Abstract
Background
Second-generation cryoballoon ablation is safe and effective in patients with paroxysmal (PAF) and persistent atrial fibrillation (AF).
Objective
This study aimed to assess the long-term clinical outcomes and freedom from AF in patients undergoing thermal-guided cryoablation without the use of an electrical mapping catheter.
Methods
All patients who had undergone thermal-guided second-generation cryoablation without electrical mapping between January 2015 and April 2018 at Eastbourne District General Hospital were retrospectively analysed. Success was defined as freedom from atrial arrhythmia lasting >30 s during the follow up period.
Results
The study included 234 patients with a mean age of 65.3 ± 10.6 years. There were 134 (57.0%) and 100 (42.7%) patients who had PAF and persistent AF respectively.
Arrhythmia recurrence occurred in 38 of 134 (28.4%) PAF and 42 of 100 (42.0%) persistent AF patients after mean follow up of 40 ± 9.2 months. The patients with PAF had a significantly greater freedom from arrhythmia than patients with persistent AF (p = .040). The mean procedure time was 55.5 ± 12.2 min and the mean fluoroscopy time was 10.9 ± 4.8 min 73.5% of patients were discharged on the same day.
Conclusion
Thermal-guided cryoablation is feasible, safe and results in freedom from arrhythmia in the majority of paroxysmal and persistent AF patients in the long term. Randomised controlled trials are required to confirm the findings of this study.
Keywords: Cryoablation, Electrical mapping, Atrial fibrillation, Paroxysmal AF, Persistent AF
1. Introduction
Second-generation cryoablation has been shown to be safe, effective and improve symptoms in patients with paroxysmal (PAF) and persistent atrial fibrillation (AF) [[1], [2], [3]]. Using the first generation cryoballoon, the STOP AF trial reported a freedom from arrhythmia rate of 69.9% [2]. More recently, the STOP Persistent AF trial reported a 12 month freedom from arrhythmia rate of 54.8% [3].
Recently it has been shown that thermal-guided cryoablation is as effective as cryoablation with pulmonary vein mapping with similar AF recurrence rates [[4], [5], [6]]. However previous studies have been limited by only including patients with PAF or a low number of patients [[4], [5], [6]].
This study examines the long-term effect of thermal-guided second-generation cryoablation, which is standard practice in our centre, in patients with PAF and persistent AF.
2. Methods
All patients who had undergone thermal-guided second-generation cryoablation between January 2015 and April 2018 at Eastbourne District General Hospital were retrospectively analysed. Data was collected from the Eastbourne District General Hospital AF ablation registry and clinical notes. The study was registered and approved by the local clinical effectiveness group (East Sussex Healthcare NHS Trust) and complies with the Declaration of Helsinki.
2.1. Definitions
Patients were defined as having PAF if they had any episode of AF which self-terminated or required cardioversion within 7 days of onset. Persistent AF patients were defined as having episodes of AF lasting longer than 7 days [7].
A major complication was defined as any adverse event related to the procedure, which required intervention for treatment, caused long-term disability, or resulted in prolonged hospitalisation.
2.2. Procedure management
Before ablation, each patient gave written informed consent for the procedure. All procedures were performed on uninterrupted anticoagulation. After obtaining femoral venous access, a decapolar catheter was placed in the coronary sinus (CS). Left atrial access was achieved using the modified Brockenbrough technique [8] and a 8.5F transseptal sheath (SL1, St Jude Medical, St Paul, MN) which was then exchanged over the wire for the 12F Flexcath Advance. After the transseptal puncture, heparin was administered to maintain an activated clotting time >300 s.
An Amplatz Super Stiff Guidewire (Boston Scientific Corporation, Boston, MA) was used in the inner lumen of the FlexCath and was advanced into each pulmonary vein. A 28-mm Arctic Front Advance cryoballoon (Medtronic, Minneapolis, MN) was positioned in each pulmonary vein ostium and inflated. Complete vessel occlusion was considered to be achieved by contrast injection and if there was no backflow of contrast in to the LA. Each ablation was performed with a single 3-min freeze for each vein [9,10].
A second freeze using a different balloon angulation was performed if there was contrast backflow in to the LA or if a temperature of −40 °C was not reached within 60 s.
