (2) | 0 | 2 | 1 |
| Wang et al. (2021) | 13 (29.5%) | NA | NA | NA | NA | NA | NA | NA | NR | 13 | 0 | 13 | Inferior wall and inferior-lateral wall of the basal segment | Mid myocardium, subepicardium | NA | NA | NA |
| Pan (2021) | 15 (71.4%) | NA | 3 | 5 | NR | 10 | NR | 10 | NR | NR | NR | NR | NR | NR | NR | NR | NA |
| Zhou (2021) | 1 (8.3%) | 0 | 0 | NR | 0 | NR | NR | 0 | NR | 1 | 0 | 1 | Basal anterolateral segment | Subepicardial | 0 | 1 | NA |
| Kotecha (2021) | 80 (54.1%) | 12 | 17 | 23/137 | NR | 12/137 | NR | 12/137 | NR | 70/144 | 0 | 70/144 | NR | Subepicardial (28), midwall (14), subendocardia and subepicardial (3), subendocardial and midwall (2) | NA | NA | 8 |
ECV extracellular volume, LGE late gadolinium enhancement, NA not available, NR not reported, RV right ventricular, T2w T2-weighted imaging
aOne patient who showed ischemic LGE with a history of myocardial infarction was excluded
Six of the 16 included studies enrolled only athletes as participants [16, 19, 21, 28, 36, 37], whereas there was no restriction on the occupation of study participants in the other 10 studies [11–18, 20, 29]. Eight studies had populations with normal