Abstract
Background
It is expected that GPs are increasingly confronted with a large group of patients with symptoms persisting three weeks after initial symptoms of a mild (managed in the outpatient setting) COVID-19 infection. Currently, research on these persistent symptoms mainly focuses on patients with severe infections (managed in an inpatient setting) whereas patients with mild disease are rarely studied.
Objective
The main objective of this systematic review was to create an overview of the nature and frequency of persistent symptoms experienced by patients after mild COVID-19 infection.
Methods
Systematic literature searches were performed in Pubmed, Embase and PsychINFO on 2 February 2021. Quantitative studies, qualitative studies, clinical lessons and case reports were considered eligible designs.
Results
In total, nine articles were included in this literature review. The frequency of persistent symptoms in patients after mild COVID-19 infection ranged between 10% and 35%. Symptoms persisting after a mild COVID-19 infection can be distinguished into physical, mental and social symptoms. Fatigue was the most frequently described persistent symptom. Other frequently occurring persistent symptoms were dyspnoea, cough, chest pain, headache, decreased mental and cognitive status and olfactory dysfunction. In addition, it was found that persisting symptoms after a mild COVID-19 infection can have major consequences for work and daily functioning.
Conclusion
There is already some evidence that symptoms of mild COVID-19 persist after 3 weeks in a third of patients. However, there is a lack of data about symptoms persisting after 3 months (long-COVID). More research is needed to help GPs in managing long-COVID.
Keywords: Long-Covid, outpatients, post-acute Covid-19, primary health care, recovery of function, systematic review
Key Messages.
GPs increasingly see mild COVID-19 patients with persistent symptoms.
There is a lack of knowledge about persistent symptoms after mild COVID-19.
The frequency of persistent symptoms ranges between 10% and 35%.
Persistent symptoms can be divided into physical, mental and social symptoms.
Future research should focus on symptoms extending beyond 3 months.
Background
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by SARS-CoV-2. The disease was first diagnosed in Wuhan, China. Currently, the virus has spread all around the world and the World Health Organisation (WHO) has declared the COVID-19 outbreak a pandemic.(1,2)
COVID-19 is a multi-organ disease and the severity can range from asymptomatic to very serious.(3) Most people who are infected will develop symptoms. About one in five patients remain asymptomatic although the evidence is still inadequate.(4) COVID-19 mainly affects the respiratory system. Other organs that may be affected by COVID-19 include the heart, kidney, liver, muscles, skin and nervous system.(5) Since COVID-19 is a multi-organ disease, various clinical manifestations are involved.(6) Clinical manifestations in the acute phase of COVID-19 are fever, dry cough, fatigue, a sore throat, loss of taste or smell, dyspnoea, nasal congestion, muscle or joint pain, chest pain, headache and nausea.(6,7)
Some patients, even those with mild symptoms of COVID-19, continue to experience symptoms after initial recovery.(8,9) For many other infectious diseases, post-infectious syndromes have already been recognized (10,11) for instance, after a mild SARS-infection where a substantial proportion of patients have residual damage in the lungs.(12,13) In addition, some studies describe psychological complaints, fatigue, limitations in fitness and reduced quality of life after a SARS-infection.(14–16) Other infectious diseases known for post-infectious symptoms are, for example, Q-fever, Legionnaires’ disease, glandular fever and epidemic polyarthritis.(10,17) A prospective study showed that patients experienced fatigue, neurocognitive problems, musculoskeletal pain and mood disturbance six months after these infections.(10) Although these findings cannot be extrapolated to patients with COVID-19, it is expected that also a proportion of COVID-19 patients will experience physical, cognitive or psychological complaints and more specifically persistent fatigue after initial recovery within 3 weeks.
In the recent literature, a distinction is made between post-acute COVID-19 and chronic COVID-19 (i.e. long-COVID). Post-acute COVID-19 is described as symptoms extending beyond three weeks from initial symptoms and long-COVID is described as symptoms extending beyond 12 weeks from initial symptoms.(8) The distinction between post-acute and long-COVID is somewhat arbitrary, however, it is relevant to distinguish between the phases to better understand and research the consequences of COVID-19 on the short- and long-term.