Prior to ablation of right-sided pulmonary veins, the decapolar catheter was placed in the right subclavian vein or superior vena cava to pace the right phrenic nerve (10–20 mA at 1.0–2.0 msec pulse width at a cycle length of 1000 msec). Ablation was immediately terminated upon any perceived reduction in the strength of diaphragmatic contraction.
If after successful cryoablation of all four pulmonary veins the patient remained in AF, external electrical cardioversion was performed.
Cardiac tamponade was assessed every half an hour and at the end of the procedure by checking the left anterior oblique (LAO) projection for reduced lateral heart border excursion [11].
2.3. Post-procedure management
After the procedure, each patient was monitored continuously for any complication and most were discharged the same evening or if the procedure was performed in the late evening, the following day if clinically stable. Post procedure echocardiography was not routinely performed. Anti-arrhythmic medications were discontinued before the end of the blanking period (3 months).
2.4. Follow-up
Clinical follow-up data were collected until April 2020 allowing for a minimum of two years follow-up in all patients. Patients were reviewed in the outpatient clinic at three months and one-year post procedure and were assessed for AF related symptoms and underwent an electrocardiogram (ECG) to document any arrhythmia recurrence and 24 h Holter monitoring at 3 months and one year. Thereafter patients were followed up and monitoring was performed upon physician discretion and their suspicion of arrhythmia recurrence.
Recurrence of AF was deemed to have occurred whenever an atrial arrhythmia lasting more than 30 s was documented on continuous monitoring or resting ECG during the follow-up period after a three-month blanking period.
2.5. Statistical analysis
Continuous variables are presented as mean ± SD or median (interquartile range), as appropriate. Categorical variables are presented as absolute number and percentage. Continuous variables between groups were analysed using Student's T-Test or the independent medians test. Categorical variables between groups were analysed using the Chi-square test.
The primary outcome of time to atrial arrhythmia was plotted using the Kaplan-Meier estimator. The log-rank test was used to compare freedom from arrhythmia between groups. All statistical analysis was performed using SPSS version 26. A p-value less than 0.05 was considered to be significant.
3. Results
In total, 235 cryoablation procedures were performed or attempted during the study period. One procedure was abandoned before cryoablation was attempted due to difficult transseptal access which required postponement of the procedure. This procedure is included in the complication data but excluded when calculating arrhythmia-related outcomes.
Thus 234 patients underwent thermal-guided cryoablation without electrical mapping. The mean age was 65.3 ± 10.6 years and 52.1% of patients were male. At index procedure 134 (57.0%) and 100 (42.7%) patients had PAF and persistent AF respectively (Table 1).
Table 1.
Baseline characteristics of the study group.
| Characteristic | All patients (N = 234) | PAF (N = 134) | Persistent AF (N = 100) | P value |
|---|---|---|---|---|
