Abstract
Background
The neoadjuvant use of immune checkpoint inhibitors (ICIs) in resectable non-small-cell lung cancer (NSCLC) is currently an area of active ongoing research. The place of neoadjuvant ICIs in the treatment guidelines needs to be determined. We carried out a systematic review of published data on neoadjuvant ICIs in resectable NSCLC to study its efficacy and safety.
Patients and methods
A literature search was carried out using the MEDLINE (PubMed) and Embase databases to retrieve articles and conference abstracts of clinical trials measuring the efficacy [major pathological response (MPR) and pathological complete response (pCR)] and safety (failure to undergo resection, surgical delay, treatment-related adverse events (trAEs) grade ≥3) of neoadjuvant immunotherapy in resectable NSCLC until July 2021.
Results
Nineteen studies with a total of 1066 patients were included in this systematic review. Neoadjuvant immunotherapy was associated with improved pathological response rates, especially in combination with chemotherapy. Using mono ICI, dual therapy–ICI, chemoradiation–ICI, radiotherapy–ICI, and chemo–ICI, the MPR rates were 0%-45%, 50%, 73%, 53%, and 27%-86%, respectively. Regarding pCR, the rates were 7%-16%, 33%-38%, 27%, 27%, and 9%-63%, respectively. Safety endpoints using monotherapy–ICI, dual therapy–ICI, chemoradiation–ICI, radiotherapy–ICI, and chemo–ICI showed a failure to undergo resection in 0%-17%, 19%-33%, 8%, 13%, and 0%-46%, respectively. The trAEs grade ≥3 rates were 0%-20%, 10%-33%, 7%, 23%, and 0%-67%, respectively.
Conclusion
In patients with resectable NSCLC stage, neoadjuvant immunotherapy can improve pathological response rates with acceptable toxicity. Further research is needed to identify patients who may benefit most from this approach, and adequately powered trials to establish clinically meaningful benefits are awaited.
Key words: neoadjuvant, immune checkpoint inhibitors, immunotherapy, non-small-cell lung cancer, lung resection
Highlights
-
•
Neoadjuvant strategies involving immunotherapy are currently being investigated in NSCLC.
-
•
Neoadjuvant immunotherapy was associated with improvement in pathological response rates.
-
•
Neoadjuvant immunotherapy can be safe and feasible with acceptable surgical delay and trAEs.
-
•
Further research is needed to identify patients benefiting most from these approaches.
Introduction
In early and locally advanced stage non-small-cell lung cancer (NSCLC), surgery is the cornerstone of curative-intent treatments, resulting in a 5-year overall survival rate varying from 92% in stage IA to 26% in stage IIIB.1 However, despite a complete resection, ∼30%-55% of patients subsequently develop disease recurrence, mainly at distant sites.2,3 The addition of neoadjuvant or adjuvant chemotherapy resulted in an absolute improvement of only 5% in the 5-year survival.4
Recently, immune checkpoint inhibitors (ICIs), a class of immune oncology drugs, have proven their efficacy in the treatment of advanced stage NSCLC. The observed response rates in stage IV NSCLC have paved the way for multiple phase I-II trials that investigated the value of ICIs in a neoadjuvant setting for resectable (stage I-IIIA) or potentially resectable (stage IIIB) lung cancer. In this context, several ICIs have been explored; either as monotherapy or in combination with other treatment modalities such as a second ICI, radiotherapy, or chemotherapy. Although results are promising thus far, significant toxicities have also been reported.5, 6, 7 Nevertheless, there are several considerations that support the use of neoadjuvant ICIs, for example, tumor-specific antigens may prime the immune system, leading to sustained antitumor T-cell immune responses and benefits in long-term tumor control.8
This systematic review aims to provide an overview of the reported data on the efficacy and safety of neoadjuvant ICIs in patients with resectable or potentially resectable NSCLCs.
Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.9 The protocol for this study is registered in PROSPERO with registration number CRD42021235759.
Search strategy
A systematic review of the literature was carried out by searching MEDLINE (PubMed) and Embase libraries. Published data of completed trials assessing the efficacy and safety of neoadjuvant immunotherapy in resectable NSCLCs were identified. In addition, conference abstracts with preliminary results of clinical trials were explored. Search terms included ‘non-small cell lung cancer’, ‘neoadjuvant’, and ‘immunotherapy’ for which Medical Subject Headings (MeSH Terms), Emtree terms, and/or free text words were identified. A detailed overview of the search strategy is provided in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2021.100244. Ultimately, the final search was conducted on 16 July 2021.