At the moment, very little is known about persistent symptoms in patients after a mild COVID-19 infection (patients managed in an outpatient setting). A lot of attention is paid to patients with severe infections (patients managed in an inpatient setting). Sparse research has been done in the much larger group with mild COVID-19 infections. Most of these patients present themselves at the GP’s office and are not referred to the hospital.(18) More attention towards this group is highly needed. Firstly, because available data indicate that the majority of people with COVID-19 has a mild disease.(19) Secondly, it is to be expected that—congruent with other infections—a certain part of these patients will experience persisting symptoms. Because of the high prevalence of mild COVID-19 infections this, could result in a considerable number of patients with persisting symptoms.
So, it is expected that GPs are increasingly confronted with a large group of patients with post-acute COVID-19 and long-COVID after a mild COVID-19 infection.(20) Identifying the symptoms of post-acute COVID-19 and long-COVID is important for the management of those patients since these can have a major impact on daily functioning. Describing these persistent symptoms is also important for the development of knowledge about the natural course of a COVID-19 infection. So, we aimed to describe the symptoms immediately after the initial phase (i.e. symptoms persisting longer than 3 weeks; post-acute COVID-19) and the symptoms in the chronic phase (i.e. symptoms persisting longer than 3 months; long-COVID) to create an overview of the nature and frequency of symptoms experienced by patients with mild COVID-19 after the first 3 weeks.
Methods
We performed a systematic search of the literature and reported the results according to the PRISMA guidelines.(21)
Search strategy
Systematic literature searches were performed on 2 February 2021 in PubMed, Embase and PsychINFO. Our search strategy consisted of three strings, one about COVID, one about persisting symptoms and one about ambulatory care; these strings were combined with the Boolean operator AND (see Supplementary material). Since this is a very new topic, we also searched for grey literature in Google Scholar, the website of the Dutch College of general practitioners (NHG), their journal ‘Huisarts en Wetenschap’ and on the website of the Dutch Journal of Medicine (NTvG). Furthermore, we also scanned Twitter for relevant articles on this topic.
Study selection
All identified records were exported to the citation management program EndNote. First, two reviewers independently screened the title and abstract, based on inclusion and exclusion criteria. Subsequently, final article selection was based on full text review by the same independent reviewers. Levels of agreement between the reviewers were analysed using Cohen’s Kappa. Any discrepancies were solved by consensus. Reference lists of included articles were screened to identify additional literature.
Studies were eligible for inclusion if they described patients who have been through a mild COVID-19 infection. Patients who were managed in an outpatient setting were considered as having a mild disease. A positive test for COVID-19 was not a prerequisite for diagnosis, since many people had not been tested at the beginning of the pandemic. Secondly, the study should describe symptoms extending beyond three weeks from initial COVID-19 symptoms. Quantitative studies, qualitative studies, clinical lessons and case reports were considered eligible designs. Articles were excluded when the described population were mainly hospitalized patients and when no full text was available in English or Dutch.
Quality assessment
For the assessment of the quality of the included studies, we used the Newcastle-Ottawa Scale for observational studies.(22) Not every item from this scale was relevant for the included studies as most included studies did not use a control group. Therefore, we used two items from Selection (representativeness and ascertainment of outcome) and the three items from Outcome (assessment of outcome, follow-up long enough, adequacy of follow-up; see Supplementary Material). The score range is 0–5, with higher scores indicating higher quality. Two researchers independently scored all items for the observational studies. Inconsistencies were discussed and resolved. We did not assess the quality of the qualitative studies (23,24) and the case study (20) because these studies did not yield data on frequency.
Data extraction and analysis
After identifying relevant articles, two authors independently extracted data from the observational studies. Extracted data included study characteristics, recruitment setting, inclusion criteria, follow-up time and outcomes: percentages of symptoms of COVID-19 persisting after 3 weeks from the initial symptoms. If possible, the percentage of patients in which the symptom was found was depicted. For studies that concerned both inpatients and outpatients, only the results of outpatients were extracted from the article. Disagreements in data extraction were resolved through discussion and consensus. We screened the case study and the two qualitative studies on numerical data on prevalence. Main observations related to the described symptoms in these papers were included. Data were interpreted and critically evaluated.