| Age (years) | 65.3 ± 10.6 | 64.4 ± 10.3 | 66.5 ± 11.0 | .138 |
| Male gender, n (%) | 122 (52.1) | 64 (47.7) | 58 (58.0) | .146 |
| Time since first AF diagnosis (months) | 37.8 ± 40.7 | 36.3 ± 39.0 | 39.1 ± 42.1 | .570 |
| Ejection fraction (%) | 55.0 ± 11.2 | 56.3 ± 9.0 | 52.0 ± 11.9 | .002 |
| Left atrial diameter (mm) | 39.1 ± 6.1 | 37.5 ± 6.4 | 41.1 ± 5.7 | <.001 |
| Diabetes, n (%) | 20 (8.5) | 11 (8.2) | 9 (9.0) | .818 |
| Hypertension, n (%) | 115 (49.1) | 60 (44.8) | 55 (55) | .146 |
| Ischaemic heart disease, n (%) | 24 (10.3) | 12 (9.0) | 12 (12.0) | .516 |
| Cerebrovascular disease, n (%) | 13 (5.6) | 9 (6.7) | 4 (4.0) | .406 |
| Hypercholesterolemia, n (%) | 30 (12.8) | 12 (9.0) | 18 (18.0) | .073 |
| Congestive cardiac failure, n (%) | 30 (12.8) | 11 (8.2) | 19 (19.0) | <.001 |
| CHA2DS2-VASc | 2.1 ± 1.4 | 2.0 ± 1.5 | 2.2 ± 1.3 | .194 |
| Previous Medical therapy | ||||
| Amiodarone, n (%) | 60 (25.6) | 18 (13.4) | 42 (42) | <.001 |
| Flecainide, n (%) | 31 (13.2) | 25 (18.7) | 6 (6) | .005 |
| Sotalol, n (%) | 28 (12.0) | 23 (17.2) | 5 (5) | .005 |
| Dronedarone, n (%) | 14 (6.0) | 9 (6.7) | 5 (5) | .584 |
| Propafenone, n (%) | 2 (0.9) | 2 (1.5) | 0 (0) | .220 |
| Bisoprolol, n (%) | 132 (56.4) | 76 (59.0) | 56 (56) | .913 |
| Anticoagulation therapy | ||||
| NOAC, n (%) | 183 (78.2) | 107 (79.9) | 76 (76) | .480 |
| Vitamin K antagonist, n (%) | 51 (21.8) | 27 (20.1) | 24 (24) | .480 |
There was no significant difference in the average age between groups (p = .138). Patients with PAF had a significantly smaller left atrial antero-posterior diameter (p < .001) than patients with persistent AF. Patients with persistent AF were significantly more likely to have congestive cardiac failure than patients with paroxysmal AF (19 (19%) vs 11 (8.2%), p < .001). At baseline all patients had failed or were intolerant of an antiarrhythmic or beta-blocker therapy (Table 1).
The mean duration of follow up was 40 ± 9.2 months. The mean continuous duration of AF in persistent AF patients was 3.6 ± 2.5 months.
3.1. Procedural characteristics
The majority of the procedures were performed under conscious sedation (98.7%) and were discharged on the same day (73.5%). The mean procedure time was 55.5 ± 12.2 min and the mean fluoroscopy time 10.9 ± 4.8 min in all patients (Table 2).
Table 2.
Procedural characteristics.
| All patients (N = 234) | PAF (N = 134) | Persistent AF (N = 100) | P value | |
|---|---|---|---|---|
| Total procedure time in minutes | 55.5 ± 12.2 | 56.7 ± 12.3 | 56.2 ± 12.2 | .746 |
| Total fluoroscopy time in minutes | 10.9 ± 4.8 | 11.0 ± 4.4 | 10.9 ± 5.2 | .921 |
| Minimum Temperature LUPV (°C) | −47.0 ± 5.2 | −47.3 ± 5.6 | −46.4 ± 4.6 | .188 |
| Minimum Temperature LLPV (°C) | −45.2 ± 4.0 | −45.5 ± 4.2 | −44.8 ± 3.8 | .238 |
| Minimum Temperature RUPV (°C) | −47.1 ± 5.6 | −47.2 ± 5.7 | −47.0 ± 5.5 | .831 |
| Minimum Temperature RLPV (°C) | −45.3 ± 5.3 | −45.6 ± 6.1 | −45.0 ± 5.0 | .422 |
| Cavotricuspid isthmus Line N (%) | 8 (3.4) | 1 (0.7) | 7 (7.0) | .022 |
LUPV Left upper pulmonary vein, LLPV Left lower pulmonary vein, RUPV Right upper pulmonary vein, RLPV Right lower pulmonary vein.
In total 7 (5.2%) PAF patients and 2 (2.0%) persistent AF patients had an common left-sided pulmonary vein. In total 8 (3.4%) patients underwent additional cavotricuspid isthmus line ablation.
