Abstract
Calvarial Doughnut Lesions with Bone Fragility (CDL) is an autosomal dominant genetic disease, characterized by low bone mineral density, multiple fractures starting in childhood, and sclerotic doughnut-shaped lesions in the cranial bones. Aubé and colleagues described in 1988 a French-Canadian family of 12 affected members who had a clinical diagnosis of doughnut lesions of the skull, with pathological fractures, osteopenia, “bone in bone” in the vertebral bodies and squaring of metatarsal and metacarpal bones. Herein we study new members of this family. Sequential genetic testing identified a nonsense variant c.148C>T, p. Arg50⁎ in SGMS2 previously reported in other families. SGMS2 encodes Sphingomyelin Synthase 2, which produces Sphingomyelin (SM), a major lipid component of the plasma membrane that plays a role in bone mineralization. The nonsense variant is associated with milder phenotype. The proband presents with bone in bone vertebral appearance that had been defined uniquely in the first cases described in the same family. The proband's son was identified to carry the same variant, which makes him the sixth generation with the diagnosis of CDL. We also report that the same pathogenic variant was identified in another previously described family, from France. These reports further confirm the genetic basis of CDL, the recurrence of the same variant (p.Arg50*) in individuals of the same ancestry, and the variable penetrance of some of the clinical findings.
Keywords: SGMS2, Calvarial doughnut lesions with bone fragility, Bone fragility, Skeletal dysplasia
1. Introduction
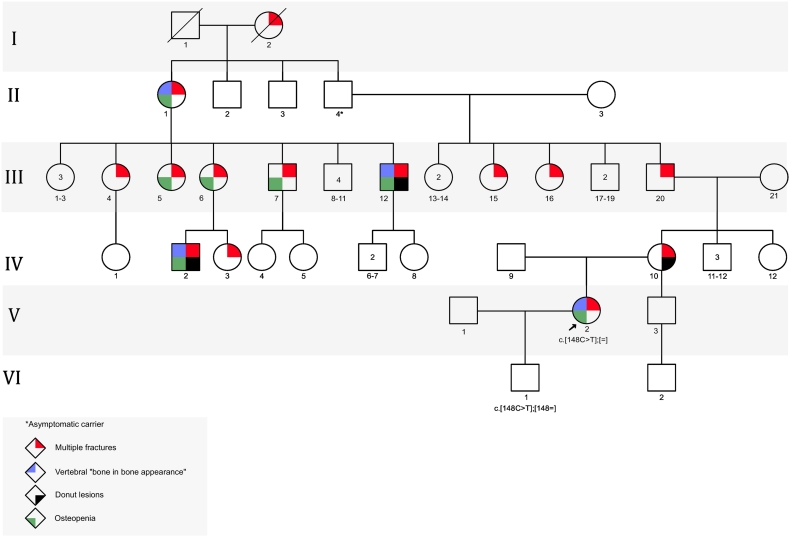
Calvarial Doughnut Lesions with Bone Fragility (CDL) (MIM 126550) is an autosomal dominant genetic disease, characterized by low bone mineral density, multiple fractures since childhood, and sclerotic doughnut-shaped lesions in the cranial bones. The Calvarial “Doughnut lesions” were first described in 1969 by Keats and Holt; two radiologist who described innocent and distinctive ring-like lesions of the calvarium in a cohort of about 24 patients (Keats and Holt, 1969). This entity has been better understood in the literature with many published case reports and case series (Bartlett and Kishore, 1976; Nishimura et al., 1996). The clinical spectrum was expanded to include: low bone density, multiple fractures, “bone in bone appearance” of vertebrae, squaring of the metatarsal and metacarpal bones, abnormal and thin bone trabeculae and other clinical and radiological findings (Bartlett and Kishore, 1976; Nishimura et al., 1996; Colavita et al., 1984). Aubé and colleagues described in 1988 a French-Canadian family of 12 members spanning 4 generations (see Fig. 1) who had a clinical diagnosis of doughnut lesions of the skull, with pathological fractures, osteopenia, “bone in bone” appearance in the vertebral bodies and squaring of metatarsal and metacarpal bones (Aube et al., 1988). This has remained a clinical diagnosis until 2019, when Pekkinen and colleagues identified SGMS2 as a causative gene for Calvarial Doughnut Lesions with Bone Fragility (Pekkinen et al., 2019). The SGMS2 encodes Sphingomyelin Synthase 2, which produces Sphingomyelin (SM) a major lipid component of the plasma membrane (Sphingomyelin synthase 2; SGMS2, 2019). Herein we describe a French-Canadian family of three generations with CDL. The clinical features demonstrate variable expressivity among the family members and collectively included; low bone mineral density, recurrent fractures, calvarial doughnut lesions, and vertebral “bone in bone” appearance. Interestingly they belong to the same family that was first described by Aubé in 1988.
