Abstract
Introduction
The epidemiology of atrial fibrillation (AF) in India has not been studied systematically in large scale population based surveys. Stroke is one of the leading causes of death and disability in India. As AF is a major contributor of stroke, it is important to know the burden of AF and stroke risk in the population. The Andhra Pradesh Atrial Fibrillation (AP-AF) study aims to assess the prevalence, etiology, risk factors and stroke risk among the rural population in Andhra Pradesh, India.
Methods
This is a cross-sectional survey done using a two-stage sampling process. Adults (≥18years) from villages in East and West Godavari districts were sampled. Field investigators used a structured questionnaire to collect information on basic demographics, cardiovascular risk factors and medical history. Anthropometric measurements were performed, blood pressure measured and fasting capillary blood glucose was assessed. Electrocardiogram was done using a hand-held mobile ECG device-KardioMobile. ECGs were interpreted by study cardiologists. Participants diagnosed to have AF were invited to participate in a camp conducted by cardiologists where echocardiogram was done and also a focused history related to AF was collected. Along with age and sex stratified prevalence of AF, descriptive statistics will be used to present demographics, clinical profile, and cardiovascular risk factors. Stroke risk will be calculated using CHA 2 DS 2 -Vasc score.
Conclusion
The AP-AF study is expected to provide important information on AF epidemiology in rural India. The information may help improve health care policies in preventing stroke and other complications of AF.
Keywords: Atrial fibrillation, Prevalence, Stroke, Mobile ECG, Screening, India
1. Background and rationale
Atrial fibrillation (AF) is a significant public health problem worldwide, with a global prevalence of 0.5% [1]. A 2010 report on the global burden of diseases, injuries, and risk factors (GBD) estimated the worldwide burden of AF as 33 million and predicted that the number would double by 2050 [1]. This potential rise in AF may burden our healthcare system by increasing morbidity and mortality due to stroke, heart failure, and dementia. The risk of stroke due to AF can be reduced with oral anticoagulation therapy (OCT) [[2], [3], [4]]. However, 10%–40% of patients with AF remain asymptomatic [[5], [6], [7], [8], [9]]. In these cases, an embolic stroke could be the first manifestation of AF [10,11]. Early identification and treatment of AF, especially in asymptomatic patients, can reduce the morbidity associated with the disease. Population-level screening and intervention can reduce the burden of AF and its associated co-morbidities. The first step towards decreasing the country's burden is to estimate the magnitude of the problem by systematic epidemiological studies.
1.1. The need for systematic population-based screening of AF in India
India is witnessing an increase in non-communicable diseases (NCD) due to multiple factors such as aging, a decreasing fertility rate, and changing lifestyles [12]. Ischemic heart disease, stroke, and diabetes are on the rise in India. These NCDs share common risk factors with AF, and hence the prevalence of AF is also likely to be high. Apart from NCDs, rheumatic heart disease (RHD), though on the decline, is a significant contributor to AF in India [[13], [14], [15], [16], [17]].
To date, only a few systematic population-based studies to detect AF have been done in India. The Nagpur-AF study, the first population-based study in India in over two decades showed a prevalence of 0.19% [16]. Notable observations from the study were that RHD was the predominant cause of AF, and though diagnosed with AF, most patients were not on stroke prophylaxis. Possible reasons for this may be reduced health awareness and accessibility in many parts of India. As these issues could be more prevalent in rural areas [[18], [19], [20], [21]], [[18], [19], [20], [21]] [[18], [19], [20], [21]] we planned to conduct a systematic, population-based survey in this population. This report details the rationale and methods used in the Andhra Pradesh-Atrial Fibrillation (AP-AF) study.
2. Methods
2.1. Study design
The AP-AF study was a population-based, cross-sectional survey of adults (≥18 years) in rural Andhra Pradesh (AP), India. The study was a joint effort of CARE Hospital and CARE Foundation, Hyderabad, India.
2.2. Study objectives
The primary objective was to determine the prevalence of AF in adults in a random sample of a rural population in AP, India. The secondary objectives were to 1) understand the demographic profile of the sampled population and assess the prevalence of cardiovascular risk factors, 2) understand the clinical profile of participants detected to have AF, and 3) study stroke risk and stroke prophylaxis of participants detected to have AF.
