Abstract
Background
Acute-on-chronic liver failure (ACLF) is a syndrome of acute portal hypertension with high short-term mortality. ACLF patients have low mean arterial pressure (MAP), systemic vascular resistance, and high cardiac output. This, in turn, leads to an increased incidence of ascites, acute kidney injury, and hyponatremia. We evaluated the role of the early addition of midodrine, which has not been analyzed to date.
Methods
ACLF patients who were started on midodrine (Gr. A) in addition to standard of care (SOC) for ascites control were included and compared with those who received only SOC (Gr. B). The aim was to assess the hemodynamics, ascites control, diuretic-related complications, and mortality at 1 month.
Results
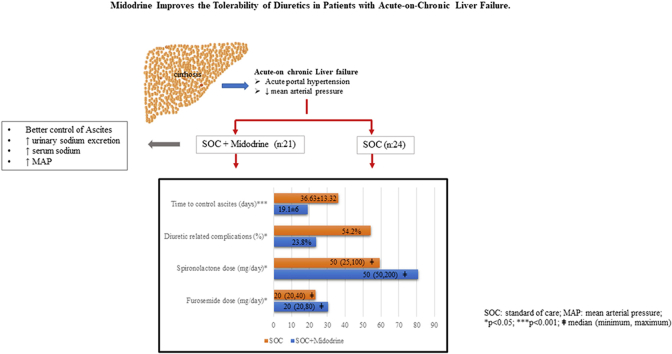
Forty-five ACLF patients (Gr. A-21; Gr. B-24) were included in the pilot study. At inclusion, the baseline characteristics were similar among the groups. The dose of midodrine was 22.5 (7.5–22.5) mg/day for 22.29 ± 8.75 days in Gr. A. Midodrine significantly improved the MAP and urinary sodium excretion. Only 33.34% of patients required paracentesis in Gr. A compared with 62.5% in Gr. B (p = 0.05). Gr. A patients tolerated a higher dose of diuretics than Gr. B. Diuretic-related complications developed in 54.2% of patients in Gr. B compared with only 23.8% in Gr. A (p = 0.03). Fourteen percent in Gr. A developed side effects to midodrine and required dose modification. Mortality at day 30 was similar in both groups.
Conclusion
Addition of midodrine improves the hemodynamics, tolerability of diuretics, and ascites control in ACLF patients.
Keywords: portal hypertension, mean arterial pressure, ascites, variceal bleed, ACLF
Abbreviations: ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; AVB, acute variceal bleed; MAP, mean arterial pressure; SOC, standard of care
Graphical abstract
Please see accompanying editorial on page 528 in this issue.
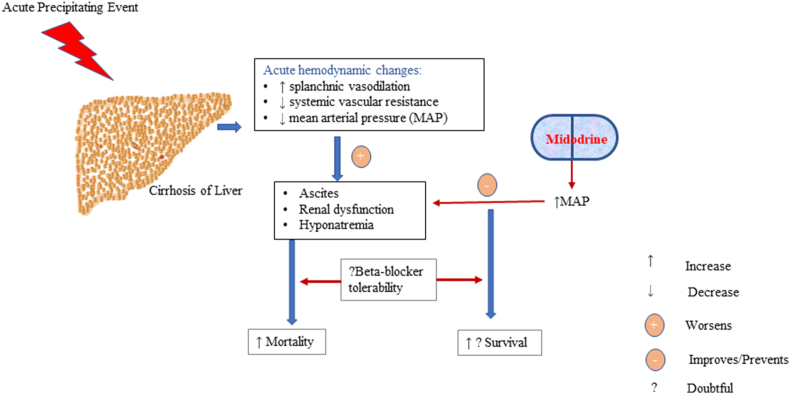
Acute-on-chronic liver failure (ACLF) is a syndrome of acute portal hypertension associated with organ dysfunction and high short-term mortality.1 ACLF patients have lower mean arterial pressure (MAP), systemic vascular resistance, and higher cardiac output compared with cirrhotics, which may lead to ascites worsening, acute kidney injury (AKI), and hyponatremia.2,3 Development of ascites represents a state of acute portal hypertension in patients with ACLF. Highly activated stellate cells, cytokine storm, and ongoing hepatic parenchymal necrosis perpetuate the portal hypertension syndrome.1 Development of ascites portends a poor prognosis in ACLF patients.4 The mortality is similar whether patient presents with ascites or hepatic encephalopathy in ACLF.4 The presence of ascites also increases the risk of infections. Midodrine, an α1 agonist, increases systemic vascular resistance, MAP, renal blood flow, and thereby aids in ascites control without any harmful effects in cirrhotic patients.5 Midodrine has been demonstrated to be beneficial in cirrhosis patients with refractory ascites.5 Diuretic-induced complications develop in 8–20% of cirrhosis patients treated with a combination loop diuretic and anti-mineralocorticoid.6,7 However, there are no reports on the tolerability of diuretics and use of midodrine in ACLF patients. We hypothesized that ACLF patients with low MAP might benefit from the addition of midodrine (Figure 1: concept diagram).