Fig. 1.
Pedigree of family.
2. Case reports
2.1. The proband
The proband (V-2 in the pedigree) is the only child to a non-consanguineous French-Canadian couple. She has a half-brother from the maternal side who has a history of Bell's palsy at the age 35 years but no history of bone disease. There is a strong family history of calvarial bone lesions and bone fragility in the maternal side of the family (see pedigree-fig. 11q).
The proband was born term after uncomplicated pregnancy. The birth weight was 3400 g. The childhood past medical history is remarkable only for milk intolerance. She had normal developmental history. At the age of 8 years and 4 months she underwent a clinical evaluation by pediatric endocrinologist for a history of recurrent fractures. She sustained multiple peripheral fractures starting at the age of 3 years, all fractures happened after low trauma. The physical exam showed normal height (120.5 cm; 10th percentile) and weight (2300 g; 25th percentile), she did not have blue sclera, and she had normal dentition. The spine and limbs were unremarkable. The working diagnosis was osteogenesis imperfecta type I. Bone mineral density of the lumbar spine was measured (Z-score: −2.98). She was prescribed vitamin D supplementation and started Pamidronate treatment (Aredia) until the age of 14 years, when she was switched to Alendronate (Fosamax) for 4–5 months. She went medication free from the age of 15–20 years. Since the age of 20 years, she received multiple types osteoporosis treatment including; Teriparatide (Forteo), Denosumab (Prolia) and Risedronate (Actonel). By the age of 29 years, she has sustained a total of about 40 fractures. Her adult height is 157.5 cm (16th percentile). When she presented for genetic counselling, she was offered four sequential genetic testing panels before identifying the right molecular diagnosis.
2.2. The proband's son
The proband son (VI-1 in the pedigree) was born at 36 + 1 weeks of gestation by vaginal delivery. The birth weight was 2580 g, the length was 46.5 cm and the head circumference was 32.8 cm. The Apgar scores were 8, 9 and 9 at 1, 5 and 10 min respectively. The clinical evaluation by a geneticist at three months of age revealed no history of fractures and no features of OI; physical examination was normal. The length (57 cm; 15-50th percentile), weight (4670 g; 15th percentile) and head circumference (39 cm; 50th percentile) were normal. He had a skeletal survey and DXA scan.
2.3. The proband's mother
The mother of the proband (IV-10 in the pedigree) is known to have osteoporosis and a total of about 19–30 fractures since the age of 4 months. Her height is 151 cm (2nd percentile, z-score of −2). She has a history of unilateral ocular paralysis. She had a skull x-ray.
3. Materials and methods
The clinical information was collected from the patients' charts. Consent was obtained to publish medical information. All the genetic tests were performed after obtaining an informed consent.
3.1. Radiology
Dual-energy X-ray absorptiometry (DXA) was performed at the lumbar spine (L1-L4) for the proband and her infant, and at the femoral neck for the proband. We used Hologic QDR 4500 Densitometer Machine for the proband (Hologic Inc., Waltham, MA, USA) and GE Lunar Prodigy Densitometer Machine (General Electric, Boston, MA, USA) for the proband's infant.