Study inclusion and exclusion criteria
Inclusion criteria consisted of clinical trials including patients (i) with pathologically diagnosed NSCLC presenting in a potentially resectable, nonmetastatic stage (I-IIIB); (ii) receiving planned ICIs in a neoadjuvant setting prior to surgical resection, and (iii) with data available on efficacy and safety. Exclusion criteria comprised studies addressing patients (i) with pathologically diagnosed NSCLC in an unresectable and metastatic stage, (ii) receiving immunotherapies other than ICIs, and (iii) with absence of reported data on the efficacy and safety of ICI treatment. The following types of reports were excluded: (i) articles reporting on duplicate results, (ii) study protocols, (iii) (systematic) reviews, (iv) editorials, (v) case reports, (vi) comments, (vii) articles in languages other than English, (viii) retrospective studies, (ix) studies missing data on >2 of 5 required endpoints, (x) case series with n < 5 receiving immunotherapy, (xi) studies providing multiple ICI regimens within a single study arm, and (xii) meta-analyses.
Primary outcome and definitions
The primary outcome was efficacy and safety of neoadjuvant immunotherapy in resectable NSCLCs. The efficacy was defined by either major pathological response (MPR) or pathological complete response (pCR). Radiological responses assessed by RECIST version 1.1 were not evaluated. Safety was defined as (i) rate of failure to undergo resection, (ii) surgical delay (as defined by the authors of the included studies), and (iii) the incidence of treatment-related adverse events (trAEs) grade ≥3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events.
Results
Study screening and selection
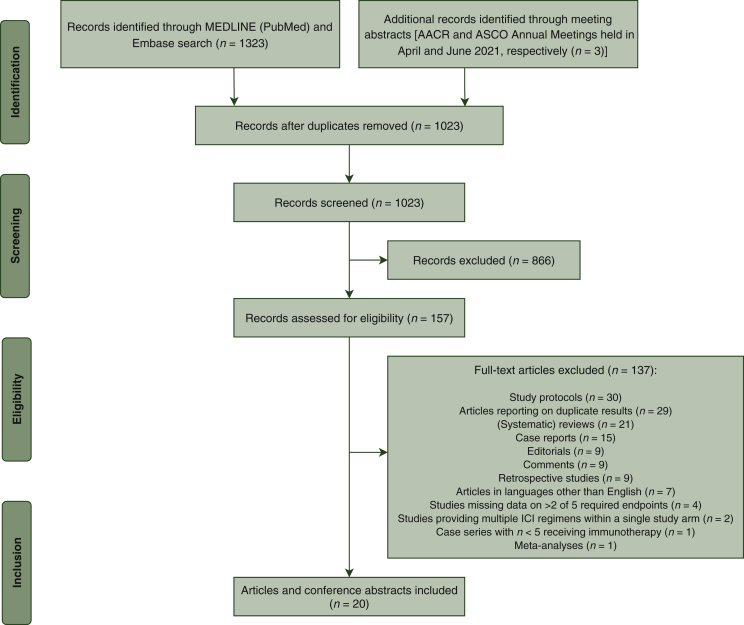
The literature search identified a total of 1323 records, with three additional records identified through searching the meeting abstracts of the American Association for Cancer Research (AACR) and American Society of Clinical Oncology (ASCO) Annual Meetings held in April and June 2021, respectively.10, 11, 12 A total of 303 records were excluded by deduplication, resulting in a final number of 1023 records. All records were independently screened by two authors (EBU and CD) to identify eligible studies based on the predefined inclusion and exclusion criteria. Differences in interpretation between the two reviewers were discussed to reach consensus and make a final decision on eligibility. From the 1023 records, 866 were excluded based on title and abstract and another 137 due to ineligibility, leaving 20 articles with a total of 1066 patients for inclusion. These 20 articles represent 15 single-arm cohort studies11,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 and four randomized studies with two arms.10,12,27, 28, 29 The PRISMA flowchart depicting study selection is presented in Figure 1. An overview of the study characteristics is presented in Table 1. An outline of the excluded clinical studies is provided in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100244.
Figure 1.
PRISMA flowchart.
AACR, American Association for Cancer Research; ASCO, American Society of Clinical Oncology; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Table 1.