3.2. Arrhythmia recurrence & predictors of success
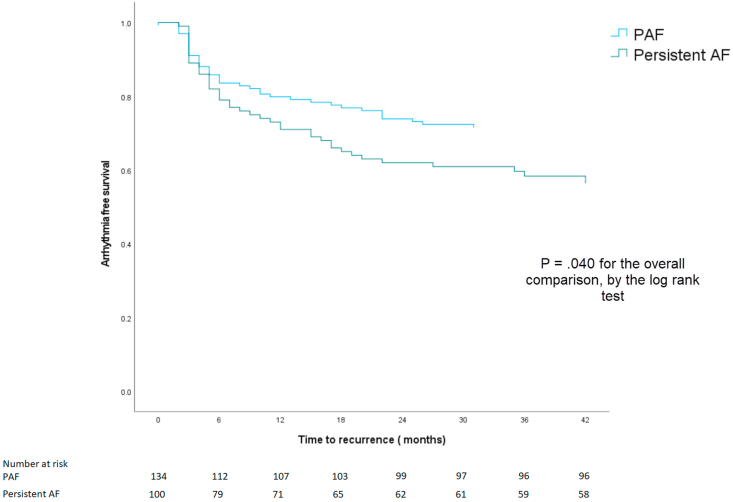
A documented recurrence of an atrial arrhythmia lasting longer than 30 s after undergoing thermal-guided cryoablation occurred in 80 of 234 (34.2%) patients. Arrhythmia recurrence occurred in 38 of 134 (28.4%) PAF and 42 of 100 (42.0%) persistent AF patients (Fig. 1). Patients with PAF had significantly greater freedom from arrhythmia than patients with persistent AF (p = .040).
Fig. 1.
Arrhythmia free survival in PAF and persistent AF patients.
3.3. Repeat ablation
In total 55 (23.5%) patients (25 PAF and 30 persistent AF) underwent a repeat ablation procedure using radiofrequency energy. 12 (21.8%) patients underwent repeat ablation using PVAC GOLD (Medtronic, Minneapolis, MN) catheter and 43 (78.2%) patients underwent point-by-point ablation guided by CARTO (Biosense Webster Inc., Diamond Bar, California) or EnSite NavX (St. Jude Medical, St Paul, Minnesota) 3D electroanatomical mapping system. The mean time to repeat ablation was 16.7 ± 11.1 months.
At repeat ablation, 16 (64%) PAF patients had at least one reconnected vein compared to 24 (80%) of persistent AF patients. In the whole cohort 17 (31.5%) left upper pulmonary veins, 19 (35.2%) left lower pulmonary veins, 23 (41.8%) right upper pulmonary veins, and 24 (43.6%) right lower pulmonary veins were found to be reconnected. One patient with left-sided common pulmonary vein required repeat ablation and reisolation.
Additionally, 23 patients underwent complex fractionated electrogram ablation, 4 patients underwent roof linear ablation, 9 patients underwent mitral isthmus line ablation, 19 patients underwent cavotricuspid isthmus line ablation and 2 patients underwent atrial tachycardia ablation.
In total 8 patients underwent a third ablation during the study period. The mean time between the second and the third ablation was 12.3 ± 4.0 months. All patients had persistent AF at index procedure. All procedures were performed using point-by-point technology (6 CARTO and 2 EnSite NavX). At third ablation 1 (12.5%) left upper pulmonary vein, 2 (25%) left lower pulmonary veins, 2 (25%) right upper pulmonary veins and 4 (50%) right lower pulmonary veins were found to be reconnected. Additionally 3 (37.5%) patients underwent complex fractionated electrogram ablation, 3 patients roof linear ablation, 3 patients underwent mitral isthmus line ablation, 3 patients underwent cavotricuspid isthmus line ablation and 3 patients underwent atrial tachycardia ablation.
3.4. Procedural complications
Device and/or procedure-related complications occurred in 16 patients (6.8%) (Table 3). The major and minor complication rate was 2.6% and 4.2% respectively each. The most frequent complication, was phrenic nerve injury which occurred in 6 patients (2.6%). In all cases phrenic nerve injury resolved at follow up. 1 patient presented one week after ablation with a pseudoaneurysm of the superficial femoral artery, which required thrombin injection. 1 patient had an inadvertent aortic root puncture with the transseptal needle which did not result in cardiac tamponade. The procedure was abandoned without cryoablation performed and was postponed to a later date with the transeptal puncture guided by a transoesophageal echocardiogram. 2 (0.9%) patients had a pericardial effusion and cardiac tamponade, which required pericardiocentesis. Both patients were discharged after a period of observation without further sequelae.
Table 3.