3.2. Genetic testing
The following tests were done for the proband; A connective tissue disease panel of 43 genes as well as an OI and decreased bone density panel of 32 genes (Fulgent Genetics, Temple city, CA, USA) and a Dominant OI comprehensive panel of 7 genes (Connective Tissue Gene Test, Allentown, PA, USA) were performed using Next Generation Sequencing at a minimal coverage of 96% at 20×. Following these, a customized panel for CREB3L1, MBTPS2, P4HB, PLS3, SEC24D, and TENT5A (Prevention Genetics, WI, USA) was performed using next generation sequencing to cover the coding regions of the targeted genes plus 10 bases of non-coding DNA flanking each exon. The DNA was sequenced using Illumina's Reversible Dye Terminator (RDT) platform NovaSeq 6000 using 150 by 150 bp paired end reads (Illumina, San Diego, CA, USA). >98% of target bases are covered. At >20×, and mean coverage of target bases >120×. Finally, SGMS2 was sequenced as a single gene (Fulgent Genetics, Temple city, CA, USA) using Next Generation Sequencing with coverage at 100% of coding bases at >20×. The variant was identified following alignment to the human genome reference sequence (assembly GRCh37/hg19). A chromosomal microarray was performed using array comparative genomic hybridization (aCGH) (CHU Sainte-Justine) by PerkinElmer (CGX™- HD 4x180K) containing about 180,000 oligonucleotides. For testing the familial variant in the proband's son, Sanger sequencing was performed; DNA was amplified for the target region and sequenced bi-directionally using an ABI 3730XL instrument. The data was analysed against the reference gene sequence and the known variant position. The proband's mother declined genetic testing.
For the patient from France, exome sequencing was performed as follows: Exome capture was performed at the genomic platform of the IMAGINE Institute (Paris, France) with the SureSelect Human All Exon kit (Agilent Technologies). Agilent SureSelect Human All Exon (V6) libraries were prepared from 3 μg of genomic DNA sheared with ultrasonicator (Covaris). Barcoded exome libraries were pooled and sequenced using a HiSeq2500 (Illumina), generating paired-end reads. After demultiplexing, sequences were mapped on the human genome reference (NCBI build 37, hg19 version). After demultiplexing, sequences were aligned to the reference human genome hg19 using the Burrows-Wheeler Aligner. The mean depth of coverage per sample was approximately ~98× with 97,8% of the targeted exonic bases covered by at least by 15 independent reads and 97.1% covered by at least 30 independent reads. Downstream processing was carried out with the Genome Analysis Toolkit (GATK), SAMtools, and Picard, following documented best practices (http://www.broadinstitute.org/gatk/guide/topic?name=best-practices). Variants were annotated and filtered using an inhouse annotation software system (Polyweb, unpublished).
4. Results
4.1. Radiology
As a part of clinical follow up of the proband by endocrinology she had multiple bone mineral density (BMD) measured by Dual-energy X-ray absorptiometry (Table 1). The baseline BMD at the time of diagnosis at the age of 8 years and 4 showed low bone density at the lumbar spine area (L1-L4) with a Z-score of −3. Her bone mineral density scores increased significantly at the age of 13 years (Z-score of +0.27) and has remained in the normal range (Z-score of +0.01) (see Table 1).
Table 1.
Bone mineral density of the proband.
| Age of proband | Lumbar spine (L1-L4) |
Left neck of femur | |
|---|---|---|---|
| 8y4ma | |||
| Bone density | N/A | ||
| Z-score | −2.98 | ||
| 13 y | |||
| Bone density | 0.906 g/cm2 | 1.019 g/cm2 | |
| Z-score | +0.27 | N/A | |
| 14 y | |||
| Bone density | 0.937 g/cm2 | 1.065 g/cm2 | |
| Z-score | + 0.01 | + 0.98 | |
| 21 y | |||
| Bone density | 1.151 g/cm2 | 1.317 g/cm2 | |
| Z-score | +0.2 | +3 | |
Age of diagnosis and start of bisphosphonate treatment.