cardiac enzyme levels [11, 12, 15, 16, 19, 28, 29, 37]. Seven other studies had patients with undetermined cardiac enzyme levels [13, 14, 17, 18, 20–22], and one study reported data for normal and undetermined cardiac enzyme level subgroups [36].
Pooled prevalence of abnormal CMR findings
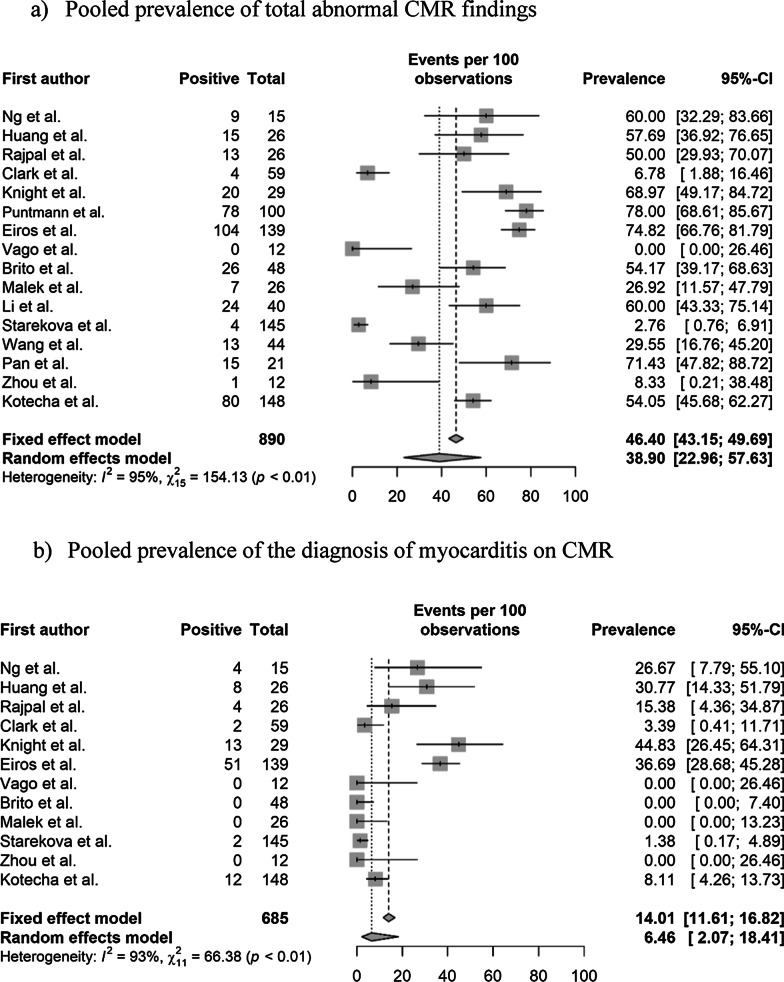
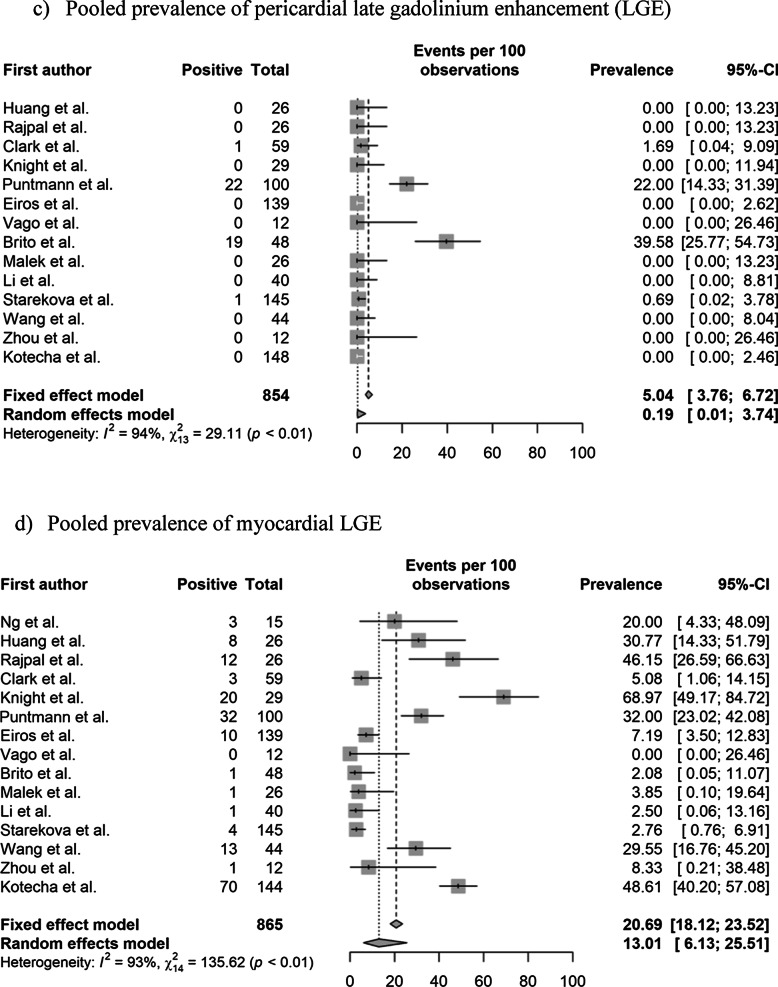
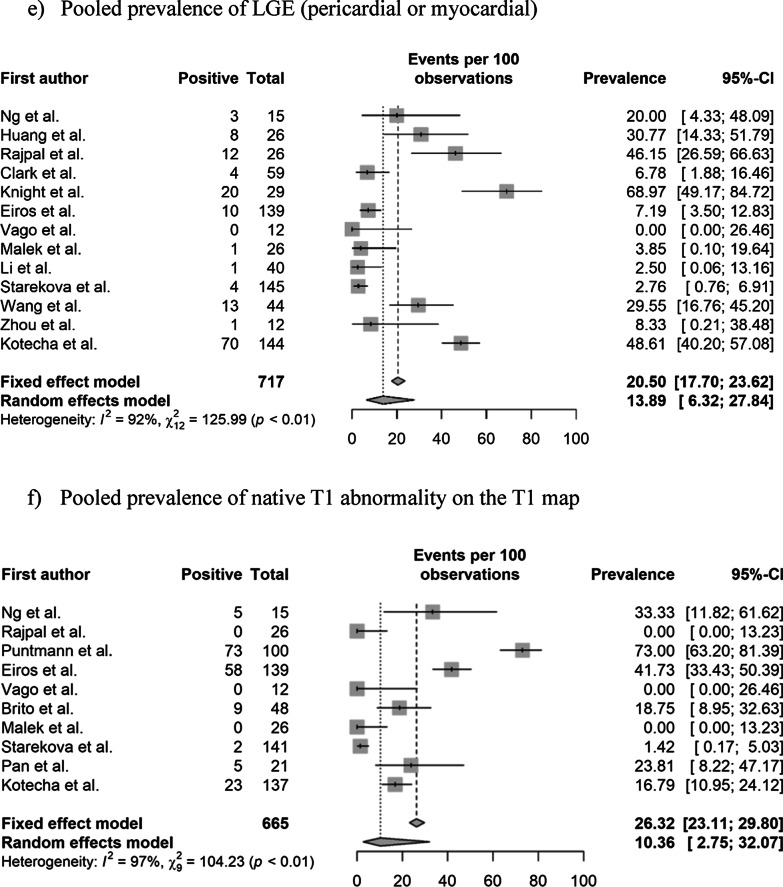
The pooled prevalence values of abnormal CMR findings are summarized in Table 3 and Fig. 2. The overall prevalence of any abnormal CMR finding in recovered COVID-19 patients was 46.4% (95% CI 43.2%–49.7%) in 16 studies [11–22, 28, 29, 36, 37]. The pooled prevalence of a CMR diagnosis of myocarditis was 14.0% (95% CI 11.6%–16.8%) in 12 studies [11–14, 16, 19, 21, 22, 28, 29, 36, 37]. The pooled prevalence of pericardial and myocardial LGE was 5.0% (95% CI 3.8%–6.7%) in 14 studies [11–16, 18–21, 28, 29, 36, 37] and 20.7% (95% CI 18.1%–23.5%) in 15 studies [11–16, 18–22, 28, 29, 36, 37], respectively. The pooled prevalence of total (pericardial or myocardial) LGE was 20.5% (95% CI 17.7%–23.6%) in 13 studies [11–16, 19, 20, 22, 28, 29, 36, 37].
Table 3.