Results
Selection of eligible studies
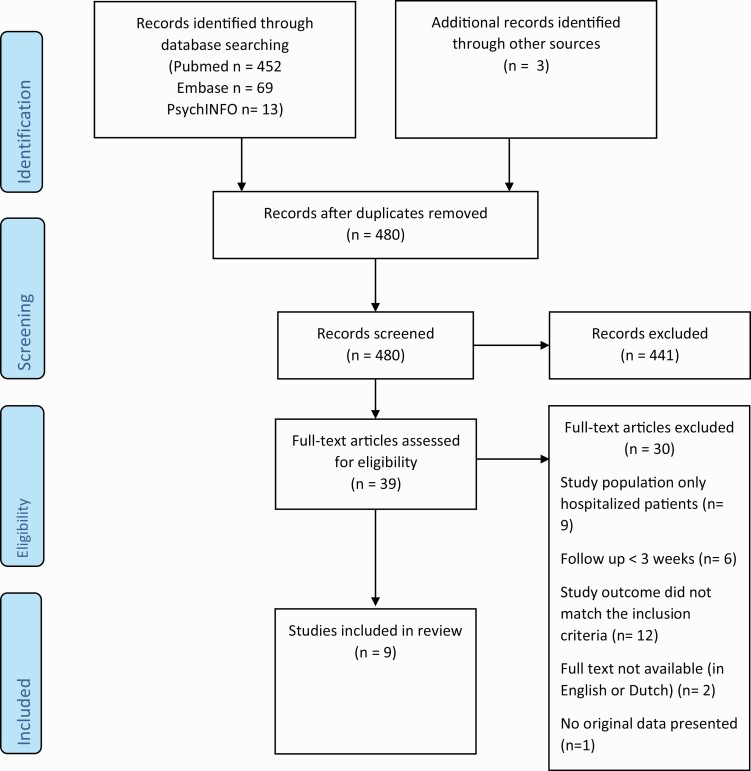
The literature search yielded 534 articles. Of these papers, we removed 57 duplicates. In addition, three articles were identified through other sources: the website of the NTvG, Twitter and the reference lists of the included articles. After screening the title and abstract we excluded 441 articles because the topic of these papers was not symptoms after COVID-19. The 39 remaining articles were reviewed based on reading the full text. Thirty of these articles did not match our inclusion criteria. Reasons for exclusion were that the study population only consisted of hospitalized patients (n = 9), that the outcome of the study was not relevant (n = 12), a follow-up shorter than 3 weeks (n = 6), that no full-text was available in English or Dutch (n = 2) and that the design was not eligible (n = 1). The non-eligible design was a reaction to another paper. In total, nine articles were included in this review.(9,20,23–29) The flowchart of the article selection process is provided in Fig. 1. Cohen’s Kappa for agreement between the two reviewers was moderate (0.44).
Figure 1.
Flow diagram of study selection (From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097).(21)
Characteristics of included studies
All included articles were published in 2020, except for one article published in 2021. Most of the included articles are quantitative, observational studies (n = 5), two articles are qualitative studies, one article is a mixed methods study and one a case study. The number of COVID-19 patients in the included studies ranged from 3 to 2113. We could not extract numerical data from the qualitative studies and the case study. Among the selected studies, four included only outpatients and five also studied inpatients. In two studies no distinction had been made between inpatients and outpatients but since, respectively, 90.4% and 94.7% of their study populations concerned outpatients, we used their results for this review.(23,26) The overall quality of the included observational studies was moderate with four studies scoring three out of five points, one study scoring two points and one study with four points. A summary of the descriptive characteristics of the included studies is presented in Table 1. This table also includes information on the different methods for recruitment and inclusion of participants.
Table 1.