Study characteristics
| Study | Phase | Study design | N | Stage I/II/III%a | ICI | Dose | Primary endpoints |
|---|---|---|---|---|---|---|---|
| Monotherapy–ICI | |||||||
| Tong et al.25 | II | Single-arm cohort study | 30 | 30/43/27 | Pembrolizumab | Two cycles 200 mg IV Q3W | Safety, feasibility |
| Eichhorn et al.14 | II | Single-arm cohort study | 15 | 0/40/60 | Pembrolizumab | Two cycles 200 mg IV Q3W | Safety, feasibility |
| Lee et al.18 | II | Single-arm cohort study | 181 | 9/41/49 | Atezolizumab | Two cycles 1200 mg IV Q3W | MPR |
| Gao et al.16 | Ib | Single-arm cohort study | 40 | 20/35/45 | Sintilimab | Two cycles 200 mg IV Q3W | Safety |
| Besse et al.13 | II | Single-arm cohort study | 30 | 50/20/30 | Atezolizumab | One cycle 1200 mg IV Q3-4W | % of patients without major toxicities or morbidities |
| Forde et al.15 | II | Single-arm cohort study | 22 | 19/48/33 | Nivolumab | Two cycles 3 mg/kg IV Q2W | Safety, feasibility |
| Dual–ICI | |||||||
| Cascone et al.27 | II | Randomized two-arm study | 23 | 48/30/22 | Nivolumab | Three cycles 3 mg/kg IV Q2W | MPR |
| 21 | 57/24/19 | Nivolumab Ipilimumab | Three cycles 3 mg/kg IV Q2W One cycle 1 mg/kg IV Q6W |
||||
| Reuss et al.20 | II | Single-arm cohort study | 9b | 11/22/67 | Nivolumab | Three cycles 3 mg/kg IV Q2W | Safety |
| Ipilimumab | One cycle 1 mg/kg IV Q6W | ||||||
| Chemotherapy with ICI | |||||||
| Rothschild et al.21 | II | Single-arm cohort study | 67 | 0/0/100 | Durvalumab | Two cycles 750 mg IV Q2W | EFS at 12 months |
| Zhao et al.11 | II | Single-arm cohort study | 33 | 0/0/100 | Toripalimab | Three cycles 240 mg Q3W | MPR |
| Forde et al.10 and Spicer et al.12 | III | Randomized two-arm study | 179 | 23/14/63 | Nivolumab | Three cycles 360 mg IV Q3W | pCR, EFS |
| 179 | 22/13/64 | Chemo alone arm | |||||
| Shen et al.22 | — | Single-arm cohort study | 37 | 0/8/92 | Pembrolizumab | Two cycles 2 mg/kg IV Q3W | pCR |
| Lei et al.29 | II | Randomized two-arm study | 7 | 0/0/100 | Camrelizumab | Three cycles 200 mg IV Q3W | pCR |
| 7 | 0/0/100 | Chemo alone arm | |||||
| Shu et al.23 | II | Single-arm cohort study | 30 | 0/23/77 | Atezolizumab | Four cycles 1200 mg IV Q3W | MPR |
| Provencio et al.19 | II | Single-arm cohort study | 46 | 0/0/100 | Nivolumab | Three cycles 360 mg IV Q3W | PFS at 24 months |
| Tfayli et al.24 | II | Single-arm cohort study | 15c | 13/33/54 | Avelumab | Four cycles 10 mg/kg IV Q2W | ORR by RECIST version 1.1 |
| Yang et al.26 | II | Single-arm cohort study | 24 | 0/21/79 | Ipilimumab | Two cycles 10 mg/kg IV | Safety, feasibility |
| Chemoradiotherapy with ICI | |||||||
| Hong et al.17 | I/II | Single-arm cohort study | 11 | 0/0/100 | Durvalumab | Two cycles 1500 mg IV Q4W | pCR |
| Radiotherapy with ICI | |||||||
| Altorki et al.28 | II | Randomized two-arm study | 30 | 37/16/47 | Durvalumab | Two cycles 1120 mg IV Q3W | MPR |
| 30 | 26/33/40 | Durvalumab + SBRT | |||||
EFS, event-free survival; ICI, immune checkpoint inhibitor; IV, intravenous; MPR, major pathological response; N, number of patients included; ORR, objective response rate; pCR, pathological complete response; PFS, progression-free survival; RECIST, response evaluation criteria in solid tumors; SBRT, stereotactic body radiotherapy.
Either the 7th or 8th edition of TNM staging was assessed in the included studies to determine the stages of disease.
Study was terminated by investigator consensus due to toxicities.
Study was terminated due to failure to achieve required response rate.
Efficacy
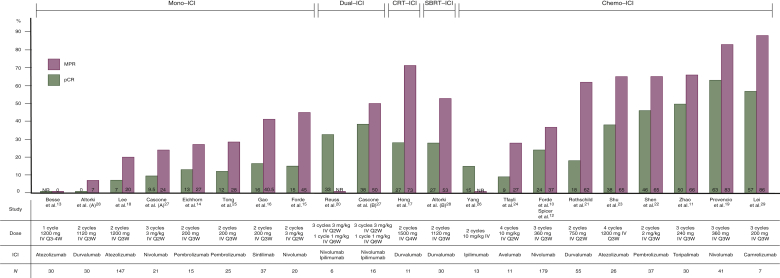
The efficacy outcome measurements are presented in Table 2. The results are graphically summarized in Figure 2.
Table 2.