2.3. Description of study sites
East and West Godavari, coastal districts of AP, a southern state in the Indian sub-continent (Fig. 1) were chosen for the study. East Godavari has a population density of 477 persons per square kilometer. The population is 75% rural, with a sex ratio of 998 females per 1000 males. The overall literacy rate in rural East Godavari is 68%, with 71% for males and 64% for females [22].
Fig. 1.
1A shows the location of Andhra Pradesh state within India. 1B shows a zoomed out map of Andhra Pradesh highlighting the districts where the study was conducted.
West Godavari District's population density is 509 persons per square kilometer, with a rural population of 79%. The rural sex ratio is 996 females per 1000 males. The overall literacy rate is 75%. For males, it is 79%, and for females, 71%. Agriculture is the predominant occupation in both the districts, with more than 50% of the category being labourers and less than 10% forming the cultivator category [23].
2.4. Rationale for study sites selection
East and West Godavari districts have a mostly rural population, the target population for our study. In addition to this, several villages in these districts have peripheral health centers (PHCs) funded and managed by CARE Rural Health Missions (CRHM), a key arm of CARE Foundation. A qualified auxiliary nurse midwife (ANM) manages each PHC. The ANM has experience in conducting field surveys and outreach medical camps. The ANM is a local villager and has a good rapport with the residents. The PHCs also have coordinators who facilitate the activities of CRHM in the designated village clusters and serve as a channel of communication between the PHCs and CRHM's head office at Hyderabad. The ANMs and coordinators were readily available resource personnel for the study.
2.5. Participant inclusion and exclusion criteria
All residents of the study villages aged 18 and above were eligible for the study.
2.6. Ethical considerations
The study was conducted as per the International standards of Good Clinical Practices (ICH - GCP) guidelines. The study protocol was reviewed and approved by the Institutional Ethics Committee (IEC) of CARE Hospital. Strict participant confidentiality was maintained throughout the study. Only authorized members of the study team had access to the study data. Informed consent was obtained from all participants before enrolment in the study.
2.7. Sample size calculation and sampling procedure
We calculated the sample size based on Nagpur-AF study, where the prevalence (P) was 0.1%. We set the confidence limit (d) at 0.001962 (P/2). As we planned a two-stage sampling, we incorporated a design effect of two to have a confidence interval of 90%. With these parameters, the sample size was determined as 11,021 (n = Z2 P(1-P)/d2.
The CRHM manages 127 villages in the East and West Godavari districts. For the first stage of sampling, 40 villages were sampled with probability proportional to size method. In the second stage, individuals aged 18 years and above were selected by age and sex-stratified simple random sampling using CRHM's latest household listing.
2.8. Participant approach and enrolment
The field investigators approached the participants at their respective houses and explained the study. After obtaining consent from the participants, they started the study procedures.
2.9. Study procedures
The study was done in two stages.
Stage I: The study procedures were carried out in the participants' houses. Investigators screened the participants for AF, collected basic demographic information and medical history.
Stage II: The participants identified with AF were brought to a local hospital on a pre-specified date. Study cardiologists interviewed the participants for a focussed medical history related to AF. An echocardiography (Two-dimensional and Doppler) was done to look for any underlying cardiac conditions.
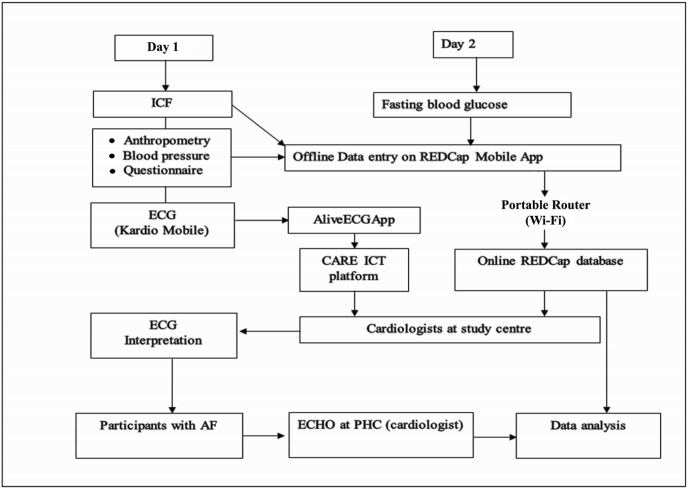
The sequence of the study procedures is outlined pictorially in Fig. 2.