Descriptive characteristics of the included articles (n = 9)
| First author and year of publication | Country | Publication type or study design | Quality assessed by adapted Newcastle- Ottawa scalea | Recruitment | Recruitment setting | Inclusion criteria | Total study population | Number of out-patients | Mean or median age in years |
|---|---|---|---|---|---|---|---|---|---|
| Goërtz 2020 (26) | The Netherlands | Prospective observational study | ** | Inpatients and outpatients | Recruitment from Facebook groups for COVID-19 patients with persistent symptoms and registries on the website of the Lung Foundation on COVID-19 informationb | – | 2113 | 2001c | Median 47/47d |
| Van den Borst 2020 (27) | The Netherlands | Prospective observational study | *** | Inpatients and outpatients | Referral to researchers by GPs | - Mild disease- Non-admitted patients- Symptoms persisting >6 weeks | 124 | 27 | Mean 59/52d |
| D’Ascanio 2020 (25) | Italy | Prospective case-control study | **** | Inpatients and outpatients | Patients included after home testing, and analysis in the Santa Croce Hospital | - Positive COVID-19 test | 68 | 23 | Mean 58.1/47.5d |
| Townsend 2021 (28) | Ireland | Cross-sectional study | *** | Inpatients and outpatients | Participants were recruited from the post-COVID-19 outpatient clinic | - Positive COVID-19 test- At least 6 weeks after date of last acute symptoms | 153 | 79 | Median 48/40d |
| Landsman 2020 (29) | The Netherlands | Explorative mixed methods study | *** | Outpatients | Recruitment through Public Health Services | - Positive COVID-19 test- Age >18- Healthcare workers | 109 | 109 | Mean 42.5 |
| Tenforde 2020 (9) | United States | Retrospective observational study | *** | Outpatients | Recruitment from lists submitted to the researchers by different urban academic health systems | - Positive COVID-19 test - Adults |
292 | 292 | Median 42.5 |
| Ladds 2020 (23) | United Kingdom | Qualitative study | Not applicable | Inpatients and outpatients | Patients were recruited from the UK-based long COVID patient support groups, social media and through snowball samplinge | - Symptoms developed between February and July 2020 - Acute illness - Continuing symptoms beyond 3 weeks |
114 | 103c | Median 46 |
| Kingstone 2020 (24) | United Kingdom | Qualitative study | Not applicable | Outpatients | Participants were invited through social media and snowball sampling | - Presence of self-reported persistent symptoms | 24 | 24 | Mean 43.2 |
| Burgers 2020 (20) | The Netherlands | Case study | Not applicable | Outpatients | Cases have been described by a GP | - Non-admitted patients - Presence of persistent symptoms | 3 | 3 | Mean 38.7 |
aScore range is 0–5 stars, with higher scores indicating higher quality. See Supplementary Material for details.
bThis is a very selective study population since it can be expected that members of those Facebook groups have a high prevalence of persistent symptoms.
cBoth inpatients and outpatients were analysed for this literature review.
dThe mean or median age in years is depicted for, respectively, the total study population and for the outpatients.
eSome focus groups in the study were restricted to health professionals.
Persistent symptoms
The percentage of patients still experiencing symptoms after the initial phase of COVID-19 (first three weeks), who have been managed in an outpatient setting, appears to be between 10% and 35%.(8,9) One study indicated that 35% of their respondents (n = 292) reported that they did not return to their preceding state of health after a median of 16 days.(9) Another study showed that 62% of patients (n = 153) felt that they had not returned to full health.(28) A cross-sectional study showed that after a mean of 30 days, 22% still experienced symptoms.(29) In a very large study (2001 outpatients and 112 inpatients), only 0.7% of the population was symptom-free approximately 3 months after the initial infection.(26)
Included studies describe many different kinds of symptoms experienced by patients. The analyses in different studies reveal an illness with symptoms that relapse and remit.(23) The symptoms described in the included studies can be categorized into physical, mental and social symptoms. We will describe these symptoms in more detail below. Furthermore, we separately describe symptoms for post-acute COVID-19 and long-COVID (Table 2).
Table 2.