Efficacy outcome measurements
| Study | Study arm | MPR | pCR |
|---|---|---|---|
| Monotherapy–ICI | |||
| Range over all studies | 0%-45% | 7%-16% | |
| Tong et al.25, n (%) | 7/25 (28) | 3/25 (12) | |
| Eichhorn et al.14, n (%) | 4/15 (27) | 2/15 (13) | |
| Lee et al.18, n (%) | 30/147 (20)a | 10/147 (7)a | |
| Gao et al.16, n (%) | 15/37 (40.5) | 6/37 (16) | |
| Besse et al.13, n (%) | 0/30 (0) | NR | |
| Forde et al.15, n (%) | 9/20 (45) | 3/20 (15) | |
| Dual–ICI | |||
| Cascone et al.27, n (%) | Nivo Ipi/nivo |
5/21 (24) 8/16 (50) |
2/21 (9.5) 6/16 (38) |
| Reuss et al.20, n (%) | NR | 2/6 (33) | |
| Chemotherapy with ICI | |||
| Range over all studies | 27%-86% | 9%-63% | |
| Rothschild et al.21, n (%) | 34/55 (62) | 10/55 (18) | |
| Zhao et al.11, n (%) | 20/30 (66) | 15/30 (50) | |
| Forde et al.10 and Spicer et al.12, n (%) | Nivo + chemo Chemo alone |
66/179 (37) 4/179 (2) |
43/179 (24) 16/179 (9) |
| Shen et al.22, n (%) | 24/37 (65) | 17/37 (46) | |
| Lei et al.29, n (%) | Cam + chemo | 6/7 (86) | 4/7 (57) |
| Chemo alone | 2/6 (33) | 1/6 (17) | |
| Shu et al.23, n (%) | 17/26 (65) | 10/26 (38) | |
| Provencio et al.19, n (%) | 34/41 (83) | 26/41 (63) | |
| Tfayli et al.24, n (%) | 3/11 (27) | 1/11 (9) | |
| Yang et al.26, n (%) | NR | 2/13 (15) | |
| Chemoradiotherapy with ICI | |||
| Hong et al.17, n (%) | 8/11 (73) | 3/11 (27) | |
| Radiotherapy with ICI | |||
| Altorki et al.28, n (%) | Durva Durva + SBRT |
2/30 (7) 16/30 (53) |
0/30 (0) 8/30 (27) |
Cam, camrelizumab; Chemo, chemotherapy; Durva, durvalumab; ICI, immune checkpoint inhibitor; Ipi/nivo, ipilimumab/nivolumab; MPR, major pathological response; Nivo, nivolumab; NR, not reported; pCR, pathological complete response; SBRT, stereotactic body radiotherapy.
Patients with oncogenic driver mutations were excluded from pathological evaluations.
Figure 2.
Graphical abstract showing the efficacy outcome measurements (MPR, pCR).
Altorki et al. (A) = arm with patients receiving durvalumab monotherapy; Altorki et al. (B) = arm with patients receiving durvalumab + SBRT; Cascone et al. (A) = arm with patients receiving nivolumab monotherapy; Cascone et al. (B) = arm with patients receiving nivolumab + ipilimumab.
Studies were ranked according to the MPR rate. Abbreviations: CRT, chemoradiotherapy; chemo, chemotherapy; ICI, immune checkpoint inhibitor; mono, monotherapy; IV, intravenous; MPR, major pathological response; N, number of resected tumors; NR, not reported; pCR, pathological complete response; SBRT, stereotactic body radiotherapy.
Major pathological response (MPR)
MPR, defined as ≤10% viable tumor tissue on pathological examination,30 was reported in 17 of 19 studies included. Two studies did not report on MPR.20,26 In the monotherapy–ICI group, MPR rates up to 45% were reported,15 and one study reported absence of MPR in the included patients.13 The addition of ipilimumab to nivolumab led to a 50% MPR rate in one study.27 Regarding the combination of chemoradiotherapy with durvalumab, MPR rate was reported to be 73%.17 The chemotherapy–ICI group shows higher MPR rates when compared with the monotherapy–ICI group, with rates varying from 27%24 to 86%.29 Radiotherapy combined with durvalumab led to MPR in 53% of patients.28
Pathological complete response (pCR)
pCR, which is defined as the absence of residual viable tumor cells after induction therapy, was reported in all studies except one.13 The monotherapy–ICI group shows consistent rates of pCR ranging from 0%28 to 16%,16 with one study reporting a pCR rate for half the patients achieving MPR.14 The addition of ipilimumab to nivolumab led to an absolute increase of pCR, with reported rates of 33% and 38%.20,27 Combining chemoradiotherapy with durvalumab led to pCR rate of 27%.17 The chemotherapy–ICI group reported rates varying from 9%24 to 63%,19 with most studies reporting pCR in more than half of the patients achieving MPR.10,11,19,22,23,29 When neoadjuvant durvalumab was combined with radiotherapy, 27% of the included patients achieved pCR.28
Safety
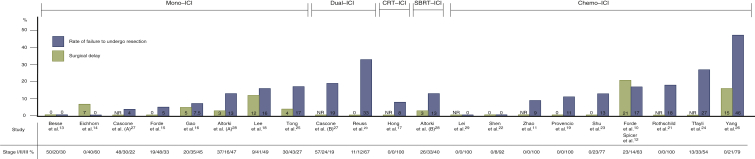
The safety outcome measurements are presented in Table 3. The results are graphically summarized in Figure 3.