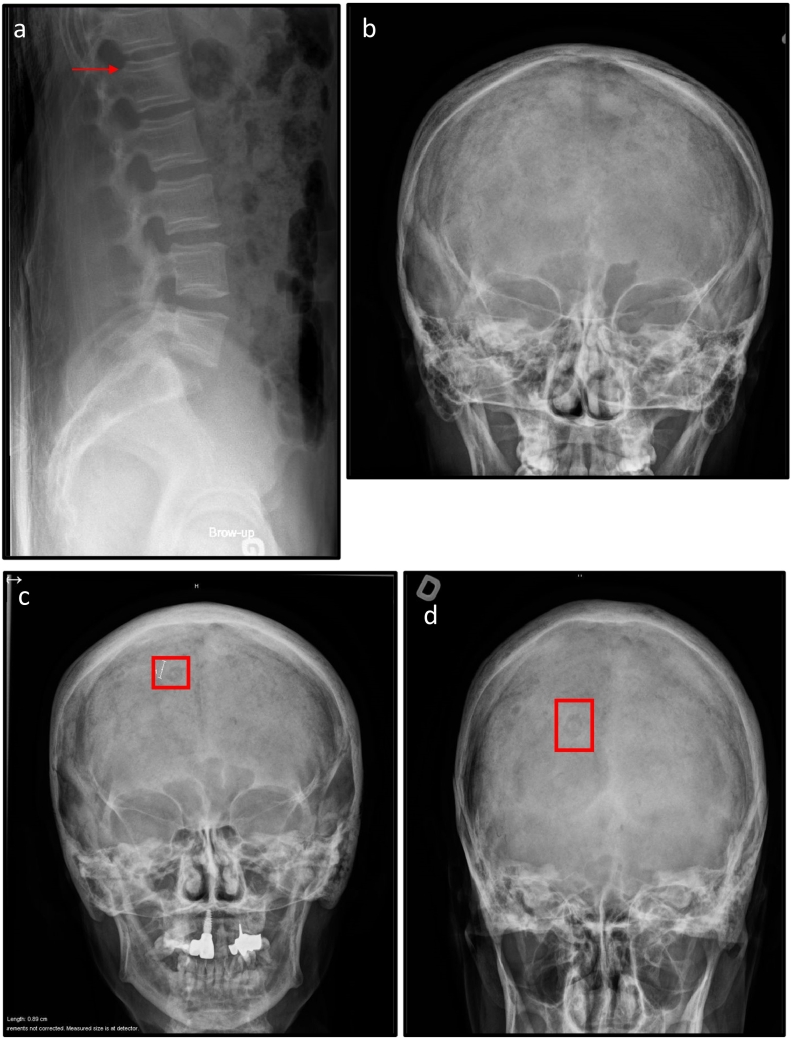
The BMD of the lumbar spine (L1-L4) of the proband's son measured at the age of 4 months showed a density of 0.194 g/cm2, which is within the normal range for age (as per Salle et al.1992)(Salle et al., 1992). X-rays of the skulls of the proband, the proband's mother and the proband's son identified doughnut lesions only in the skull x-ray of the proband's mother. X-ray of the proband's spine at the age of 30 years revealed “bone in bone” appearance of the vertebral bodies (see Fig. 1). The skeletal survey for the proband's son revealed diffuse osteopenia with linear osteocondenstaion of the tibial proximal epiphyses (data not shown).
4.2. Genetic testing
All the sequential genetic testing panels did not identify any pathogenic variants than could explain the clinical findings. Eventually after the publication implicating SGMS2 in a human disorder consistent with the diagnosis, she was offered SGMS2 (NM_001136258) gene sequencing (Pekkinen et al., 2019). The test identified the previously described recurrent heterozygous stop gain pathogenic variant, namely c.148C>T, p. Arg50⁎. This variant is predicted to introduce a stop codon with subsequent nonsense mediated decay at the level of the mRNA leading to presumptive haploinsufficiency. The chromosomal microarray showed normal female results. The proband's son had the same variant that was identified in the proband.
For the patient from France; exome sequencing identified the missense variant c.148C>T, p. Arg50⁎in SGMS2.
5. Discussion
In this report we describe a French-Canadian family with CDL due to the recurrent stop variant in SGMS2; they belong to the same family that was described by Aubé and colleagues in 1988 (see Table 2). This diagnosis spans 6 generations of the family (Aube et al., 1988). At the moment, we haven't traced the French region of origin of this family's ancestors, but this could eventually be done with the BALSAC database (Nelson et al., 2018).
Table 2.