Figure 1.
Acute hemodynamic alterations in acute-on-chronic liver failure are splanchnic vasodilation, reduced systemic vascular resistance, and mean arterial pressure, which lead to worsening of ascites, renal dysfunction, and dyselectrolytemia. Addition of midodrine in the early phase of diagnosis improves hemodynamics and improves ascites control and diuretic tolerability. Further trials on the combination of midodrine and beta-blocker may be evaluated in a larger sample size, which may have a mortality benefit.
Aims
The aim was to assess the time taken to control ascites (1-grade improvement and/or clearance of ascites with 10% reduction in body weight and waist circumference),8 number of patients requiring paracentesis at 1 month, diuretic-related complications at 1 month, mortality at 1 month and the change in hemodynamics at 1 month.
Methods
The study is a retrospective analysis (pilot study) of the prospectively collected data from our tertiary liver care center. The study was approved by the ethics committee vide letter number AIG/IEC: Post BH&R 03/05.2020–02 on 5 May 2020.
Group A consisted of ACLF patients who were started on midodrine with standard of care (SOC), and group B consisted of age-matched randomly selected ACLF patients on SOC for ascites control (after AKI resolution) from January 2019 to December 2019. Salt restriction of 5 g/day with intravenous albumin infusions 20 g/week and diuretics as tolerated is the SOC in our center for ACLF patients. A Fixed-dose combination of furosemide 20 mg plus spironolactone 50 mg/day is started initially, and every 7th day the dose of diuretics is increased if there are no adverse events. Patients were started on oral midodrine 7.5 mg thrice daily, and the dose was downscaled if patients were intolerant. The reason for the change in the dose of midodrine was also recorded. Baseline demographic, clinical, and biochemical data were collected. At each paracentesis, 8 g/L of albumin was infused for each liter above 5 L of ascites was tapped.9 Patients with creatinine >1.5 mg/dl who were started on midodrine therapy were excluded. The data was accessible to all the authors of the study.
Definitions: ACLF: ACLF is an acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dl) and coagulopathy (INR ≥ 1.5) complicated within 4 weeks by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis and is associated with high 28-day mortality.1
Ascites control was defined as a 1-grade improvement on ultrasonography and/or clearance of ascites with a 10% reduction in body weight. Diuretic-related complications were defined as per EASL criteria.9 Diuretic-induced hepatic encephalopathy: development of encephalopathy in the absence of any other precipitating factor. An increase in serum creatinine by >100% to a value >2 mg/dl in patients with ascites responding to treatment was considered to be diuretic-induced renal injury. A decrease in serum sodium by >10 mmol/L to serum sodium of ≤125 mmol/L was considered diuretic-induced hyponatremia.
Statistical methods
The data were analyzed using SPSS 25.0. Descriptive statistics have been expressed as mean (±standard deviation) and/or median (range) for continuous data as and when applicable. For categorical data, we have expressed the results as number (n,%). We used Student's t-test to compare the means between the two groups. We used paired sample t-test for comparing the change in mean from enrollment (baseline) to end of the study (i.e., 1 month). We used Pearson's Chi-square test or Fisher's exact test for comparing categorical. We considered p < 0.05 as significant.
Results
Forty-five ACLF patients (Gr. A-21; Gr. B-24) were included for analysis. Baseline characteristics were similar among the groups (Table 1). Three in Gr. A and four in Gr. B had presented with acute variceal bleed (AVB). Prophylactic variceal ligation was done in another nine patients who had high-risk esophageal varices. None of the patients received beta-blockers due to low MAP. The median dose of midodrine was 22.5 (7.5–22.5) mg/day for an average duration of 22.29 ± 8.75 days in Gr. A. Fourteen percent of patients in Gr. A developed side effects (anxiety in one and urinary retention in two patients) and required midodrine dose modification.