Fig. 2.
Sequence of the study procedures in Stage I and Stage II of the study. ICF: Informed consent Form; ICT: Information and Communications Technology; PHC: Peripheral Health Centre.
2.9.1. Study instruments and measurements
The field investigators visited each house twice (consecutive days) to complete the study procedures: anthropometric measurements, blood pressure (BP) recording, participant interview, ECG recording, and blood sampling for fasting glucose.
Bodyweight was measured using a digital weighing scale. Body height, waist, and hip circumference were measured using a non-stretchable measuring tape. Blood pressure was recorded with a digital, electronic BP monitor (Omron HEM-8721, Omron Healthcare Co, Ltd., Kyoto, Japan) and fasting capillary blood glucose (FBS) using Omron HGM-112 glucose meter (Omron Healthcare Co, Ltd., Kyoto, Japan). Blood pressure was recorded thrice with at least a 1-min interval between measurements and the average of the three was taken as the participant's BP.
The field investigators interviewed the participants using a structured questionnaire developed using the REDCap survey and data management tool [24]. The collected data was entered offline using REDCap Mobile App installed on the mobile tablets (Apple iPad Mini 2 tablet-WiFi only).
Electrocardiograms were recorded using a hand-held ECG device, Kardia Mobile (Alivecor, Inc., Mountain View, CA, United States). The Kardia Mobile was synchronized to the Kardia app (downloaded and installed on the mobile tablet). The single-lead (Lead I) ECG recorded using the Kardia Mobile was automatically transferred to the tablet after each recording. The procedure for recording ECGs with Kardia Mobile is described elsewhere [25]. The key variables are given in Table 1.
Table 1.
Description of key variables used in the study.
| Variables | Description |
|---|---|
| Systemic Hypertension | Raised blood pressure (systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg [26] recorded during the house visit) ∗or, diagnosis of systemic hypertension as mentioned in the medical records/self-reported by participants or currently on medications for hypertension |
| Diabetes Mellitus | Raised fasting capillary blood glucose (100 mg/dl) [27] or diagnosis of diabetes mellitus as mentioned in medical records/self-reported by participants or currently on medications for diabetes mellitus |
| Dyslipidaemia | Fasting levels of serum cholesterol >/200 mg/dl or serum triglycerides >/150 mg/dl or high density lipoprotein <40 mg/dl for men and <50 mg/dl for women or low density lipoprotein >130 mg/dl [28] or a diagnosis of dyslipidaemia as mentioned in medical records/self-reported by participants or currently on lipid lowering medications. |
| Tobacco use | Smoking, sucking, chewing or snuffing any form of tobacco |
| Smoking | Current Smoking - An adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes. Former smoker: An adult who has smoked at least 100 cigarettes in his or her lifetime but who had quit smoking at the time of interview. Never smoker: An adult who has never smoked, or who has smoked less than100 cigarettes in his or her lifetime.[29] |
| Alcohol Abuse/Alcohol use disorder | A person who “can no longer control their use of alcohol, compulsively abuse alcohol despite its negative ramifications, and/or experience emotional distress when they are not drinking.” [30] |
| Atrial Fibrillation | Diagnosis of atrial fibrillation recorded in participant's medical record or, irregular rhythm with no p waves on a the ECG recorded by KardiaMoblie (confirmed by study cardiologist) or, the same findings recorded by a standard 12-lead ECG when KardiaMobile rhythm is unclear (confirmed by study cardiologist) |
∗Average of three blood pressure measurements were taken as the participant's blood pressure.
2.9.2. Data transfer from field to the study center
At the end of each field visit, the field investigators transferred the collected data from their mobile tablets via Jio Dongle to the REDCap server, hosted at CARE Foundation, Hyderabad. This enabled coordination of the data from the Mobile App with the main REDCap project. The study team members at Hyderabad accessed the data from the web-based REDCap application. The ECGs stored on the mobile tablet were uploaded on to CARE-ICT (Information and Communication Technology) platform for remote access by the study cardiologists at Hyderabad.
2.9.3. ECG interpretation
Two study cardiologists at CARE hospital read the ECGs independently. The cardiologists read all the ECGs irrespective of the classification by Kardia Mobile's automated algorithm (“Normal”, “AF”, or Unreadable”). Mismatches, if any, were resolved by mutual consensus.