Clinical features of the patients described by Aube et al.
| Aube et al. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
I − 1 (Aube's pedigree) (I-2) (Basalom's pedigree) |
FC | N/A | N/A | Multiple fractures | N/A | N/A | N/A | N/A | N/A |
|
II-2 (II-1) |
FC | N/A | N/A | Multiple fractures | No | Yes | Frontal hyperostosis | Osteopenia | N/A |
|
III-4 (III-4) |
FC | N/A | N/A | Multiple fractures | N/A | N/A | N/A | N/A | N/A |
|
III-6 (III-5) |
FC | N/A | N/A | Femur fracture | No | N/A | N/A | Osteopenia | N/A |
|
III-7 (III-6) |
FC | 42 years | Normal | Multiple fractures | No | N/A | N/A | Osteopenia | N/A |
|
III-9 (III-7) |
FC | N/A | N/A | Femur fracture | No | N/A | N/A | Osteopenia | N/A |
|
III-15 (III-12) |
FC | N/A | N/A | Femur fracture | No | Yes | Doughnut lesions | Osteopenia | N/A |
|
III-20 (III-16) |
FC | N/A | N/A | Multiple fractures | N/A | N/A | N/A | N/A | N/A |
|
III-24 (III-24) |
FC | N/A | N/A | Multiple fractures | N/A | N/A | N/A | N/A | N/A |
|
IV-2 (IV-2) |
FC | 18 years | Normal | 3 fractures | No | Yes | Doughnut lesions | Osteopenia | N/A |
|
IV-4 (IV-4) |
FC | N/A | N/A | Multiple fractures | N/A | N/A | N/A | N/A | N/A |
|
IV-9 (IV-9) |
FC | N/A | N/A | Multiple fractures | N/A | N/A | N/A | N/A | N/A |
FC, French Canadian: N/A, not available.
Both the proband (V-2) and her mother (IV-10) present with major bone fragility that is represented by low bone density and recurrent fractures. The proband (V-2) has a significant number of recurrent fractures that is more recurrent that the reported cases, this might be attributed to variable expressivity or to the influence of an active lifestyle. The osteopenia presents in the proband and her mother and it is a major symptom that presents in most of the reported cases(Jaakkola et al., 2009). Some of the other reported clinical features included migraines and facial nerve palsies; it is unclear if these symptoms are related to the skull findings or not.
Fortunately, the early diagnosis in the proband (V-2) with bone fragility at a young age and the start of bisphosphonate treatment significantly improved her bone density. Although vertebral compression fracture is a common feature in all previously reported cases, by the time the proband (V-2) presented to medical genetics, the spine x-ray did not show vertebral fractures, this could be due to real absence of vertebral fractures or most likely reshaping of the vertebrae after treatment. Even with the bisphosphonate treatment, the proband still developed multiple long bones fractures, our clinical experience, and the literature of bone fragility disorders, mainly osteogenesis imperfecta, favours giving the treatment. Mainly owing to its effect on bone mineral density, on reshaping of the vertebrae and outcomes of increased height. These clinical outcomes been proven by multiple studies including case controls studies with untreated patients(Palomo et al., 2015).
Interestingly, the vertebral bodies show the ‘bone in bone’ sign that was described by Aubé 32 years ago in the same French-Canadian family. The ‘bone in bone’ is a radiological sign that represents a thin vertebral body cortex and fine lines parallel to the cortex(Aube et al., 1988) (see Fig. 2-a). This feature has not been reported in any other cases in the literature including the 13 reported patients with the same nonsense variant in SGMS2(Pekkinen et al., 2019; Robinson et al., 2020), which makes it a unique feature in this French-Canadian family. The proband's son was diagnosed based on the family history and by the time of our clinical evaluation at three months of age, he did not have any of the clinical features of CDL.
Fig. 2.
a: Lateral view x-ray of the lumbar spine of the proband showing the vertebral bone in bone appearance.
b: AP skull X-ray of the proband, normal.
c: AP skull X-ray of the proband's mother showing the doughnut lesions.
d: PA skull X-ray of the proband's mother showing the doughnut lesions.
Despite the fact that the proband (V-2) had a clinical diagnosis in childhood and received the appropriate treatment and clinical follow up, it took many years and many rounds of genetic testing to identify the right gene through serial genetic panels testing, partly since whole exome sequencing is not covered by the health care system in our institution and that the responsible gene was only recently identified. Pathogenic variants in SGMS2 were identified by Pekkinen (Pekkinen et al., 2019) and colleagues in 2019 by performing whole exome sequencing in six families with rare skeletal phenotypes and osteoporosis. 10 members of four families had the same nonsense variant in SGMS2 like our French-Canadian family. Interestingly, the patients with the nonsense variant (p.Arg50*) in the Pekkinen study and our family have a milder phenotype than the other previously reported patients who have missense variants in SMGS2 (Pekkinen et al., 2019). The other reported cases in the literature with confirmed genetic diagnoses of the recurrent nonsense variant are two unrelated probands who presented childhood osteoporosis and calvarial doughnut lesions. Interestingly, one of the proband's parent had a positive genetic test in the absence of any clinical features, a phenomenon known as incomplete penetrance; this also presented in a member of our family (II-4) where the individual had no symptoms, but he transmitted the condition to his offspring.