Overview of follow-up time and persistent symptoms of COVID-19 in mild disease patients described in nine articles
| First author and year of publication | Follow-up time | Symptoms described in articlea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical symptoms | Mental symptoms | Social symptoms | ||||||||||
| Fatigue | Dyspnoea | Cough | Chest pain | Fever like symptoms | Loss of taste and/or smell | Headache | Psychological symptoms | Cognitive dysfunction | Hinder in daily functioning | Consequences for work | ||
| Post-acute COVID-19 | ||||||||||||
| D’Ascanio 2020 (25) | 30 days | + (16%) | ||||||||||
| Landsman 2020 (29) | Mean 30 days | + | + (33%) | + (6%) | ||||||||
| Tenforde 2020 (9) | 14–21 days | + (>30%) | + (29%) | + (43%) | + (~20%) | − | + (13%) | + (>10%) | ||||
| Ladds 2020 (23) | At least 3 weeks | + | + | + | + | |||||||
| Kingstone 2020 (24) | Not applicable | + | + | + | + | |||||||
| Burgers 2020 (20) | 8–16 weeks | + | + | + | + | + | + | |||||
| Long-COVID | ||||||||||||
| Goërtz 2020 (26) | Mean 79 days | + (87%) | + (71%) | + (29%) | + (24%) | + (22%) | + (>20%) | + (38%) | ||||
| Van den Borst 2020 (27) | Mean 13 weeks | + | + (5%) | + (~10%) | + (~10%) | |||||||
| Townsend 2021 (28) | Median 75 days for inpatients and 92 days for outpatients | + (47%) |
a(+) is depicted if the article describes the symptom as a persistent symptom of COVID-19 in their study population, (−) is depicted if the article describes that the symptom is NOT found to be present in their study population as a persistent symptom of COVID-19, nothing is depicted if the symptom is not mentioned in the article. If possible the percentage of patients in which the symptom was found in the study is depicted. However, we should be careful with interpreting these percentages since the percentages are highly dependent on the selected population and often based on very small sample sizes.
Post-acute COVID-19
Physical symptoms
In several studies, fatigue is found to be the most, or second most prevalent symptom of post-acute COVID-19.(29) In one article no exact frequency is given, but over 30% of the patients still experience fatigue after 14–21 days.(9) In qualitative studies fatigue is the most described persistent symptom in quotes of participants.(20,24) Participants also describe that they are sleeping a lot.(24) In the clinical lesson of Burgers et al., all three cases report fatigue after approximately 2 months as a persistent symptom.(20)
Apart from fatigue, several quantitative and qualitative studies described respiratory symptoms for post-acute COVID-19.(20,24) One study showed that shortness of breath was not resolved after 14–21 days in 29% of patients.(9) The same study mentioned that 43% of patients still had a cough and 20% experienced pain in the lungs at follow-up.(9) Patients mention that they are feeling stuffy when talking a lot and during effort, and that it comes in random waves.(20)
Studies show contradicting results about fever-like symptoms of post-acute COVID-19. A case description described patients with fluctuating fever and patients who were sometimes soaked with sweat.(20) Conversely, another study showed that among those who reported fever and chills on the day of COVID-19 diagnosis, these symptoms have resolved during follow-up in 97% and 96% of patients, respectively.(9)
Only one study explicitly studied olfactory dysfunction in ex COVID-19 patients with a mild course of the disease.(25) The study of D’Ascanio et al. analysed the frequency and severity of subjective loss of smell in 23 outpatients, 20 inpatients and 25 healthy controls.(25) Among the outpatients, 83% reported olfactory dysfunction. By day 30, most outpatients reported recovery of olfactory dysfunction (84%).(25) The results indicate that olfactory dysfunction is more common in patients with a mild course of COVID-19 and that symptoms persist longer in this group. However, most patients reported recovery within 30 days. Another study found that 13% of the patients reported that they still experienced the loss of smell and taste 14–21 days after COVID-19 diagnosis.(9)
Also, headache was mentioned as a persistent symptom in different articles. Tenforde et al. reported that over 10% of patients reported headache after 14–21 days.(9)
Mental symptoms
Psychological and cognitive functioning were recurrent themes for post-acute COVID-19 in the studies included in this review. Participants in different studies describe their fears associated with their ongoing symptoms.(23,24) Some patients express concerns that full recovery may not be possible.(24) Patients also describe that it is both physically and emotionally very stressful that they are trapped in a cycle of improvements and declines in health.(23) Other patients describe a change in their identity when they reflect on how they perceived themselves before being diagnosed with COVID-19.(24)
Social symptoms