Pooled prevalence of abnormal CMR findings
| Parameter (number of studies) | Overall | Study population | Cardiac enzyme level† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | Heterogeneity* | Non-athletes (n = 10) | Athletes (n = 6) | Normal (n = 9) | Undetermined (n = 8) | |||||
| Prevalence (%) | Heterogeneity* | Prevalence (%) | Heterogeneity* | Prevalence (%) | Heterogeneity* | Prevalence (%) | Heterogeneity* | |||
| Abnormal CMR findings (n = 16) | 46.4 [43.2–49.7] | < 0.01, 95% | 62.5 [58.5–66.4] (n = 10) | < 0.01 86% | 17.1 [13.3–21.7] (n = 6) | < 0.01, 92% | 35.9 [31.7–40.3] (n = 9) | < 0.01, 95% | 59.4 [54.5–64.04] (n = 8) | < 0.01, 77% |
| Diagnosis of myocarditis on CMR (n = 12) | 14.0 [11.6–16.8] | < 0.01, 93% | 23.9 [19.8–28.5] (n = 6) | < 0.01, 85% | 2.5 [01.3–5.0] (n = 6) | 0.11, 64% | 15.2 [12.1–19.0] (n = 8) | < 0.01, 90% | 12.0 [8.5–16.8] (n = 5) | < 0.01, 90% |
| Presence of pericardial LGE (n = 14) | 5.0 [3.8–6.7] | < 0.01, 94% | 4.1 [2.7–6.1] (n = 8) | > 0.99, 98% | 6.7 [4.4–10.0] (n = 6) | < 0.01, 86% | 10.4 [7.1–15.1] (n = 5) | < 0.01, 88% | 24.8 [20.6–29.6] (n = 7) | < 0.01, 96% |
| Presence of myocardial LGE (n = 15) | 20.7 [18.1–23.5] | < 0.01, 93% | 28.8 [25.2–32.7] (n = 9) | < 0.01, 92% | 6.7 [4.4–10.0] (n = 6) | < 0.01, 82% | 8.6 [6.0–12.1] (n = 8) | < 0.01, 83% | 36.5 [31.8–41.4] (n = 7) | < 0.01, 93% |
| Presence of LGE (myocardial or pericardial) (n = 13) | 20.5 [17.7–23.6] | < 0.01, 92% | 28.1 [24.1–32.4] (n = 8) | < 0.01, 92% | 7.8 [5.2–11.7] (n = 5) | < 0.01, 84% | 8.3 [5.8–11.8] (n = 8) | < 0.01, 85% | 45.5 [39.2–51.9] (n = 5) | < 0.01, 75% |
| Increased native T1 value on the T1 map (n = 10) | 26.3 [23.1–29.8] | < 0.01, 97% | 39.8 [35.2–44.6] (n = 5) | < 0.01, 92% | 4.4 [2.4–7.7] (n = 5) | 0.02, 81% | 1.0 [0.1–8.7] (n = 4) | > 0.99, 0% | 35.7 [30.7–41.1] (n = 6) | < 0.01, 88% |
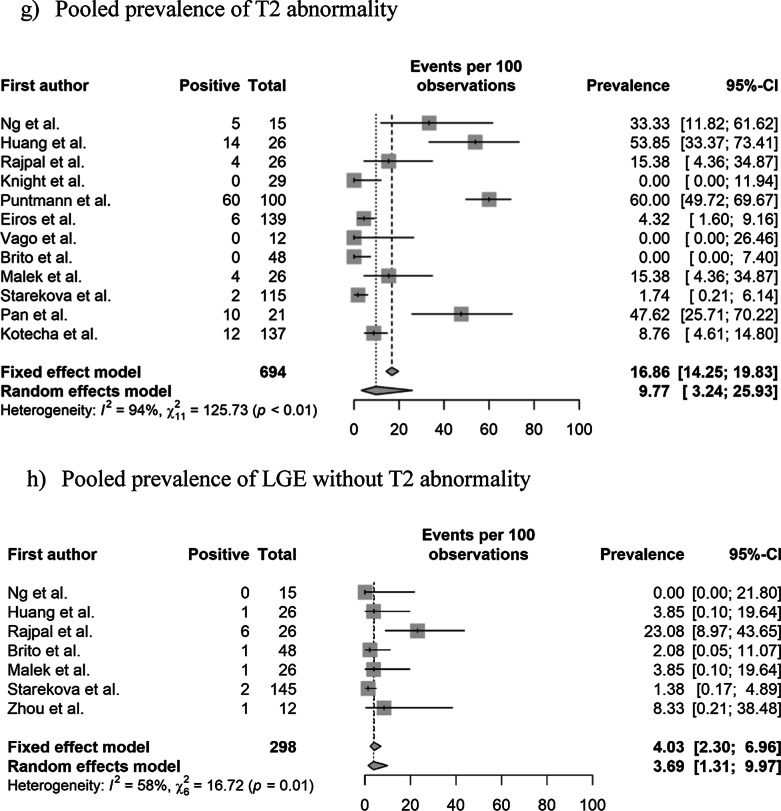
| T2 abnormality (n = 12) | 16.9 [14.3–19.8] | < 0.01, 94% | 22.9 [19.3–26.9] (n = 7) | < 0.01, 95% | 4.4 [2.4–8.0] (n = 5) | 0.08, 75% | 10.4 [7.1–15.1] (n = 5) | < 0.01, 88% | 24.8 [20.6–29.6] (n = 7) | < 0.01, 96% |
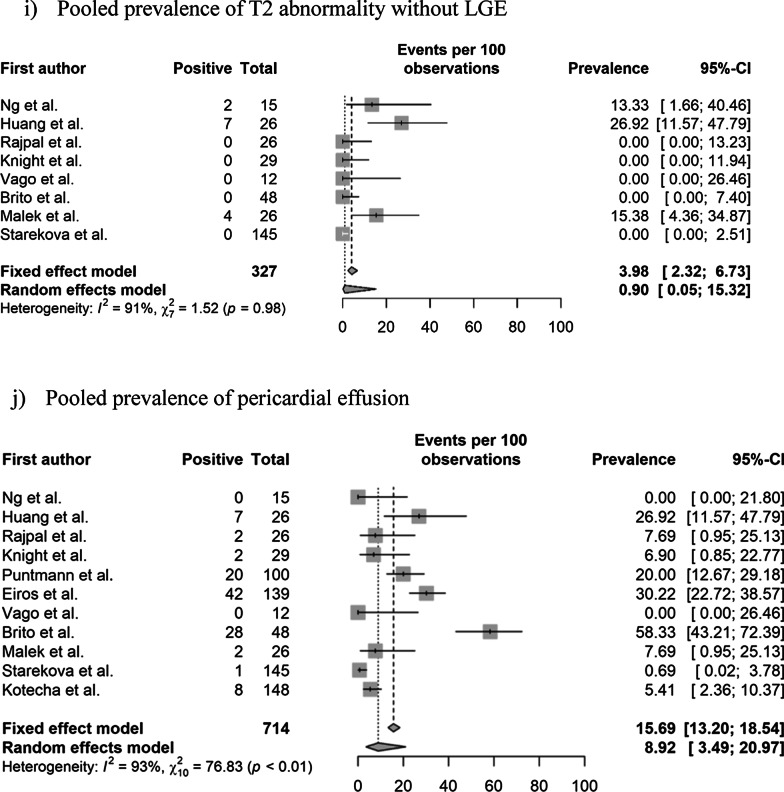
| T2 abnormality without LGE (n = 8) | 4.0 [2.3–6.7] | 0.98, 91% | 12.9 [6.8–22.9] (n = 3) | 0.061, 73% | 1.6 [0.6–4.1] (n = 5) | > 0.99, 90% | 5.7 [3.3–9.5] (n = 5) | < 0.01, 83% | 2.2 [0.5–8.2] (n = 4) | > 0.99, 76% |
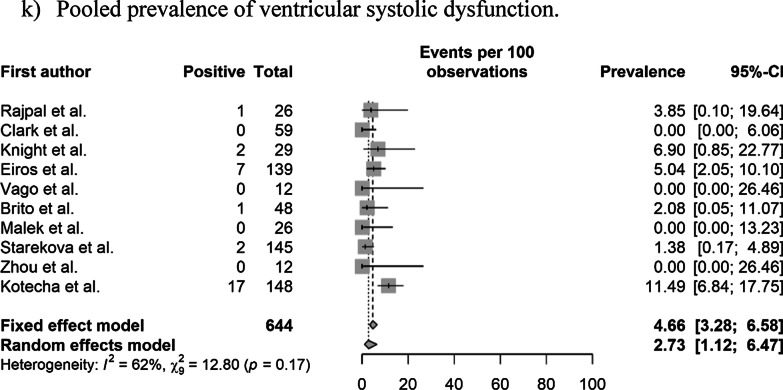
| Presence of myocardial LGE without T2 abnormality (n = 7) | 4.0 [2.3–7.0] | 0.01, 58% | 3.8 [0.2–46.6] (n = 3) | 0.85, 0% | 4.1 [2.2–7.4] (n = 4) | < 0.01, 71% | 4.4 [2.4–8.0] (n = 5) | < 0.01, 64% | 1.6 [0–100] (n = 2) | > 0.99, 0% |
| Pericardial effusion (n = 11) | 15.7 [13.2–18.5] | < 0.01, 93% | 17.3 [14.1–21.0] (n = 6) | < 0.01, 86% | 12.8 [9.3–17.5] (n = 5) | < 0.01, 89% | 5.2 [3.0–9.0] (n = 5) | < 0.01, 75% | 17.0 [13.4–21.4] (n = 6) | < 0.01, 91% |
| Ventricular systolic dysfunction on cine (n = 10) | 4.7 [3.3–6.6] | 0.17, 62% | 7.4 [2.9–17.3] (n = 4) | 0.28, 27% | 1.3 [0.4.–4.5] (n = 6) | 0.98, 0% | NA | NA | 7.4 [3.1–16.8] (n = 4) | 0.34, 22% |
Numbers in brackets represent 95% confidence intervals