Table 3.
Safety outcome measurements
| Study | Study arm | Failure to undergo resection | Surgical delay | trAEs grade ≥3 |
|---|---|---|---|---|
| Monotherapy–ICI | ||||
| Range over all studies | 0%-17% | 0%-12% | 0%-20% | |
| Tong et al.25, n (%) | 5/30 (17) | 1/25 (4) | 1/30 (3) | |
| Eichhorn et al.14, n (%) | 0/15 (0) | 1/15 (7)a | 3/15 (20) | |
| Lee et al.18, n (%) | 29/181 (16) | 19/159 (12)b | 9/181 (5)f | |
| Gao et al.16, n (%) | 3/40 (7.5) | 2/37 (5) | 4/40 (10) | |
| Besse et al.13, n (%) | 0/30 (0) | 0/30 (0)c | 0/30 (0) | |
| Forde et al.15, n (%) | 1/21 (5) | 0/20 (0) | 1/22 (4.5) | |
| Dual–ICI | ||||
| Cascone et al.27, n (%) | Nivo | 1/23 (4) | 8/37 (22)d | 3/23 (13) |
| Ipi/nivo | 4/21 (19) | 2/21 (10) | ||
| Reuss et al.20, n (%) | 3/9 (33) | 0/6 (0) | 3/9 (33) | |
| Chemotherapy with ICI | ||||
| Range over all studies | 0%-46% | 0%-21% | 0%-67% | |
| Rothschild et al.21, n (%) | 12/67 (18) | NR | 45/67 (67)j 8/62 (13)k |
|
| Zhao et al.11, n (%) | 3/33 (9) | NR | 3/33 (9) | |
| Forde et al.10 and Spicer et al.12, n (%) | Nivo + chemo | 30/179 (17) | 31/149 (21) | 60/179 (33.5) |
| Chemo alone | 44/179 (25) | 24/135 (18) | 66/179 (37) | |
| Shen et al.22, n (%) | 0/37 (0) | 0/37 (0) | 4/37 (11) | |
| Lei et al.29, n (%) | Cam + chemo | 0/7 (0) | NR | NR |
| Chemo alone | 0/6 (0)g | NR | NR | |
| Shu et al.23, n (%) | 4/30 (13) | 0/26 (0) | 15/30 (50) | |
| Provencio et al.19, n (%) | 5/46 (11) | 0/41 (0) | 14/46 (30) | |
| Tfayli et al.24, n (%) | 4/15 (27) | NR | 4/15 (27) | |
| Yang et al.26, n (%) | 11/24 (46) | 2/13 (15)e | 11/24 (46) | |
| Chemoradiotherapy with ICI | ||||
| Hong et al.17, n (%) | 1/12 (8)g | NR | 1/14 (7) | |
| Radiotherapy with ICI | ||||
| Altorki et al.28, n (%) | Durva Durva + SBRT |
4/30 (13) 4/30 (13) |
1/30 (3)h 1/30 (3)i |
6/30 (20) 7/30 (23) |
Cam, camrelizumab; Chemo, chemotherapy; Durva, durvalumab; ICI, immune checkpoint inhibitor; Ipi/nivo, ipilimumab/nivolumab; Nivo, nivolumab; NR, not reported; SBRT, stereotactic body radiotherapy; trAEs, treatment-related adverse events.
Incidence of surgical delay of 13 days.
Incidence of surgical delay outside the ±10-day window.
Incidence of surgical delay >15 days.
Incidence of surgical delay > 42 days; no distinction between the two arms was made.
Incidence of surgical delay ≥28 days.
Postoperative trAEs grade ≥3 excluded.
Patients awaiting surgery are excluded.
Incidence of surgical delay of 4 weeks.
Incidence of surgical delay of 7 weeks.
trAEs grade ≥3 during neoadjuvant chemotherapy.
trAEs grade ≥3 during neoadjuvant immunotherapy.
Figure 3.
Graphical abstract showing safety outcome measurements (rate of failure to undergo resection, surgical delay).
Altorki et al. (A) = arm with patients receiving durvalumab monotherapy; Altorki et al. (B) = arm with patients receiving durvalumab + SBRT; Cascone et al. (A) = arm with patients receiving nivolumab monotherapy; Cascone et al. (B) = arm with patients receiving nivolumab + ipilimumab.
Studies were ranked according to the rate of failure to undergo resection. Abbreviations: chemo, chemotherapy; CRT, chemoradiotherapy; ICI, immune checkpoint inhibitor; mono, monotherapy; NR, not reported; SBRT, stereotactic body radiotherapy.