Post-acute COVID-19 can hinder daily functioning. In a qualitative study, many participants describe that they experience a dramatic decline in their ability to perform basic everyday activities. Participants describe how they need to discover new routines and self-disciplinary measures, such as counting steps and planning out visits, to avoid exhaustion.(23) This is in line with descriptions of participants of another study, who describe how they had worked out ways to plan their activities to conserve the little energy they had.(24) The results of a cross-sectional study with a mean follow-up of 30 days showed that only 67% was fully active again, and 22% was able to do light household chores but not able to do physical exercise.(29)
Also, consequences for work are described in the literature.(20) One patient lacks income for everyday that she is sick. Other patients mention that they cannot work fully due to fatigue.(20) Another article mentioned that symptom such as fatigue and cognitive blunting severely limited the prospect of returning to work or finding new employment.(23)
Long-COVID
Physical symptoms
Also after 3 months, fatigue is found to be the most frequent symptom. In one of the studies, fatigue affected 87% of their patients.(26) Another study measured health status with the Short Form Health Survey (SF-36) (30) and the Nijmegen Clinical Screening Instrument (NCSI).(31) Mild COVID-19 patients reported severe problems on different domains, including the domain ‘fatigue’ for both instruments.(27) A third study measured fatigue with the Chalder Fatigue Scale (CFQ-11) and found that 47% of patients met the case definition for fatigue.(28)
The study of Goërtz et al. showed that a large percentage of patients still experienced respiratory symptoms after 3 months: 71% of patients still experienced shortness of breath, 29% had a cough and 24% had pain in their lungs.(26)
For long-COVID, no study explicitly studied olfactory dysfunction. However, the symptom was reported. One of the studies indicated that more than 20% of patients still experienced a loss of smell after approximately 3 months.(26) This study also showed that 22% of patients had increased body temperature and 38% reported headache after approximately 3 months.(26)
A lot of other persistent symptoms were listed but not further described. They were only present in a figure or table or mentioned once in the text, but no further description or analysis was given in the results or discussion section of the article. These symptoms include body aches, diarrhoea, congestion, nausea, vomiting, confusion, a sore throat, sneezing, swollen glands, difficulties in concentration, pains in hands and feet, joint pain, blue finger nails, muscle pains, ear pain, rashes, metabolic disruption, sudden loss of body weight, thromboembolic conditions, palpitations and an increased resting heart rate.(9,23,24,27)
Mental symptoms
As in post-acute COVID-19 patients, psychological and cognitive functioning can be reduced in long-COVID patients. In the study of van den Borst et al. abnormal scores on different questionnaires on mental and cognitive health were observed in approximately 10% of patients after a mean follow-up of 13 weeks. Normal scores for all used questionnaires were found for only 59% of the participants.(27)
Social symptoms
Social symptoms were mainly studied in qualitative studies, which are all on post-acute COVID-19. No evidence was available in the retrieved publications about social symptoms experienced by long-COVID patients.
Discussion
With the COVID-19 pandemic still ongoing, we are only at the beginning of an understanding of the long-term sequelae of COVID-19. This systematic review gives an overview of the nature and frequency of symptoms experienced by patients after 3 weeks of the initial mild COVID-19 infection. Frequencies of patients with persistent symptoms vary from 10% to 35%.
In different studies, fatigue is found to be the most or second most frequent symptom of post-acute COVID-19 and long-COVID. Dyspnoea is also frequently occurring. Furthermore, cough, chest pain and headache are described in most articles as are mental and cognitive symptoms. There were few articles that described the loss of taste and/or smell and their sample sizes are low. The frequency of olfactory dysfunction is between 13% and 20%. Finally, it was found that post-acute COVID-19 and long-COVID can have major consequences for work and daily functioning. It can be concluded that the results indicate that a substantial proportion of patients with a mild COVID-19 still experience symptoms after initial recovery and that the nature of the symptoms is very broad.
For infectious diseases, other than COVID-19, post-infectious syndromes have already been recognized.(10,11) The results of this systematic review show similarities with those syndromes. Persistent symptoms of COVID-19 and SARS are overlapping.(14–16,24) Also, other infectious diseases like Q-fever, glandular fever, epidemic polyarthritis and Legionnaires show similarities with persistent symptoms of COVID-19.(10,17) Symptoms that are overlapping are fatigue, pain, limitations in the quality of life and limitations in fitness. Loss of taste and/or smell is not described in studies on post-infectious syndromes of diseases other than COVID-19, and, therefore, is likely to be a specific persistent symptom for COVID-19.