2.10. Data variables
The questionnaire included questions on the participant demographics, cardiovascular and AF risk factors, previous medical history, and medications. In Stage II, medical history was reviewed with a focus on previous cardiac surgery, history of stroke, and medications, especially OACs. In echocardiography, the left ventricular function, left atrial clot, valvular pathology, and any other cardiac conditions, if present, were assessed.
2.11. Study personnel and training
The field investigators and field supervisors were given a one-day training on the study rationale, project overview, interview skills, study tools, and data transferring process. The field supervisors were additionally trained in the supervision of data collection and quality checks. The list of core study team members and their responsibilities are given in Table 2.
Table 2.
Key study personnel and responsibilities.
| Study personnel | Responsibilities |
|---|---|
| PI (cardiologist) |
|
| Field investigators (ANMs) |
|
| Field supervisors |
|
| Field officer (Public health specialist) |
|
| Study cardiologists CARE hospital |
|
2.12. Data quality
2.12.1. Design, pre-testing, and training
We designed the questionnaire as a multilingual survey instrument (English and Telugu) and tested for validity with ANMs at CARE hospital as enumerators and patients' attenders as respondents. The field investigators and supervisors were made to familiarize themselves with the questionnaire through team exercises on the training day.
2.12.2. Data validation, security, and access
Data validation checks (alerts) were programmed into the REDCap system for missing values, out of range values, and data outliers. Field supervisors performed back checks on randomly selected participants surveyed by the field investigators.
The collected data were stored in the secure database of the REDCap application and CARE-ICT system. The field officer was the database administrator who exclusively had the authority for controlled and customized data sharing with the rest of the study team.
The study cardiologists from CARE hospital and the field officer from CRHM were jointly responsible for data validity and analysis.
2.13. Statistical analysis plan
Prevalence will be calculated as the total number of participants diagnosed with AF divided by the total number of participants surveyed. The prevalence will be reported as the percentage of study participants diagnosed with AF. The prevalence of AF will also be calculated for different groups categorized by age and sex. Descriptive statistics will be used to present demographics, clinical profile, and cardiovascular risk factors. The clinical profile of participants diagnosed with AF, echocardiographic parameters, anticoagulant use will also be presented using descriptive statistics. Stroke risk will be calculated using the CHA2DS2-Vasc score [31].
2.14. Study administration
CARE Hospital, Road No.1, Banjara Hills, is responsible for the overall study administration, data coordination, statistical data analysis, and preparing study reports.
3. Discussion
AP-AF study is a large-scale population-based study in India to assess AF's prevalence, clinical profile of people with AF, and their stroke risk. This study is now in the data analysis phase.
There were no systematic, population-based surveys on AF epidemiology in India at the time of planning the study. The recently published SMARTIndia screened 2500 people more than 40 years of age with Kardia Mobile [32]. The study was done from villages in Western India using serial ECG recording over five days. The prevalence was 1.6%, with two-third of the diagnosis made with the first ECG recording.
The AP-AF study's main limitation is that it uses a single time point strategy for screening and therefore would miss on paroxysmal AFs. While the SMART India study screened thrice over five days, some studies from other parts of the world have reported a much higher prevalence with recording over weeks using an ambulatory ECG monitor [[33], [34], [35], [36]]. Apart from the screening duration, the population's risk profile and the instrument used to screen also influence the results.
In addition to studying the prevalence in a different geographical region, the AP-AF study will complement the SMART India study on profiling the risk factors of people with AF and understand the etiology for AF. It is expected to fill in critical knowledge gaps about AF, such as stroke risk, OAC use in patients with known AF, treatment practices, and adherence to treatment. These factors have not been studied in a general population in India, except for the Nagpur AF study, which has the disadvantage of selection bias.
Funding
This study was financially supported by Boehringer Ingelheim India Private Limited, and Zydus Cadila Healthcare Limited (Medica). The Kardia mobile units were provided by Asia Pacific Heart Rhythm Society.
Declaration of competing interest
The authors disclose no conflicts of interest.