CMR cardiovascular magnetic resonance, LGE late gadolinium enhancement, n number of studies
*Values indicate p-values for the Cochran Q test and I2
Fig. 2.
Pooled prevalence of abnormal CMR findings in patients who recovered from COVID-19. a Pooled prevalence of total abnormal CMR findings. b Pooled prevalence of the diagnosis of myocarditis on CMR. c Pooled prevalence of pericardial late gadolinium enhancement (LGE). d Pooled prevalence of myocardial LGE. e Pooled prevalence of LGE (pericardial or myocardial). f Pooled prevalence of native T1 abnormality on the T1 map. g Pooled prevalence of T2 abnormality. h Pooled prevalence of LGE without T2 abnormality. i Pooled prevalence of T2 abnormality without LGE. j Pooled prevalence of pericardial effusion. k Pooled prevalence of ventricular systolic dysfunction. CMR, cardiovascular magnetic resonance; COVID-19, coronavirus disease 2019; LGE, late gadolinium enhancement
The pooled prevalence of an elevated native T1 was 26.3% (95% CI 23.1%–29.8%) in 10 studies [11, 14, 16–19, 21, 22, 28, 36] and that of a T2 abnormality (increased T2 value on the T2 map or abnormal SI on T2 weighted (T2w) imaging was 16.9% (95% CI 14.3%–19.8%) in 12 studies [11–14, 16–19, 21, 22, 28, 36]. The pooled prevalence of a T2 abnormality without LGE was 4.0% (95% CI 2.3%–6.7%) in eight studies [12, 13, 16, 19, 21, 22, 28, 36], and that of LGE without a T2 abnormality was 4.0% (95% CI 2.3%–7.0%) in seven studies [12, 16, 19, 21, 22, 28, 29]. The pooled prevalence of pericardial effusion was 15.7% (95% CI 13.2%–18.5%) in 11 studies [11–14, 16, 18, 19, 21, 22, 28, 36], and that of ventricular systolic dysfunction on cine CMR was 4.7% (95% CI 3.3%–6.6%) in 10 studies [11, 13, 14, 16, 19, 21, 28, 29, 36, 37]. Significant heterogeneities among the included studies were observed for all parameters of abnormal findings (I2 > 50%).
Prevalence of abnormal CMR findings relative to patient characteristics
The pooled prevalence values of abnormal CMR findings within subgroups are summarized in Table 3.
Non-athletes vs. athletes
Of the 890 patients in 16 studies, 316 (35.5%) subjects were athletes [16, 19, 21, 28, 36, 37]. The pooled prevalence of abnormal CMR findings and a CMR diagnosis of myocarditis was higher in non-athletes than in athletes (62.5% vs. 17.1% and 23.9% vs. 2.5%, respectively). Similarly, compared with athletes, non-athletes had a higher pooled prevalence of other CMR abnormalities, including myocardial LGE (28.8% vs. 6.7%), an elevated native T1 (39.8% vs. 4.4%), a T2 abnormality (22.9% vs. 4.4%), a T2 abnormality without LGE (12.9% vs. 1.6%), pericardial effusion (17.3% vs. 12.8%), and ventricular systolic dysfunction (7.4% vs. 1.3%). In contrast, the pooled prevalence values were slightly higher in athletes than in non-athletes for pericardial LGE (6.7% vs. 4.1%) and were similar in both groups for myocardial LGE without T2 abnormality (4.1% vs. 3.8%). After subgroup analysis, the heterogeneity of studies became insignificant for abnormal CMR and ventricular dysfunction in both subgroups and the presence of myocardial LGE without T2 abnormality in the non-athlete subgroup (all, p > 0.1, I2 < 50%).