Strengths and limitations
This study has several strengths. First, the literature search was very extensive, and several databases were explored. In addition, a search for grey literature was included. Therefore, this overview is as complete as possible. Second, all articles were screened and read by two independent researchers. Cohen’s Kappa for agreement between the two reviewers was moderate. However, after discussion consensus was reached on all articles. This highlights the importance of two reviewers. Due to this systematic approach, we are convinced that we included all relevant articles. Also, data extraction was performed by two independent researchers.
A limitation of this review is the low amount of data that was available. An extensive literature search yielded no more articles on persistent symptoms in patients with a mild course of the disease. A lot of articles that returned from the literature search were about persistent symptoms in hospitalized patients. This confirms that a lot of attention is paid to patients with serious infections, and that long-COVID in mild disease patients is an underexposed research topic. It is important that there will be more attention for this group because the majority of people with COVID-19 belongs to this group.(19) This literature review was based on only nine articles with different research methods and sometimes very small sample sizes. One of the studies had a follow-up period of only 14–21 days. This means that only a part of the study population met our inclusion criteria, this could have inflated the frequency of persistent symptoms, which was defined as symptoms extending beyond 3 weeks. Also, qualitative studies and case reports were included, as we aimed to make this overview as completely as possible. Although these studies gave us a more in-depth insight into the experiences of patients with regard to persistent symptoms after a mild COVID infection, the frequency of these persistent symptoms cannot be extracted or calculated from these studies.
The papers that fitted our inclusion criteria were mostly about post-acute COVID-19, which was defined as symptoms extending beyond three weeks from initial symptoms.(8) Little literature was found on long-COVID, which was defined as symptoms extending beyond 12 weeks from initial symptoms.(8) However, this is reasonable since the COVID-19 pandemic recently started and several studies are still ongoing. More long-term observational studies are needed to draw reliable conclusions with regard to helping GP’s in recognizing post-acute COVID-19 and long-COVID. The impact across different domains of health (as defined in this review) should be systematically explored. Studies should concern the course of the symptomatology and the influence on the daily life functioning of patients. Different groups should be compared: patients in primary care, inpatients who were not admitted to the ICU, ICU patients and unexposed controls. These unexposed controls serve to account for the influence of noise on symptoms, which is always present.
Another limitation is that some of the included studies have very selective populations. Therefore, we have to be very careful with drawing conclusions. One study with a frequency of persistent symptoms of 99.3% was found.(26) However, this study recruited patients via Facebook groups of patients with persistent symptoms. This is a highly selected study population since it can be expected that members of those Facebook groups have a high frequency of long-COVID symptoms. In addition, there is a possible bias to confirmed results. Not all quantitative studies did predefine the symptoms of interest. Therefore, only persistent symptoms that were found are described, but the absence of certain symptoms is not systematically reported.
As the pandemic progresses, there is a need for more evidence-based treatment options for patients with post-acute COVID-19 and long-COVID. Management of COVID-19 after the first three weeks is currently based on limited evidence.(8) This is because there is currently hardly any literature available about optimal aftercare for patients with COVID-19, or about the effectiveness of rehabilitation programs.(32) However, documents on aftercare that were found on the internet describe similar persistent symptoms as described in this review.(32,33) A document of the Royal Australian College of General Practitioners describes that the majority of patients seeking support from GPs will be experiencing fatigue, dyspnoea, joint pain, chest pain, cough and a change in sense of smell and/or taste.(33) A document of the Dutch Federation of Medical Specialists describes psychological complaints, chronic fatigue and restrictions in fitness and quality of life.(32) Now that we know which symptoms belong to post-acute COVID-19 and long-COVID, GPs can respond more specifically to those symptoms. First of all, they can better recognize symptoms. Second, more specific research can be conducted into evidence-based treatment options that address the symptoms found. Based on this, guidelines can be made. GPs can use those in practice. It seems that the focus should be on fatigue and respiratory symptoms.
Conclusion
To conclude, there is evidence that symptoms persist after mild COVID-19 infections in a substantial part of the patients. Therefore, post-acute COVID-19 and long-COVID will possibly become important public health problems in the near future. These results need to be interpreted cautiously because only nine studies could be included in this review. More knowledge on persistent symptoms can help GPs in recognizing long-COVID in mild disease patients and in being able to respond to long-COVID adequately.