Acknowledgements
The authors would like to thank the following individuals for their contribution: Dr. Prasad Sistla, Chief of telemedicine, CARE Foundation for support in data management and telemedicine services, Ms. Nalla Swapna, Senior Physician Assistant, CARE Hospital for training the field workers, Mr. Sudhir Chintakindi, Biostatistician, CARE Hospital for statistical inputs, and Mr. Prasanth Kumar Palepu, Programme Head, CARE Rural Health Mission for logistical support.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014 Feb 25;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine. American College of Physicians. Ann Intern Med. 2007 Jun 19;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mitrousi K., Lip G.Y.H., Apostolakis S. Age as a risk factor for stroke in atrial fibrillation patients: implications in thromboprophylaxis in the era of novel oral anticoagulants. J Atr Fibrillation. 2013;6(1):783. doi: 10.4022/jafib.783. Published 2013 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Singer D.E., Hughes R.A., Gress D.R., Sheehan M.A., Oertel L.B., Maraventano S.W. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990 Nov 29;323(22):1505–1511. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 5.Bakhai A., Darius H., De Caterina R., Smart A., Le Heuzey J.Y., Schilling R.J., Zamorano J.L., Shah M., Bramlage P., Kirchhof P. Characteristics and outcomes of atrial fibrillation patients with or without specific symptoms: results from the PREFER in AF registry. Eur Heart J Qual Care Clin Outcomes. 2016 Oct 1;2(4):299–305. doi: 10.1093/ehjqcco/qcw031. [DOI] [PubMed] [Google Scholar]
- 6.Boriani G., Laroche C., Diemberger I., Fantecchi E., Popescu M.I., Rasmussen L.H. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med. 2015 May;128(5):509–518.e2. doi: 10.1016/j.amjmed.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Lévy S., Maarek M., Coumel P., Guize L., Lekieffre J., Medvedowsky J.L. Characterization of different subsets of atrial fibrillation in general practice in France: the College of French Cardiologists. Circulation. 1999 Jun 15;99(23):3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 8.Freeman J.V., Simon D.N., Go A.S., Spertus J., Fonarow G.C., Gersh B.J. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) Circ Cardiovasc Qual Outcomes. 2015 Jul;8(4):393–402. doi: 10.1161/CIRCOUTCOMES.114.001303. [DOI] [PubMed] [Google Scholar]
- 9.Rienstra M., Vermond R.A., Crijns H.J.G.M., Tijssen J.G.P., Van Gelder I.C. Asymptomatic persistent atrial fibrillation and outcome: results of the RACE study. Heart Rhythm. 2014 Jun;11(6):939–945. doi: 10.1016/j.hrthm.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Flaker G.C., Belew K., Beckman K., Vidaillet H., Kron J., Safford R. Asymptomatic atrial fibrillation: demographic features and prognostic information from the atrial fibrillation follow-up investigation of Rhythm management (AFFIRM) study. Am Heart J. 2005 Apr;149(4):657–663. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Brambatti M., Connolly S.J., Gold M.R., Morillo C.A., Capucci A., Muto C. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 12.India State-Level Disease Burden Initiative Collaborators Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017 Dec 2;390:2437–2460. doi: 10.1016/S0140-6736(17)32804-0. 10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao D.V., Reddy R.M., Srikanth K., Prakash R.B., Prasad S.A., Prasad G.S. To study the prevalence and clinical profile of chronic atrial fibrillation in hospitalized patients. NUJHS. 2014;4:17–20. [Google Scholar]
- 14.Narasimhan C., Verma J.S., Ravi Kishore A.G., Singh B., Dani S., Chawala K. Cardiovascular risk profile and management of atrial fibrillation in India: real world data from RealiseAF survey. Indian Heart J. 2016 Sep-Oct;68(5):663–670. doi: 10.1016/j.ihj.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negi P.C., Sondhi S., Asotra S., Mahajan K., Mehta A. Current status of rheumatic heart disease in India. Indian Heart J. 2019 Jan-Feb;71(1):85–90. doi: 10.1016/j.ihj.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saggu D.K., Sundar G., Nair S.G., Bhargava V.C., Lalukota K., Chennapragada S., Narasimhan C., Chugh S.S. Prevalence of atrial fibrillation in an urban population in India: the Nagpur pilot study. Heart Asia. 2016 Apr 18;8(1):56–59. doi: 10.1136/heartasia-2015-010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vora A., Kapoor A., Nair M., Lokhandwala Y., Narsimhan C., Ravikishore A.G. Clinical presentation, management, and outcomes in the Indian heart Rhythm society-atrial fibrillation (IHRS-AF) registry. Indian Heart J. 2017 Jan-Feb;69(1):43–47. doi: 10.1016/j.ihj.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnaiah S., Kovai V., Srinivas M., Shamanna B.R., Rao G.N., Thomas R. Awareness of glaucoma in the rural population of Southern India. Indian J Ophthalmol. 2005;53(3):205–208. doi: 10.4103/0301-4738.16685. [DOI] [PubMed] [Google Scholar]