Procedural complications.
| Major complications | |
|---|---|
| Pericardial effusion requiring pericardiocentesis | 2 (0.9%) |
| Transient ischaemic attack or stroke | 2 (0.9%) |
| Vascular injury requiring blood transfusion or intervention | 1 (0.4%) |
| Aortic root perforation | 1 (0.4%) |
| Minor complications | |
| Haematoma | 4 (1.7%) |
| Temporary phrenic nerve injury | 6 (2.6%) |
4. Discussion
The main findings in this study were
-
1)
The long-term freedom from arrhythmia success rate was 65.8% in all patients.
-
2)
Freedom from arrhythmia success rate in PAF patients was significantly greater than in patients with persistent AF.
-
3)
The complication rate was very low.
In this study, the long-term freedom from arrhythmia success rate is reported to be 71.6% in PAF patients. This is similar to the reported outcomes in the STOP-AF post approval study which reported a three-year freedom from arrhythmia rate of 68.1% in PAF patients [12]. Additionally, the results in this study are comparable to those reported by Papparella et al. who reported outcomes in 192 PAF patients who also underwent thermal-guided ablation [6]. Similarly, Iacopino et al. also demonstrated a high success rate in PAF patients undergoing thermal-guided ablation when compared to a propensity matched cohort who underwent cryoablation with the Achieve catheter [13]. This study supports the finding of Iacopino and Papparella et al. with the majority of patients free from arrhythmia during the follow-up period.
This study also reports PVI without using concurrent pulmonary vein electrical mapping (using the Achieve catheter, Medtronic, Minneapolis, MN) in patients with persistent AF, which is standard practice in our institution. The long-term freedom from arrhythmia is reported to be 58% in persistent AF patients, significantly less than patients with PAF. This is to be expected given that patients with longer-term AF have been shown to have greater left atrial dilatation, fibrosis and substrate than patients with PAF [14]. Despite this, previous studies have not found a significant difference in performing additional linear and substrate ablation and PVI compared to PVI alone at index procedure [15].
The results achieved in this study may be explained by the fact that the second generation cryoballoon (Arctic Front Advance) results in a high rate of chronic lesion durability [16] and complete circular homogenous lesions to a greater extent than the first generation cryoballoon (Arctic Front) [17,18] Additionally, all operators at our centre are highly experienced in using the cryoballoon catheter performing more than 300 cryoablations each. Furthermore, utilising parameters such as achieving −40 °C at 60s (which has been shown to be a predictor of decreased PV reconnection) [19] may have contributed to the results seen in this study.
In this study, 74.1% of all patients were discharged on the same day of their procedure, which is greater than the same day discharge rate of 20.8% and 28.5% reported by Bartoletti et al. and Reddy et al. [20,21] Although this study only reports the outcomes of patients undergoing cryoablation, this is representative of the majority of AF ablations conducted during the study period in our institution as all patients undergoing the first time AF ablation undergo cryoablation as described.
Deyell et al. reported a same day discharge rate of 79.2% [22] however the majority of the procedures were performed using radiofrequency energy and under general anaesthesia with prolonged average procedure times of more than 3 h [22]. The mean procedure time reported in this study was 55.5 ± 12.2 min which is significantly less compared to previous studies. The Cryo AF Global Registry reported a procedure time of 82 ± 34 min and Sawhney et al. reported a procedure time of 95 ± 65 min [23,24]. In addition, the fluoroscopy times in this study compared to previous studies using electrical mapping is more favourable. Knight et al. and Sawhney et al. reported a fluoroscopy time of 20.1 ± 12.1 min and 13 ± 10 min compared to 10.9 ± 4.8 in this study [12,24].
Thermal-guided cryoablation may also result in cost savings with the Achieve Mapping catheter costing £960 representing a saving of £240,960 in this study alone [25]. Thus using a stepwise approach using thermal-guided cryoablation at the first procedure and radiofrequency ablation at the second procedure as used in this study may improve catheter lab efficiency and lead to considerable cost savings.
In this study, 23.5% of patients underwent a repeat ablation. The percentage of PAF patients undergoing repeat ablation in the study (18.7%) is similar to the reported number by Papparela et al. (18%). In addition, the number of PAF patients who had reconnected veins was also similar to that reported by Pappaela et al. [6] The pulmonary vein reconnection rate in the persistent AF group was higher than in the PAF group. In addition, substrate ablation was more likely to be performed in the patients with persistent AF than PAF which is to be expected given the greater fibrosis seen in these patients [14].