Supplementary Material
Declaration
Funding: None.
Ethical approval: Not applicable.
Conflict of interest: None.
References
- 1. Ge H, Wang X, Yuan Xet al. . The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis 2020; 39(6): 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronavirus disease 2019 (COVID-19): situation report, 51. World Health Organisation; 2020, (accessed on 11 March 2020). [Google Scholar]
- 3. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol 2020; 45(8): 100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buitrago-Garcia D, Egli-Gany D, Counotte MJet al. . Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020; 17(9): e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gavriatopoulou M, Korompoki E, Fotiou Det al. . Organ-specific manifestations of COVID-19 infection. Clin Exp Med 2020; 20(4): 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machhi J, Herskovitz J, Senan AMet al. . The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol 2020; 15(3): 359–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324(6): 603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ 2020; 370: m3026. [DOI] [PubMed] [Google Scholar]
- 9. Tenforde MW, Kim SS, Lindsell CJet al. ; IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020; 69(30): 993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hickie I, Davenport T, Wakefield Det al. ; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006; 333(7568): 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bannister BA. Post-infectious disease syndrome. Postgrad Med J 1988; 64(753): 559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan KS, Zheng JP, Mok YWet al. . SARS: prognosis, outcome and sequelae. Respirology 2003; 8 Suppl: S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang P, Li J, Liu Het al. . Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lau HM, Lee EW, Wong CNet al. . The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil 2005; 86(6): 1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee AM, Wong JG, McAlonan GMet al. . Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry 2007; 52(4): 233–40. [DOI] [PubMed] [Google Scholar]
- 16. Lam MH, Wing YK, Yu MWet al. . Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169(22): 2142–7. [DOI] [PubMed] [Google Scholar]
- 17. van Loenhout JA, van Tiel HH, van den Heuvel Jet al. . Serious long-term health consequences of Q-fever and Legionnaires’ disease. J Infect 2014; 68(6): 527–33. [DOI] [PubMed] [Google Scholar]
- 18. Mahase E. Covid-19: what do we know about “long covid”? BMJ 2020; 370: m2815. [DOI] [PubMed] [Google Scholar]
- 19. Kaswa R, Govender I. Novel coronavirus pandemic: a clinical overview. S Afr Fam Pract (2004) 2020; 62(1): e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgers JS, Reijers MH, Cals JW. Persisting symptoms after uncomplicated COVID-19: dealing with uncertainty by general practitioner and patient. Ned Tijdschr Geneeskd 2020; 164: D5338. [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Institute OHR. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 May 2021).
- 23. Ladds E, Rushforth A, Wieringa Set al. . Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality criteria for services. medRxiv 2020. Doi: 2020.10.13.20211854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kingstone T, Taylor AK, O’Donnell CAet al. . Finding the ‘right’ GP: a qualitative study of the experiences of people with long-COVID. BJGP Open 2020; 4(5): bjgpopen20X101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D’Ascanio L, Pandolfini M, Cingolani Cet al. . Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg 2020. Doi: 194599820943530. [DOI] [PubMed] [Google Scholar]
- 26. Goërtz YMJ, Van Herck M, Delbressine JMet al. . Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020; 6(4): 00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Borst B, Peters JB, Brink Met al. . Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis 2020; 21: ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Townsend L, Dowds J, O’Brien Ket al. . Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021; 18(6): 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landsman JA, Verheij NP, Alma MAet al. . COVID-19: recovering at home is not easy. Ned Tijdschr Geneeskd 2020;164: D5358. [PubMed] [Google Scholar]
- 30. Aaronson NK, Muller M, Cohen PDet al. . Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol 1998; 51(11): 1055–68. [DOI] [PubMed] [Google Scholar]
- 31. Peters JB, Daudey L, Heijdra YFet al. . Development of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: the Nijmegen Clinical Screening Instrument. Qual Life Res 2009; 18(7): 901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leidraad Nazorg voor patiënten met COVID-19. Utrecht, The Netherlands: Federatie Medisch Specialisten, 2020. [Google Scholar]
- 33. Caring for Adult Patients with postCOVID-19 Conditions. East Melbourne, Victoria, Australia: The Royal Australian College of General Practitioners, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.