Normal cardiac enzyme level vs. undetermined cardiac enzyme level
Among the 890 patients in 16 studies, 474 (53.3%) from nine studies [11, 12, 15, 16, 19, 28, 29, 36, 37] had normal enzyme levels (e.g. troponin) and 406 (45.6%) from eight studies had undetermined cardiac enzyme levels [13, 14, 17, 18, 20–22, 36]. The undetermined cardiac enzyme level subgroup exhibited a higher pooled prevalence than the normal cardiac enzyme level subgroup for abnormal CMR findings (59.4% vs. 35.9%), the presence of pericardial (24.8% vs. 10.4%) or myocardial LGE (36.5% vs. 8.6%), an elevated native T1 value (35.7% vs. 1%), T2 abnormality (24.8% vs. 10.4%), and pericardial effusion (17% vs. 5.2%). In contrast, the pooled prevalence values were higher in the normal cardiac enzyme level subgroup than in the undetermined cardiac enzyme level subgroup for a diagnosis of myocarditis on CMR (15.2% vs. 12.0%) and the presence of myocardial LGE without T2 abnormality (4.4% vs. 1.6%). After subgroup analysis, the heterogeneity between studies became insignificant for ventricular dysfunction in the undetermined enzyme level subgroup (p = 0.34, I2 = 22%).
Meta-regression analysis results are summarized in Table 4. In the univariable meta-regression analyses, the athlete subgroup was significantly associated with heterogeneity for abnormal CMR findings, myocarditis diagnosis on CMR, myocardial LGE, and a T2 abnormality (all, p < 0.2). In contrast, undetermined cardiac enzyme level was significantly associated with heterogeneity for abnormal CMR findings and the presence of myocardial LGE (all, p < 0.2). In the multivariable meta-regression analyses, being an athlete was a significant independent factor associated with heterogeneity for abnormal CMR findings (p < 0.05). However, undetermined cardiac enzyme levels were not significantly associated with heterogeneity in multivariable meta-regression analyses.
Table 4.
Meta-regression analysis for prevalence of each CMR finding
| Parameter | Univariable meta-regression analysis | Multivariable meta-regression analysis | Residual heterogeneity after multivariable meta-regression analysis | |
|---|---|---|---|---|
| p-value | I2 | p-value | I2 | |
| Abnormal CMR findings | 92.8% | |||
| Athlete group | 0.002 | 93.4% | 0.018 | |
| Undetermined cardiac enzyme level group | 0.061 | 94.6% | 0.173 | |
| Diagnosis of myocarditis on CMR | N/A | |||
| Athlete group | < 0.001 | 90.6% | N/A | |
| Undetermined cardiac enzyme level group | 0.405 | 93.7% | N/A | |
| Presence of myocardial LGE | 93.9% | |||
| Athlete group | 0.050 | 95.4% | 0.206 | |
| Undetermined cardiac enzyme level group | 0.033 | 94.0% | 0.138 | |
| T2 abnormality | N/A | |||
| Athlete group | 0.035 | 97.2% | N/A | |
| Undetermined cardiac enzyme level group | 0.629 | 96.9% | N/A | |
| Pericardial effusion | N/A | |||
| Athlete group | 0.753 | 93.8% | N/A | |
| Undetermined cardiac enzyme level group | 0.353 | 92.1% | N/A | |
CMR cardiovascular magnetic resonance, NA not available
Quality of the studies
The quality assessments of the included studies are summarized in Additional file 1: Table S1. Most studies were classified as “high quality” (87.5% of the studies received scores of 6 or 7, and 12.5% received a score of 5).
Systematic review of the ECV, patterns of LGE, and cine findings
ECV findings
Six studies reported that ECV was significantly higher in recovered COVID-19 patients than in healthy controls [11, 12, 15, 16, 19, 37]. Huang et al. showed that ECV was significantly higher in recovered COVID-19 patients who showed abnormal CMR findings than in controls (median ECV: 28.2% vs. 23.7%, p = 0.001) [12]. Eiros et al. reported that the prevalence of elevated ECV was 37.4% (52/139) in recovered COVID-19 patients [11]. Li et al. reported that ECV was significantly elevated in patients recovered from moderate (median ECV, 29.7%) or severe COVID-19 (median ECV, 31.4%) relative to healthy controls (median ECV 25%, p < 0.001) and that the prevalence of elevated ECV was 60% (24/60) in recovered COVID-19 patients [15]. Three studies on athlete participants reported a relatively lower prevalence of abnormal ECV (Rajpal et al.: 3.8%, 1/26; Clark et al.: 4.5%, 1/22; Malek et al.: 0%, 0/26) than two studies on non-athletes (Eiros et al.: 37.4%, Li et al.: 60%) [16, 19, 37].
Patterns of LGE, the involved segments of LGE, and T2 abnormalities on CMR
A non-ischemic LGE pattern was the most frequent pattern of myocardial LGE reported in 11 studies (87.9%, 123/140, Table 2) [12–14, 16, 18–20, 22, 28, 29, 37]. Specifically, subepicardial, epicardial, and mid-wall LGE were the patterns reported in these studies. Frequently reported myocardial LGE locations in eight studies included the mid and basal inferior, septal, and lateral segments [14–17, 19, 20, 29, 37].
Two studies reported eight locations of T2 abnormalities in six patients [19, 28]. Similar to the LGE location, the mid-inferoseptum (37.5%, 3/8) and mid-anteroseptum (25%, 2/8) were the most common locations reported in the study by Rajpal et al. [19]. A study by Clark et al. on athletes reported that the T2 value was significantly higher in athletes who recovered from COVID-19 than healthy athlete controls (p = 0.02) [37].