The complication rate in this study was low. This is similar to previous studies and also real world registry data [3,26]. The major complication rate in the Multicentre STOP-AF persistent trial was reported to be 5.5% and the total complication rate in large nationwide analysis of 5608 patients undergoing cryoablation in Germany was reported to be 12.3% [3,26]. The rate of pericardial effusion is likely to be under reported considering routine echocardiography was not performed at the end of the procedure however this did not have any clinical impact as no patient represented with signs of cardiac tamponade after discharge. Additionally, cardiac tamponade was checked for at the end of each case using the LAO fluoroscopic projection [11]. There was one patient who had a pseudoaneurysm of the superficial femoral artery, which occurred before the introduction of ultrasound guided vascular access in our institution.
5. Limitations
This study was retrospective in nature and non-randomised, thus follow up and management was dependent on physician discretion which may have impacted the results. Invariably as long-term follow up was symptom guided, patients underwent a lesser degree of ECG monitoring which would be expected in a clinical trial however the results presented is more reflective of real world practice.
This study only reports the findings of patients undergoing thermal-guided ablation without electrical mapping and does not have a comparison group. Thus, the results seen in this study when compared to a cohort of patients undergoing cryoablation with electrical mapping may be no different.
6. Conclusion
This study reports the long-term outcomes after thermal-guided second-generation cryoballoon ablation in patients with PAF and persistent AF. The study shows that thermal-guided cryoablation is feasible, safe and results in freedom from arrythmia in the majority of patients. More studies, including randomised controlled trials, are required to confirm our findings.
Financial support
Eastbourne Cardiology Research Charity Fund.
Declaration of competing interest
Dr Rick A Veasey has received research grant funding from Medtronic.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Kuck K.H., Brugada J., Fürnkranz A., Metzner A., Ouyang F., Chun K.R.J. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 2.Packer D.L., Kowal R.C., Wheelan K.R., Irwin J.M., Champagne J., Guerra P.G. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American arctic front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 3.Su W.W., Reddy V.Y., Bhasin K., Champagne J., Sangrigoli R.M., Braegelmann K.M. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP persistent AF trial. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Mccready J., Champney F. 216-34: cryoablation without pulmonary vein mapping in paroxysmal atrial fibrillation: a comparison with a standard approach. EP Eur. 2016;18 i149–i149. [Google Scholar]
- 5.Dulai R., Sulke N., Furniss S., Veasey R.A. The effect of second-generation cryoablation without electrical mapping in persistent AF using continuous monitoring. J Intervent Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00721-1. [DOI] [PubMed] [Google Scholar]
- 6.Paparella G., Iacopino S., Osório T.G., de Torres J.P.A., Ströker E., Sieira J. Temperature-guided ablation with the second-generation cryoballoon for paroxysmal atrial fibrillation: 3-year follow-up in a multicenter experience. J Intervent Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00770-6. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P., Benussi S., Zamorano J.L., Aboyans V., Achenbach S., Agewall S. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Russ J Cardiol. 2016;147:7–86. 2017. [Google Scholar]
- 8.Yao Y., Ding L., Chen W., Guo J., Bao J., Shi R. The training and learning process of transseptal puncture using a modified technique. Europace. 2013;15:1784–1790. doi: 10.1093/europace/eut078. [DOI] [PubMed] [Google Scholar]
- 9.Ciconte G., De Asmundis C., Sieira J., Conte G., Di Giovanni G., Mugnai G. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015;12:673–680. doi: 10.1016/j.hrthm.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Ciconte G., Sieira-Moret J., Hacioglu E., Mugnai G., Di Giovanni G., Velagic V. Single 3-minute versus double 4-minute freeze strategy for second-generation cryoballoon ablation: a single-center experience. J Cardiovasc Electrophysiol. 2016;27:796–803. doi: 10.1111/jce.12986. [DOI] [PubMed] [Google Scholar]