- 19.Kanungo S., Bhowmik K., Mahapatra T., Mahapatra S., Bhadra U.K., Sarkar K. Perceived morbidity, healthcare-seeking behavior and their determinants in a poor-resource setting: observation from India. Reddy H., editor. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasthuri A. Challenges to healthcare in India - the five A's. Indian J Community Med. 2018;43:141–143. doi: 10.4103/ijcm.IJCM_194_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medhi G.K., Hazarika N.C., Borah P.K., Mahanta J. Health problems and disability of elderly individuals in two population groups from same geographical location. J Assoc Phys India. 2006;54:539–544. [PubMed] [Google Scholar]
- 22.Pradesh A. Census of India 2011 Andhra Pradesh district census handbook village and town directory directorate of census operations. https://censusindia.gov.in/2011census/dchb/2805_PART_A_DCHB_HYDERABAD.pdf Available from:
- 23.Andhra Pradesh series-29 Part Xii-B district census handbook West Godavari village and town wise primary census abstract (pca) directorate of census operations Andhra Pradesh. https://censusindia.gov.in/2011census/dchb/2815_PART_B_DCHB_WEST%20GODAVARI.pdf Available from:
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau J.K., Lowres N., Neubeck L., Brieger D.B., Sy R.W., Galloway C.D. IPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193–194. doi: 10.1016/j.ijcard.2013.01.220. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jones D.W., Materson B.J., Oparil S., Wright J.T., Roccella E.J. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. J Am Med Assoc. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 27.Toscano C.M., Duncan B.B., Mengue S.S., Polanczyk C.A., Nucci L.B., Costa e Forti A. CNDDM Working Group. Initial impact and cost of a nationwide population screening campaign for diabetes in Brazil: a follow up study. BMC Health Serv Res. 2008 Sep 22;8:189. doi: 10.1186/1472-6963-8-189. PMID: 18808662; PMCID: PMC2562380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandra K.S., Bansal M., Nair T., Iyengar S.S., Gupta R., Manchanda S.C. Consensus statement on management of dyslipidemia in Indian subjects. Indian Heart J. 2014;66:S1–S51. doi: 10.1016/j.ihj.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NHIS - adult tobacco use-glossary. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm
- 30.What is alcoholism? Find alcohol addiction treatment. https://www.alcohol.org/alcoholism/ (n.d.)
- 31.Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M., Andresen D. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 32.Soni A., Karna S., Fahey N., Sanghai S., Patel H., Raithatha S. Age-and-sex stratified prevalence of atrial fibrillation in rural Western India: results of SMART-India, a population-based screening study. Int J Cardiol [Internet] 2019;280:84–88. doi: 10.1016/j.ijcard.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scalvini S., Piepoli M., Zanelli E., Volterrani M., Giordano A., Glisenti F. Incidence of atrial fibrillation in an Italian population followed by their GPs through a telecardiology service. Int J Cardiol. 2005;98(2):215–220. doi: 10.1016/j.ijcard.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Hendrikx T., Hörnsten R., Rosenqvist M., Sandström H. Screening for atrial fibrillation with baseline and intermittent ECG recording in an out-of-hospital population. BMC Cardiovasc Disord. 2013;13:41. doi: 10.1186/1471-2261-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sposato L.A., Saposnik G. Stroke rates vary substantially across cohorts of patients with atrial fi brillation Implications for practice. Europace. 2013;6(11):5–8. [Google Scholar]
- 36.Turakhia M.P., Ullal A.J., Hoang D.D., Than C.T., Miller J.D., Friday K.J. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the screening study for undiagnosed atrial fibrillation (STUDY-AF) Clin Cardiol. 2015;38(5):285–292. doi: 10.1002/clc.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]