Failure to undergo resection
The rates of failure to undergo resection varied from 0%13,14,22,29 to 46%.26 There was no difference in these rates when considering the different groups (monotherapy–ICI, dual–ICI, chemoradiotherapy–ICI, radiotherapy–ICI, chemotherapy–ICI). There were various reasons reported for not proceeding to a resection: disease progression,10,11,18, 19, 20, 21,23, 24, 25, 26, 27, 28 inadequate lung function,24,27 persistent N2 disease,26 unresectable disease,21,23,25 trAEs,10,27 location of tumor,15,26 or patient refusal.11 One study did not report on the specific reason for a patient not to proceed to the planned surgery.16
Surgical delay
Surgical delays were reported using author-specified definitions varying from surgical delay outside the ±10-day window to a surgical delay of 7 weeks. The rates of patients experiencing surgical delay ranged from 0%13 to 22%,27 with an outlier study reporting a pooled incidence for the two arms. No surgical delay was reported in 6 of the 19 included studies.13,15,19,20,22,23 Reported surgical delays were most often attributed to trAEs.14,16,25,26,28 Three studies did not disclose any details on the reasons leading to the reported surgical delays,13,18,27 and another five did not report any data on surgical delay.11,17,21,24,29
Treatment-related adverse events (trAEs) grade ≥3
The monotherapy–ICI group reported rates of trAEs grade ≥3 up to 20%,14 with one study reporting no trAEs grade ≥3 at all.13 Most of these trAEs were pulmonary related (i.e. pneumonitis or pneumonia).15,16,18,27 In the chemotherapy–ICI group, reported rates ranged from 0%29 to 67%.21 Here, the reported trAEs were attributed to hematological disorders,19,22, 23, 24 ipilimumab-related diarrhea,26 and chemotherapy-related toxicities.26 The combination of chemoradiation with durvalumab led to one grade 3 trAE due to neutropenia.17 The dual–ICI group reported trAEs grade ≥3 rates of 10%27 and 33%,20 with study closure of the latter trial due to the observed toxicity rate. Regarding the combination of durvalumab with radiotherapy, the most frequent reported trAEs included grade 3 hyperlipasaemia.28 One study did not report on adverse events related to the neoadjuvant regimen.29
Discussion
This systematic review on neoadjuvant use of ICIs in resectable NSCLC summarizes the efficacy and safety outcomes of the currently published trials. The results suggest that this approach is safe and feasible, with acceptable surgical delays and grade ≥3 trAEs.
MPR, defined as the presence of ≤10% residual vital tumor cells in the resection specimen, serves as a major primary endpoint in many neoadjuvant immunotherapy studies. MPR is proposed as a surrogate endpoint for survival, as it has been associated with improved survival,31 and could therefore provide a faster means of comparing different neoadjuvant treatment regimens, and shorten the period needed to evaluate neoadjuvant therapies.32 A major limitation of using MPR is the lack of precision due to the inherent interobserver variability. Weissferdt et al.32 reported on a prospective study in 151 NSCLC patients treated with neoadjuvant chemotherapy, where two trained pathologists separately scored the residual tumor cells percentage in the resected specimens. The authors reported that the MPR assessed by both pathologists was associated with long-term overall survival in patients with NSCLC undergoing neoadjuvant chemotherapy (hazard ratio 2.68; P = 0.01). The levels of agreement between the two pathologists were high (R2 = 0.994), and it was proposed that at least three slides should be read to accurately determine MPR.
In the published trials, MPR rates ranged from between 0% and 45% of patients in the monotherapy–ICI cohort, 50% for dual–ICI cohort, 73% for the chemoradiation–ICI cohort, 53% for the radiotherapy–ICI cohort, and 27%-86% in the chemotherapy–ICI cohort. These MPR rates are higher as compared with those after preoperative chemotherapy alone, which was reported to be ∼16%.33 The synergistic effect of chemotherapy and ICIs, with the cytotoxic chemotherapy functioning as a sensitizer for immune checkpoint blockade, might explain the high rates of MPR in this group.34 Sixteen of 19 included studies reported on pCR, with rates ranging between 12% and 63%, which are higher than the 10.5% reported after neoadjuvant chemotherapy.35
Some aspects of treatment efficacy merit further discussion. First, although high rates of pathological responses by MPR and pCR were reported in most included studies, it is still unclear as to which stages of NSCLC benefit the most from neoadjuvant therapy. A stage-based assessment of pathological responses is important as it may allow for improved design of future trials in specific disease stages.30 Second, responses to neoadjuvant ICIs can be influenced by the tumor molecular contexture, as several genomic alterations such as STK11 mutations have been associated with resistance to anti-PD-(L)1 agents.36 Such patient subgroups may be less likely to respond to ICIs, while suffering the risks of immune-related AEs. By contrast, biomarkers which have been associated with responses to ICIs include PD-L1 expression in stromal cells,16 CD28 expression on CD8+ tumor-infiltrating lymphocytes (TILs),37 and stromal TILs.38 This highlights the need to identify appropriate predictive biomarkers for selecting patients upfront. Third, the lack of a standardized approach for pathological reporting of resected lung carcinomas following neoadjuvant treatment may reflect a lack of sufficient input from pathologists in study design.39 The ‘IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy’ were published in 2020, and can serve to standardize reporting on the pathological outcomes.30
Rates of failure to undergo resection were generally acceptable, although higher rates of unresected patients were reported in studies that included more patients with stage III NSCLC, and disease progression was the main reason accounting for not undergoing a resection. However, it is questionable if the decision to not proceed with surgery in patients who developed distant metastases during induction therapy can be labeled therapy failure as it is likely that these patients may not have benefited from a resection in the first place.