We also report here that a patient previously reported by Couture et al., with a similar presentation of bone fragility and calvarial doughnut lesions with no reported “bone in bone“vertebral appearance and no neurological symptom, was recently found to have the same SGMS2 variant (Couture et al., 2020). (see Table 3).
Table 3.
Clinical features of all reported cases of CDL with the nonsense variant c.148C > T, p. Arg 50⁎.
| Ethnicity | Age | Height (SD) | Peripheral Fractures (n) | Vertebral fracture | Vertebral bone in bone | Skull finding | BMD Z-score (L1-L4) |
Others | |
|---|---|---|---|---|---|---|---|---|---|
| Present report | |||||||||
| Proband | FC | 29 years | -1 | ~ 40 | Not seen on x-ray | yes | None | −3 (at age 8 yrs., before treatment) | |
| Proband's Son | FC | 3 months | 0 | No | No | None | −1 | ||
| Proband's mother | FC | 63 years | −2.3 | 19 | No | N/A | Doughnut lesions | N/A | Unilateral ocular palsies |
| Couture et al. | |||||||||
| 16 years | −1.5 | >4 | Yes | N/A | Doughnut lesions | −2 (at age 16, before treatment) | |||
| Robinson et al. | |||||||||
| I-1 | FC | 22 years | 154 cm −1.4 |
1 | yes | No | N/A | −4 (at age 5, before treatment) | Migraines |
| I-2 | French, Swedish, English & FC | 12.2 years | 156 cm +0.8 |
3 | yes | No | Cobble stone appearance (at 6 years X-ray) | −3.1 (at age 8.7, before treatment) | Delayed loss of primary teeth, myopia |
| Father of I-2 | N/A | N/A | 0 | No | No | N/A | N/A | myopia | |
| Pekkinen et al. | |||||||||
| F1–1 | Finnish | 22 years | +0.4 | 6 | yes | Few sclerotic lesions | −1.4 (at age 12 yrs., before treatment) | Migraine, transient facial nerve palsy, right hand tremor, dystonia | |
| F1–2 | Finnish | 59 years | −0.1 | >9 | yes | Multiple sclerotic lesions | −1.9 (at age 48 before treatment) | Facial nerve palsies, depression | |
| F1–3 | Finnish | 85 years (deceased) | N/A | >14 | yes | Irregular diffuse thickening | N/A | Facial nerve palsies, depression, Alzheimer's disease, TIA, subdural hematoma | |
| F2–1 | Finnish | 27 years | −1.1 | 9 | yes | One sclerotic lesion | −3.4 (at age 15 yrs., before treatment) | Mild facial dysmorphia | |
| F3–1 | Caucasian (USA) |
9 years | −2.7 | 0 | yes | Normal | −5.3 (at age 5, no treatment) | Asymptomatic syringohydromyelia | |
| F3–2 | Caucasian (USA) |
12 years | −2 | 1 | yes | Few sclerotic lesions | −5.2 (at age 8 yrs) | ||
| F3–3 | Caucasian (USA) |
4 years | −3.2 | 0 | yes | Normal | −5.8 (at age 4, no treatment) | ||
| F3–4 | Caucasian (USA) |
37 years | −1.6 | 2 | yes | Normal | −3.8 (at age 34 yrs., no treatment) | ||
| F3–5 | Caucasian (USA) |
60 years | −0.2 | 15 | No | sclerotic lesions | N/A | Migraines, transient facial nerve palsies | |
| F4–1 | Northern European | 6 years | −0.8 | 6 | yes | Normal | −15.5 (at age 5 yrs., before treatment) | ||
SD, Standard deviation; FC, French Canadian; N/A, not available; TIA, transient ischemic attack; yrs., years.