- 11.Nanthakumar K., Kay G.N., Plumb V.J., Zheng X., Killingsworth C.R., Smith W.M. Decrease in fluoroscopic cardiac silhouette excursion precedes hemodynamic compromise in intraprocedural tamponade. Heart Rhythm. 2005;2:1224–1230. doi: 10.1016/j.hrthm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Knight B.P., Novak P.G., Sangrigoli R., Champagne J., Dubuc M., Adler S.W. Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval study. JACC Clin Electrophysiol. 2019;5:306–314. doi: 10.1016/j.jacep.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Iacopino S., Mugnai G., Takarada K., Paparella G., Ströker E., De Regibus V. Second-generation cryoballoon ablation without the use of real-time recordings: a novel strategy based on a temperature-guided approach to ablation. Heart Rhythm. 2017;14:322–328. doi: 10.1016/j.hrthm.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Jalife J., Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475–484. doi: 10.1016/j.tcm.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma A., Jiang C.Y., Betts T.R., Chen J., Deisenhofer I., Mantovan R. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 16.Reddy V.Y., Sediva L., Petru J., Skoda J., Chovanec M., Chitovova Z. Durability of pulmonary vein isolation with cryoballoon ablation: results from the sustained pv isolation with arctic front advance (SUPIR) study. J Cardiovasc Electrophysiol. 2015;26:493–500. doi: 10.1111/jce.12626. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury R., Coutino H.E., Darciuc R., Ströker E., De Regibus V., Mugnai G. Continuous monitoring after second-generation cryoballoon ablation for paroxysmal atrial fibrillation in patients with cardiac implantable electronic devices. Heart Rhythm. 2019;16:187–196. doi: 10.1016/j.hrthm.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Coulombe N., Paulin J., Su W. Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2013;24:919–925. doi: 10.1111/jce.12157. [DOI] [PubMed] [Google Scholar]
- 19.Ciconte G., Mugnai G., Sieira J., Velagic V., Saitoh Y., Irfan G. On the quest for the best freeze: predictors of late pulmonary vein reconnections after second-generation cryoballoon ablation. Circ Arrhythmia Electrophysiol. 2015;8:1359–1365. doi: 10.1161/CIRCEP.115.002966. [DOI] [PubMed] [Google Scholar]
- 20.Bartoletti S., Mann M., Gupta A., Khan A.M., Sahni A., El-Kadri M. Same-day discharge in selected patients undergoing atrial fibrillation ablation. PACE - Pacing Clin Electrophysiol. 2019;42:1448–1455. doi: 10.1111/pace.13807. [DOI] [PubMed] [Google Scholar]
- 21.Reddy S.A., Nethercott S.L., Chattopadhyay R., Heck P.M., Virdee M.S. Safety, feasibility and economic impact of same-day discharge following atrial fibrillation ablation. Heart Lung Circ. 2020 doi: 10.1016/j.hlc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Deyell M.W., Leather R.A., Macle L., Forman J., Khairy P., Zhang R. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020 doi: 10.1016/j.jacep.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Chun K.R.J., Okumura K., Scazzuso F., Keun On Y., Kueffer F.J., Braegelmann K.M. Safety and efficacy of cryoballoon ablation for the treatment of paroxysmal and persistent AF in a real-world global setting: results from the Cryo AF Global Registry. J Arrhythmia. 2021;37:356–367. doi: 10.1002/joa3.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawhney V., Schilling R.J., Providencia R., Cadd M., Perera D., Chatha S. Cryoablation for persistent and longstanding persistent atrial fibrillation: results from a multicentre European registry. Europace. 2020;22:375–381. doi: 10.1093/europace/euz313. [DOI] [PubMed] [Google Scholar]
- 25.Mapping catheter Achieve mapping catheter 20mm. https://my.supplychain.nhs.uk/Catalogue/product/fvi2269 (accessed 20 Jul2020)
- 26.Steinbeck G., Sinner M.F., Lutz M., Müller-Nurasyid M., Kääb S., Reinecke H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: a nationwide in-hospital analysis of administrative data for Germany in 2014. Eur Heart J. 2018;39:4020–4029. doi: 10.1093/eurheartj/ehy452. [DOI] [PMC free article] [PubMed] [Google Scholar]