The surgical delays were within acceptable ranges in all studies, with 6 of 19 studies reporting no surgical delays at all. The grade ≥3 treatment-related AEs were similar to those reported previously for chemo–ICI regimens.40,41 However, the reported trAE rates in most included studies were lower compared with preoperative chemotherapy.42
Limitations of the current systematic review include the substantial heterogeneity in the included trials, some of which included only a few patients. In addition, most were single-arm cohort studies, and no randomized controlled trials were included. Consequently, there is a risk of biases which limit the ability to draw reliable conclusions. Moreover, this systematic review included studies reporting only preliminary results of clinical trials, and a publication bias cannot be ruled. Our conclusions may therefore require revision once the final analyses of study data are presented. Another limitation is the fact that this systematic review assessed only two indicators for the efficacy outcome measurements, and three indicators for the safety outcome measurements. We did not report on the radiological response rates using RECIST version 1.1, as radiological assessments after neoadjuvant therapies have a poor correlation with the pathological response and survival.43
Currently, numerous phase II/III clinical trials are underway to further investigate the efficacy of neoadjuvant immunotherapy in resectable NSCLC, and to compare these approaches with placebo or standard treatments. CheckMate 816 was the first phase III randomized clinical trial that reported on pathological response and toxicity of neoadjuvant nivolumab/chemotherapy versus chemotherapy alone. This trial included 358 resectable stage IB-IIIA NSCLC patients, and confirmed the findings from previous phase II trials, showing a 21.6% increase in pCR (by blinded independent central review) in the nivolumab arm and no differences in safety outcomes.10 Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100244 provides an overview of the major ongoing trials.
In conclusion, the currently published data suggest that neoadjuvant ICIs can be considered safe and feasible, with encouraging pathological responses. Further research is needed to identify patients who may benefit most from this approach, and adequately powered trials to establish clinically meaningful benefits are awaited.
Acknowledgements
We thank Ralph de Vries from the Medical Library, Vrije Universiteit Amsterdam, for his assistance with searching the medical databases.
Funding
None declared.
Disclosure
SS reports grants and personal fees from AstraZeneca; personal fees from MSD, BeiGene, and Celgene; grants and nonfinancial support from Varian Medical Systems; and grants from ViewRay Inc. IB reports grants from BMS and AstraZeneca. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Goldstraw P., Chansky K., Crowley J. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Carnio S., Novello S., Papotti M., Loiacono M., Scagliotti G.V. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: tumor based approaches including gene signatures. Transl Lung Cancer Res. 2013;2(5):372–381. doi: 10.3978/j.issn.2218-6751.2013.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uramoto H., Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NSCLC Meta-analysis Collaborative Group Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–1571. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies A.M., Amaria R.N., Rozeman E.A. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC) Nat Med. 2021;27(2):301–309. doi: 10.1038/s41591-020-01188-3. [DOI] [PubMed] [Google Scholar]
- 6.Amaria R.N., Reddy S.M., Tawbi H.A. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benitez J.C., Remon J., Besse B. Current panorama and challenges for neoadjuvant cancer immunotherapy. Clin Cancer Res. 2020;26:5068–5077. doi: 10.1158/1078-0432.CCR-19-3255. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forde P.M., Spicer J., Lu S. CT003 − Nivolumab (NIVO) + Platinum-Doublet Chemotherapy (Chemo) vs Chemo as Neoadjuvant Treatment (tx) for Resectable (IB-IIIA) Non-Small Cell Lung Cancer (NSCLC) in the Phase 3 CheckMate 816 Trial. 2021. https://www.abstractsonline.com/pp8/#!/9325/presentation/5134 Available at.
- 11.Zhao Z, Chen S, Qi H, et al. Phase II trial of toripalimab plus chemotherapy as neoadjuvant treatment in resectable stage III non-small cell lung cancer (NeoTPD01 Study) [Conference presentation]. ASCO Annual Meeting 4-8 June 2021.
- 12.Spicer J, Wang C, Tanaka F, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC) [Conference presentation]. ASCO Annual Meeting 4-8 June 2021.