Ventricular systolic dysfunction on cine sequence
Among the 16 included studies, six were excluded from the meta-analysis for ventricular dysfunction because the prevalence could not be extracted [12, 13, 15, 18, 20, 28]. Four studies reported that significant RV dysfunction was observed in recovered COVID-19 patients [12, 17, 19, 37]. Huang et al. reported that the RV ejection fraction (RVEF) was significantly lower in recovered COVID-19 patients with abnormal CMR findings than in healthy controls (RVEF 36.5% vs. 46.1%, p = 0.01). In contrast, the left ventricular (LV) ejection fraction (LVEF) was low in only one patient (3.9%, 1/26) with abnormal CMR findings [12]. Pan et al. reported a decrease in RVEF in two patients (9.5%), and the mean RVEF was significantly lower in recovered COVID-19 patients than in controls (p < 0.05). However, the mean LVEF was similar between recovered COVID-19 patients and controls [17].
LV or biventricular dysfunction in recovered COVID-19 patients has been evaluated in previous studies [11, 13, 18, 21]. Puntmann et al. measured and reported that the LVEF and RVEF were significantly lower in recovered COVID-19 patients than in matched controls (LVEF: 57% vs. 62%; RVEF: 54% vs. 59%) (all, p < 0.05) [18]. Malek et al. and Eiros et al. reported that the prevalence of LV systolic dysfunction in recovered COVID-19 patients was 8% and 5%, respectively.
Malek et al. reported that two athletes (8%) exhibited an enlarged LV with a slightly decreased LVEF, whereas RVEF was normal [16]. Although Eiros et al. reported LV wall motion abnormalities in seven patients (5%, 7/139), data on RV function were not provided [11]. Although ventricular systolic function was normal, abnormal strain values were reported in two studies [15, 20]. Li et al. reported that global LV longitudinal strain was significantly lower in patients who recovered from moderate or severe COVID-19 than in healthy controls (moderate COVID-19 group: − 12.5%; severe COVID-19 group: − 12.5%; healthy controls: − 15.4%; p = 0.002 and p = 0.001, respectively) [15]. Wang et al. reported that recovered COVID-19 patients with LGE had significantly lower peak global circumferential strain values in the LV and RV and lower peak global longitudinal strain values in the RV than recovered COVID-19 patients with no LGE or healthy controls (both, p < 0.05) [20]. No cine abnormalities were reported in the populations studied by Vago et al., Ng et al. and Kotecha et al. [13, 14, 36].
Publication bias
Funnel plots of the prevalence values of abnormal CMR findings, a diagnosis of myocarditis on CMR, myocardial LGE, a T2 abnormality, and pericardial effusion are presented in Additional file 1: Fig. S1. All parameters had symmetric funnel plots without significant publication bias (p > 0.05), except for T2 abnormality without LGE (p = 0.04).
Discussion
This meta-analysis revealed that nearly half of the patients exhibited one or more abnormal CMR findings after recovery from COVID-19. Athletes and patients in the normal cardiac enzyme level subgroups showed a lower prevalence of abnormal CMR findings than non-athletes and patients in the undetermined cardiac enzyme level subgroups. The most frequent abnormal CMR finding was the presence of an elevated native T1 value on the T1 map (26.3%), followed by a presence of myocardial LGE (20.7%).
Non-invasive CMR is a valuable diagnostic tool to evaluate the presence and extent of myocardial injury in COVID-19 patients [9]. A previously published systematic review reported CMR findings for 199 COVID-19 patients, including patients with myocarditis (40.2%), myopericarditis, stress-induced cardiomyopathy, and ischemia [10]. However, the studies included in this systematic review primarily conducted CMR during the active phase of COVID-19 [10]. Therefore, the data did not contribute to our understanding of whether myocardial inflammation or scarring would be observed on CMR in recovered COVID-19 patients.
Patients with myocarditis may develop arrhythmia or heart failure after recovery due to residual myocardial fibrosis or scarring [7]. LGE with T2 abnormality on CMR suggests that myocardial edema is present and the myocarditis is in the acute inflammatory phase. Consequently, the extent of LGE can diminish after recovery [38]. In contrast, LGE without a T2 abnormality after recovery from myocarditis indicates myocardial scarring or fibrosis and is associated with a poorer prognosis [9, 39]. The prevalence of LGE in myocarditis patients other than COVID-19 dropped from 72 to 48% and that of a T2 abnormality decreased from 57 to 7% at 12 months follow-up in a previous study [38].
The time interval between a diagnosis of COVID-19 and CMR varied among the studies included in this meta-analysis. Nevertheless, CMR was performed within 22 weeks of COVID-19 diagnosis, a shorter interval than that reported in previous studies on non-COVID-19 myocarditis [38]. The pooled prevalence of CMR findings of acute myocarditis in recovered COVID-19 patients diagnosed with myocarditis (14.0%), elevated native T1 (26.3%), myocardial LGE (20.7%) and T2 abnormality (16.9%) was higher than that of myocardial LGE without T2 abnormality (4.0%), which indicates permanent myocardial scarring and is associated with a poor prognosis. A mid-wall septal pattern of LGE, a poor prognostic factor in non-COVID-19 myocarditis, has been reported in several studies [14, 16, 20, 28]. These results suggest that active myocardial inflammation persists in the early phase of recovery from COVID-19. Therefore, the results of large-scale, ongoing studies (C-MORE, CISCO-19 and COVID-HEART) with long-term follow-up may address whether these findings will disappear or remain as permanent myocardial fibrosis [40–42].