Huitema and colleagues identified a novel family of integral membrane proteins that exhibit all the enzymatic activities previously known to animal sphingomyelin synthase. Humans have two different types of sphingomyelin synthase (SMS); SMS1 which is localized in the Golgi, and SMS2 also known as SGMS2 which resides in the plasma membrane (Huitema et al., 2004). SGMS2 synthesizes Sphingomyelin (SM) from ceramide and phosphatidylcholine, forming diacylglycerol as a by-product(Taniguchi and Okazaki, 2014). Sphingomyelin is a major lipid component of plasma membrane. SGMS2 is cleaved by sphingomyelin phosphodiesterase 3 (SMPD3) to generate ceramide and phosphocholine, which provides continuous supply of phosphocholine required for normal bone mineralization(Pekkinen et al., 2019). The SGMS2 protein is composed of 366-amino acid protein contains 5 membrane-spanning alpha helices connected by hydrophilic extramembrane loops in addition to 4 highly conserved motifs, designated D1 to D4 (Sphingomyelin synthase 2; SGMS2, 2019). The nonsense variant c.148C>T, p.Arg50⁎, is predicted to produce a truncated protein that lacks entire membrane-spanning core domain, including the active sites. In cell studies of HeLa cells, heterologous expression of SGMS2, p.Arg50⁎, nonsense variant produces a protein that is completely mislocalized in the cystolic and nuclear compartment (Pekkinen et al., 2019). SMPD3 homozygous knockout mice are found to have severe bone deformities, fractures and abnormal bone mineralization(Aubin et al., 2005). The knockdown of SGMS2 expression in mice osteoblast suggests that SGMS2 regulates osteoclast differentiation by inducing receptor activator of nuclear factor κB ligand (RANKL) (Yoshikawa et al., 2019). SGMS2 is expressed the highest in cortical bone, followed by vertebrae, kidney and liver in mouse tissue (Pekkinen et al., 2019). This is consistent in humans with the findings of thin and porous cortical bone which is more affected than the trabecular bone(Robinson et al., 2020).
6. Conclusion
In conclusion, we describe a French-Canadian family with the recurrent p.Arg50⁎, nonsense variant in SGMS2, the clinical diagnosis of CDL spans 6 generations and encompasses bone fragility, calvarial doughnut lesions and the unique findings of vertebral bone in bone appearance. These reports further confirm the genetic basis of CDL, the recurrence of the same variant (p.Arg50*) in individuals of the same ancestry, and the variable penetrance of some of the clinical findings. Although the genetic diagnosis is recent, the clinical diagnosis in this family makes it one of the first reported families in the literature.
Authors statement
Shuaa Basalom: writing – original draft preparation. Melissa Fiscaletti: review and editing. Valancy Miranda: review and editing. Céline Huber: review and editing. Guillaume Couture: review and editing. Régen Drouin review and editing. Élise Marie Monceau: review and editing, Sandrine Wavrant; data curation. Johanne Dubé: review and editing. Outi Mäkitie: review, editing and supervision, Valérie Cormier-Daire: review, editing and supervision and Philippe Campeau: review, editing and supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank the patients and their families for their participation. PMC is funded by awards from the CIHR (Canadian Institutes of Health Research) and the FRQS (Fonds de Recherche du Québec - Santé).
Contributor Information
Shuaa Basalom, Email: Shuaa.basalom@mail.mcgill.ca.
Mélissa Fiscaletti, Email: melissa.fiscaletti@umontreal.ca.
Valancy Miranda, Email: valancy.miranda.hsj@ssss.gouv.qc.ca.
Céline Huber, Email: celine.huber@inserm.fr.
Guillaume Couture, Email: couture.g@chu-toulouse.fr.
Régen Drouin, Email: regen.drouin.1@ulaval.ca.
Johanne Dubé, Email: johanne.dube.hsj@ssss.gouv.qc.ca.
Outi Mäkitie, Email: outi.makitie@helsinki.fi.
Valérie Cormier-Daire, Email: valerie.cormier-daire@inserm.fr.
Philippe M. Campeau, Email: p.campeau@umontreal.ca.