- 13.Besse B., Adam J., Cozic N. 1215O − SC neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): results from the phase II PRINCEPS trial. Ann Oncol. 2020;31:S794–S795. [Google Scholar]
- 14.Eichhorn F., Klotz L.V., Kriegsmann M. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: first clinical experience. Lung Cancer. 2021;153:150–157. doi: 10.1016/j.lungcan.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Forde P.M., Chaft J.E., Smith K.N. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao S., Li N., Gao S. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Hong M.H., Ahn B., Kim H.R. FP03.02 interim analysis of neoadjuvant chemoradiotherapy and durvalumab for potentially resectable stage III non-small cell lung cancer (NSCLC) J Thorac Oncol. 2021;16(3):S194–S195. [Google Scholar]
- 18.Lee J., Chaft J., Nicholas A. PS01.05 surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. J Thorac Oncol. 2021;16(3):S59–S61. [Google Scholar]
- 19.Provencio M., Nadal E., Insa A. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–1422. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 20.Reuss J.E., Anagnostou V., Cottrell T.R. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer. 2020;8(2):e001282. doi: 10.1136/jitc-2020-001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothschild S.I., Zippelius A., Eboulet E.I. SAKK 16/14: Durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer: a multicenter single-arm phase II trial. J Clin Oncol. 2021 doi: 10.1200/JCO.21.00276. Jco2100276. [DOI] [PubMed] [Google Scholar]
- 22.Shen D., Wang J., Wu J. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J Thorac Dis. 2021;13(3):1760–1768. doi: 10.21037/jtd-21-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu C.A., Gainor J.F., Awad M.M. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–795. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 24.Tfayli A., Al Assaad M., Fakhri G. Neoadjuvant chemotherapy and avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med. 2020;9(22):8406–8411. doi: 10.1002/cam4.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong B.C., Gu L., Wang X. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2021 doi: 10.1016/j.jtcvs.2021.02.099. In press. [DOI] [PubMed] [Google Scholar]
- 26.Yang C.J., McSherry F., Mayne N.R. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg. 2018;105(3):924–929. doi: 10.1016/j.athoracsur.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Cascone T., William W.N., Weissferdt A. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27:504–514. doi: 10.1038/s41591-020-01224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altorki N.K., McGraw T.E., Borczuk A.C. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 2021;22(6):824–835. doi: 10.1016/S1470-2045(21)00149-2. [DOI] [PubMed] [Google Scholar]
- 29.Lei J., Yan X., Zhao J., Tian F., Lu Q., Jiang T. 62MO A randomised, controlled, multicenter phase II trial of camrelizumab combined with albumin-bound paclitaxel and cisplatin as neoadjuvant treatment in locally advanced NSCLC. Ann Oncol. 2020;31:S1441–S1442. [Google Scholar]
- 30.Travis W.D., Dacic S., Wistuba I. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–740. doi: 10.1016/j.jtho.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmann M.D., Chaft J.E., William W.N. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–e50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissferdt A., Pataer A., Vaporciyan A.A. Agreement on major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin Lung Cancer. 2020;21(4):341–348. doi: 10.1016/j.cllc.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu Y., Emoto K., Eguchi T. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol. 2019;14(3):482–493. doi: 10.1016/j.jtho.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailly C., Thuru X., Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. 2020;2(1):zcca002. doi: 10.1093/narcan/zcaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felip E., Rosell R., Maestre J.A. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010;28(19):3138–3145. doi: 10.1200/JCO.2009.27.6204. [DOI] [PubMed] [Google Scholar]
- 36.Skoulidis F., Goldberg M.E., Greenawalt D.M. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi J.S., Ready N., Healy P. Immune activation in early-stage non–small cell lung cancer patients receiving neoadjuvant chemotherapy plus ipilimumab. Clin Cancer Res. 2017;23(24):7474–7482. doi: 10.1158/1078-0432.CCR-17-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashemi S., Fransen M.F., Niemeijer A. Surprising impact of stromal TIL's on immunotherapy efficacy in a real-world lung cancer study. Lung Cancer. 2021;153:81–89. doi: 10.1016/j.lungcan.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Weissferdt A., Pataer A., Swisher S.G. Controversies and challenges in the pathologic examination of lung resection specimens after neoadjuvant treatment. Lung Cancer. 2021;154:76–83. doi: 10.1016/j.lungcan.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Carretero-Gonzalez A., Lora D., Ghanem I. Comparative safety analysis of immunotherapy combined with chemotherapy versus monotherapy in solid tumors: a meta-analysis of randomized clinical trials. Oncotarget. 2019;10(35):3294–3301. doi: 10.18632/oncotarget.26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puzanov I., Diab A., Abdallah K. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scagliotti G.V., Pastorino U., Vansteenkiste J.F. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol. 2012;30(2):172–178. doi: 10.1200/JCO.2010.33.7089. [DOI] [PubMed] [Google Scholar]
- 43.Villaruz L.C., Socinski M.A. The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res. 2013;19(10):2629–2636. doi: 10.1158/1078-0432.CCR-12-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.