Myocarditis in athletes can be critical because athletes place themselves at a higher risk of sudden cardiac death or adverse cardiac events during strenuous exercise [25]. Currently, the consensus among experts does not recommend routine CMR for evaluating whether to allow athletes who recovered from COVID-19 to return to play [43–46]. Typically, CMR is not a first-line modality for evaluating patients with suspected myocardial injuries. Instead, CMR is performed after electrocardiography, cardiac biomarker analysis, or transthoracic echocardiography to provide a more advanced and comprehensive evaluation in patients with ongoing clinical concerns [43–46]. Although the prevalence of abnormal CMR findings was lower in athletes than in non-athletes in this meta-analysis, the prevalence of LGE without T2 abnormality was similar between the two groups. Moreover, the prevalence of pericardial LGE was higher in athletes than in non-athletes. Therefore, long-term follow-up studies with larger numbers of participants (athletes) who recovered from COVID-19 are necessary to determine the significance of LGE observed on CMR.
In this meta-analysis, we observed that patients with normal cardiac enzyme levels had less frequent CMR abnormalities than patients with unknown cardiac enzyme levels (59.4% vs. 35.9%). Although our meta-analysis could not include a subgroup analysis for patients with elevated cardiac enzymes, elevated troponin levels are well-established markers of myocardial injury. High troponin levels are associated with severe disease and a poor prognosis in COVID-19 patients [47, 48]. Elevated troponin levels in recovered COVID-19 patients suggests ongoing subclinical inflammation; however, it is uncertain whether normal cardiac enzyme levels indicate an absence of myocardial scars. CMR may provide risk stratification for patients who recovered from COVID-19.
Besides myocardial abnormality, ventricular systolic dysfunction and pericardial abnormalities have also been reported in recovered COVID-19 patients. RV systolic dysfunction is the most common cine abnormality in recovered COVID-19 patients and is associated with increased pulmonary vascular resistance [49], acute respiratory distress syndrome, and poor outcomes in patients with COVID-19 [50]. Although the prevalence of functional abnormalities is low relative to those observed for other CMR parameters, studies clarifying the mechanism underlying the restoration of cardiac function in these patients are needed. This meta-analysis revealed that pericardial effusion was frequently observed in recovered COVID-19 patients, whereas pericardial LGE was relatively rare. Pericarditis, pericardial effusion, and cardiac tamponade have occasionally been reported during the active phase of COVID-19 [51, 52]; however, the underlying mechanisms remain unclear. Inadequate immune response to COVID-19 may lead to slower clearance of the virus from the peri-myocardium, development of pericarditis secondary to myocardial inflammation, or pericardial effusion caused by generalized COVID-19-related multi-systemic inflammatory syndrome [13, 18, 21]. The outcome of this evidence is unknown; however, our study findings would support further study.
Comprehensive and definitive cardiac imaging guidelines for recovered COVID-19 patients, especially the non-athlete population, are lacking. Future large-scale, long-term studies may reveal the clinical significance of abnormal CMR findings. Based on our study and future studies, appropriate surveillance guidelines for using CMR and other cardiac imaging modalities in recovered COVID-19 patients should be established.
Limitations
Our study has several limitations. First, the subgroup of patients with elevated cardiac enzyme levels could not be analyzed due to the small number of studies and patients. Second, an analysis of ventricular systolic dysfunction in the subgroup of patients with normal cardiac enzyme levels was not conducted due to the small number of patients with ventricular systolic dysfunction. Third, certain data necessary for subgroup analysis, such as the presence of cardiac symptoms or underlying cardiac disease, or abnormalities revealed on electrocardiography or echocardiography, could not be extracted. Lastly, CMR scans were performed within 22 weeks of COVID-19 recovery, and longer-term studies are needed to determine the clinical significance of these findings.
Conclusions
Nearly half of those recovering from COVID-19 exhibit one or more abnormal CMR findings. The prevalence of abnormal CMR findings was lower in athletes and patients with normal cardiac enzyme levels than in non-athletes and patients with undetermined cardiac enzyme levels. We propose that comprehensive surveillance with CMR could help stratify the risks of cardiovascular complications in recovered COVID-19 patients.
Supplementary Information
Additional file 1.Supplementary Table 1. Quality assessment of the included studies. Supplemental Appendix 1. Search terms used in PubMed, the Cochrane library and EMBASE. Supplementary Figure 1. Funnel plots to detect publication bias.
Acknowledgements
Not applicable.
Abbreviations
- CI
Confidence interval
- CMR
Cardiovascular magnetic resonance
- COVID-19
Coronavirus disease 2019
- CRP
C reactive protein
- ECG
Electrocardiogram
- ECV
Extracellular volume
- LGE
Late gadolinium enhancement
- LV
Left ventricle/left ventricular
- LVEF
Left ventricular ejection fraction
- RV
Right ventricle/right ventricular
- RVEF
Right ventricular ejection fraction
- T2w
T2-weighted
Authors' contributions
JYK analyzed and interpretated data, drafted the manuscript, and approved the final manuscript submitted. HH analyzed and interpretated data, drafted and revised the manuscript, and approved the final manuscript submitted. YJS concepted and designed or analyzed and interpretated data, drafted and revised the manuscript, and approved the final manuscript submitted. All authors read and approved the final manuscript.
Funding
This research was supported by the Bisa Research Grant of Keimyung University in 2020.
Availability of data and materials
The dataset analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jin Young Kim, Email: jinkim0411@naver.com.
Kyunghwa Han, Email: khhan@yuhs.ac.
Young Joo Suh, Email: rongzusuh@gmail.com.
References
- 1.Organization WH. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020.
- 2.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Accessed 7 Jul 2021.
- 3.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peretto G, Sala S, Rizzo S, De Luca G, Campochiaro C, Sartorelli S, et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16(5):793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgrad Med J. 2001;77(903):4–10. doi: 10.1136/pmj.77.903.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation. Exp Recommend. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072%JJournaloftheAmericanCollegeofCardiology. [DOI] [PubMed] [Google Scholar]
- 10.Ojha V, Verma M, Pandey NN, Mani A, Malhi AS, Kumar S, et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging. 2021;36(2):73–83. doi: 10.1097/rti.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 11.Eiros R, Barreiro-Perez M, Martin-Garcia A, Almeida J, Villacorta E, Perez-Pons A, et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: a cross-sectional descriptive study in health-care workers. medRxiv. 2020:2020.07.12.20151316. doi: 10.1101/2020.07.12.20151316.