References
- Aube L., Vallieres M., Lemay M. Doughnut lesions of the cranial vault: an hereditary bone dysplasia. Can. Assoc. Radiol. J. 1988;39(3):204–208. [PubMed] [Google Scholar]
- Aubin I., Adams C.P., Opsahl S., et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat. Genet. 2005;37(8):803–805. doi: 10.1038/ng1603. [DOI] [PubMed] [Google Scholar]
- Bartlett J.E., Kishore P.R. Familial "doughnut" lesions of the skull. A benign, hereditary dysplasia. Radiology. 1976;119(2):385–387. doi: 10.1148/119.2.385. [DOI] [PubMed] [Google Scholar]
- Colavita N., Kozlowski K., La Vecchia G., Fileni A., Ricci R. Calvarial doughnut lesions with osteoporosis, multiple fractures, dentinogenesis imperfecta and tumorous changes in the jaws. (Report of a case) Australas. Radiol. 1984;28(3):226–231. doi: 10.1111/j.1440-1673.1984.tb02511.x. [DOI] [PubMed] [Google Scholar]
- Couture G., Degboe Y., Baujat G., Cormier-Daire V., Laroche M. Juvenile osteoporosis with calvarial doughnuts: progressive high-turnover bone loss responsive to bisphosphonate therapy. Joint Bone Spine. 2020;87(3):271–272. doi: 10.1016/j.jbspin.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Huitema K., van den Dikkenberg J., Brouwers J.F., Holthuis J.C. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23(1):33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola E., Laine C.M., Mayranpaa M.K., Falck A., Ignatius J., Makitie O. Calvarial doughnut lesions and osteoporosis: a new three-generation family and review. Am. J. Med. Genet. A. 2009;149a:2371–2377. doi: 10.1002/ajmg.a.33040. [DOI] [PubMed] [Google Scholar]
- Keats T.E., Holt J.F. The calvarial "doughnut lesion": a previously undescribed entity. Am. J. Roentgenol. Radium Therapy, Nucl. Med. 1969;105(2):314–318. doi: 10.2214/ajr.105.2.314. [DOI] [PubMed] [Google Scholar]
- Nelson D., Moreau C., de Vriendt M., et al. Inferring transmission histories of rare alleles in population-scale genealogies. Am. J. Hum. Genet. 2018;103(6):893–906. doi: 10.1016/j.ajhg.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura G., Haga N., Ikeuchi S., Yamaguchi T., Aoki K., Yamato M. Fragile bone syndrome associated with craniognathic fibro-osseous lesions and abnormal modeling of the tubular bones: report of two cases and review of the literature. Skelet. Radiol. 1996;25(8):717–722. doi: 10.1007/s002560050167. [DOI] [PubMed] [Google Scholar]
- Palomo T., Fassier F., Ouellet J., et al. Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: skeletal findings during follow up throughout the growing years. J. Bone Miner. Res. 2015;30(12):2150–2157. doi: 10.1002/jbmr.2567. [DOI] [PubMed] [Google Scholar]
- Pekkinen M., Terhal P.A., Botto L.D. Osteoporosis and skeletal dysplasia caused by pathogenic variants in SGMS2. JCI Insight. 2019;4(7) doi: 10.1172/jci.insight.126180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.E., Bardai G., Veilleux L.N., Glorieux F.H., Rauch F. Musculoskeletal phenotype in two unrelated individuals with a recurrent nonsense variant in SGMS2. Bone. 2020;134 doi: 10.1016/j.bone.2020.115261. [DOI] [PubMed] [Google Scholar]
- Salle B.L., Braillon P., Glorieux F.H., Brunet J., Cavero E., Meunier P.J. Lumbar bone mineral content measured by dual energy X-ray absorptiometry in newborns and infants. Acta Paediatr. 1992;81(12):953–958. doi: 10.1111/j.1651-2227.1992.tb12152.x. [DOI] [PubMed] [Google Scholar]
- Sphingomyelin synthase 2; SGMS2. 2019. https://www.omim.org/entry/611574.
- Taniguchi M., Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta. 2014;1841(5):692–703. doi: 10.1016/j.bbalip.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y., Yoshizawa T., Domae E., et al. Knockdown of sphingomyelin synthase 2 inhibits osteoclastogenesis by decreasing RANKL expression in mouse primary osteoblasts. Biomed. Res. 2019;40(5):189–196. doi: 10.2220/biomedres.40.189. [DOI] [PubMed] [Google Scholar]