- 12.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imag. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142(11):1120–1122. doi: 10.1161/circulationaha.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Wang H, Zhao R, Wang T, Zhu Y, Qian Y, et al. Elevated extracellular volume fraction and reduced global longitudinal strains in patients recovered from COVID-19 without clinical cardiac findings. Radiology. 2021;2021:203998. doi: 10.1148/radiol.2021203998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Małek ŁA, Marczak M, Miłosz-Wieczorek B, Konopka M, Braksator W, Drygas W, et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021 doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan C, Zhang Z, Luo L, Wu W, Jia T, Lu L, et al. Cardiac T1 and T2 mapping showed myocardial involvement in recovered covid-19 patients initially considered devoid of cardiac damage. J Magn Reson Imaging. 2021 doi: 10.1002/jmri.27534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, et al. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23(1):14. doi: 10.1186/s12968-021-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, et al. high prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021;14(3):541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng MY, Ferreira VM, Leung ST, Yin Lee JC, Ho-Tung Fong A, To Liu RW, et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2476–2478. doi: 10.1016/j.jcmg.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babu-Narayan SV, McCarthy KP, Ho SY, Magee AG, Kilner PJ, Sheppard MN. Myocarditis and sudden cardiac death in the young. Circulation. 2007;116(6):e122–e125. doi: 10.1161/CIRCULATIONAHA.107.693085. [DOI] [PubMed] [Google Scholar]
- 24.di Gioia CR, Giordano C, Cerbelli B, Pisano A, Perli E, De Dominicis E, et al. Nonischemic left ventricular scar and cardiac sudden death in the young. Hum Pathol. 2016;58:78–89. doi: 10.1016/j.humpath.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Eichhorn C, Bière L, Schnell F, Schmied C, Wilhelm M, Kwong RY, et al. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):494–507. doi: 10.1016/j.jcmg.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 26.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 27.van de Schoor FR, Aengevaeren VL, Hopman MTE, Oxborough DL, George KP, Thompson PD, et al. Myocardial fibrosis in athletes. Mayo Clin Proc. 2016;91(11):1617–1631. doi: 10.1016/j.mayocp.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease with cardiac magnetic resonance imaging. JAMA Cardiol. 2019 doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou M, Wong CK, Un KC, Lau YM, Lee JC, Tam FC, et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS ONE. 2021;16(2):e0246732. doi: 10.1371/journal.pone.0246732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells BS, O’ Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Berlin: Springer; 2017. [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viechtbauer W. Conducting meta-analyses in R with the metafor package. PLoS ONE. 2010;36:88. [Google Scholar]
- 35.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vago H, Szabo L, Dohy Z, Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: Initial experiences in elite athletes. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2020 doi: 10.1161/circulationaha.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White JA, Hansen R, Abdelhaleem A, Mikami Y, Peng M, Rivest S, et al. Natural history of myocardial injury and chamber remodeling in acute myocarditis. Circulation. 2019;12(7):e008614. doi: 10.1161/CIRCIMAGING.118.008614. [DOI] [PubMed] [Google Scholar]
- 39.Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74(20):2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov. U.S. National Library of Medicine. Capturing MultiORgan Effects of COVID-19 (C-MORE) https://clinicaltrials.gov/ct2/show/NCT04510025. Accessed 04 June 2021.
- 41.U.S. National Library of Medicine. Cardiac Imaging in SARS-CoV-2 (COVID-19) (CISCO-19). https://clinicaltrials.gov/ct2/show/NCT04403607. Accessed 04 June 2021.
- 42.Gorecka M, McCann GP, Berry C, Ferreira VM, Moon JC, Miller CA, et al. Demographic, multi-morbidity and genetic impact on myocardial involvement and its recovery from COVID-19: protocol design of COVID-HEART—a UK, multicentre, observational study. J Cardiovasc Magn Reson. 2021;23(1):77. doi: 10.1186/s12968-021-00752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. JACC Cardiovasc Imaging. 2020;13(12):2635–2652. doi: 10.1016/j.jcmg.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dores H, Cardim N. Return to play after COVID-19: a sport cardiologist's view. Br J Sports Med. 2020;54(19):1132–1133. doi: 10.1136/bjsports-2020-102482. [DOI] [PubMed] [Google Scholar]
- 45.McKinney J, Connelly KA, Dorian P, Fournier A, Goodman JM, Grubic N, et al. COVID-19—Myocarditis and return-to-play: reflections and recommendations from a canadian working group. Can J Cardiol. 2020 doi: 10.1016/j.cjca.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, et al. Coronavirus disease and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2019 doi: 10.1001/jamacardio.2020.5890. [DOI] [PubMed] [Google Scholar]
- 47.Sandoval Y, Januzzi JL, Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, et al. Association of troponin levels with mortality in italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5(11):1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park S, Lee SM, Lee KH, Jung KH, Bae W, Choe J, et al. Deep learning-based detection system for multiclass lesions on chest radiographs: comparison with observer readings. Eur Radiol. 2020;30(3):1359–1368. doi: 10.1007/s00330-019-06532-x. [DOI] [PubMed] [Google Scholar]
- 50.Bleakley C, Singh S, Garfield B, Morosin M, Surkova E, Mandalia MS, et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hua A, O'Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41(22):2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2(9):1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.Supplementary Table 1. Quality assessment of the included studies. Supplemental Appendix 1. Search terms used in PubMed, the Cochrane library and EMBASE. Supplementary Figure 1. Funnel plots to detect publication bias.
Data Availability Statement
The dataset analyzed during the current study are available from the corresponding author on reasonable request.