Table 1.
Baseline Characteristics of the Included Patients.
| Variables | Midodrine + SOC (n = 21) | SOC (n = 24) | p |
|---|---|---|---|
| Acute event (alcohol/HAV/sepsis/HBV/unknown) (n) | 12/1/8/0/0 | 13/3/6/1/1 | 0.53 |
| Chronic underlying disease (alcohol/NASH/HBV) (n) | 17/2/2 | 20/3/1 | 0.74 |
| Age (years) | 44.76 ± 7.32 | 42 ± 9.85 | 0.29 |
| Grade of ascites at the initiation of therapy (Grade 2/3) (n) | 4/17 | 11/13 | 0.057 |
| Time to midodrine addition after AKI resolution (in days)ǂ | 3 (1,6) | ||
| Endoscopy: no varices/small varices/large varices | 4/10/7 | 5/11/8 | 0.98 |
| Pedal edema (Yes) | 15 (55.6%) | 12 (44.4%) | 0.14 |
| Heart rate (/minute) | 90.52 ± 8.98 | 89.67 ± 7.68 | 0.73 |
| SBP (mmHg) | 92.67 ± 7.54 | 94.92 ± 5.37 | 0.25 |
| DBP (mmHg) | 60.76 ± 3.71 | 62.71 ± 5.37 | 0.17 |
| MAP (mmHg) | 71.39 ± 3.91 | 73.45 ± 4.72 | 0.12 |
| Total leukocyte counts (×103 per cm) | 10.1 ± 5.81 | 9.67 ± 3.73 | 0.76 |
| Total bilirubin (mg/dl) | 17.6 ± 10.68 | 17.54 ± 10.76 | 0.98 |
| Serum albumin (g/dL) | 2.64 ± 0.35 | 2.8 ± 0.44 | 0.21 |
| Serum creatinine (mg/dL) | 1.24 ± 0.4 | 1.18 ± 0.37 | 0.63 |
| Serum sodium (meq/dL) | 131.24 ± 4.67 | 132.58 ± 6.03 | 0.41 |
| Urinary sodium (mmol/day) | 41.35 ± 18.24 | 41.21 ± 17.49 | 0.98 |
| Bodyweight (kg) | 78.52 ± 11.73 | 74.02 ± 9.56 | 0.16 |
| INR | 2 ± 0.47 | 2.15 ± 0.47 | 0.28 |
| MELD NA | 28.48 ± 5.47 | 28.46 ± 4.06 | 0.99 |
| CLIF-SOFA | 8.9 ± 1.37 | 9.08 ± 1.41 | 0.67 |
| CLIF-C ACLF | 51.29 ± 6.82 | 51.67 ± 9.66 | 0.88 |
| AARC score | 8.1 ± 1.6 | 8.38 ± 1.71 | 0.57 |
All the data have been presented as mean ± standard deviation except ǂ which is in median (minimum, maximum).
SOC, standard of care; HAV, hepatitis A virus; HBV, hepatitis B virus; NASH, nonalcoholic steatohepatitis; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; INR, international normalized ratio; AARC, APASL ACLF Research Consortium; MELD, model for end-stage liver disease; Na, sodium; CTP, Child–Turcotte–Pugh, CLIF-SOFA score, chronic liver failure-sequential organ failure assessment; CLIF-C ACLF score, chronic liver failure-acute on chronic liver failure score.
Control of ascites and number of paracentesis
The time taken to control ascites was shorter in Gr. A (19.1 ± 5.99 days) compared with Gr. B (36.63 ± 13.32 days) (p < 0.001). Only 7 (33.34%) patients required paracentesis at 1 month in Gr. A compared with 15 (62.5%) in Gr. B (p = 0.051) at 1-month. The median number of taps was lower in Gr. A [0 (0, 2)] than Gr. B [1 (0,3) p = 0.04]. The mean (SEM) volume of ascites removed was 1.7 ± 0.57 L in Gr. A vs. 3.74 ± 0.8 L in Gr. B (p = 0.04) at 1 month.
Diuretic-related complications at 1 month
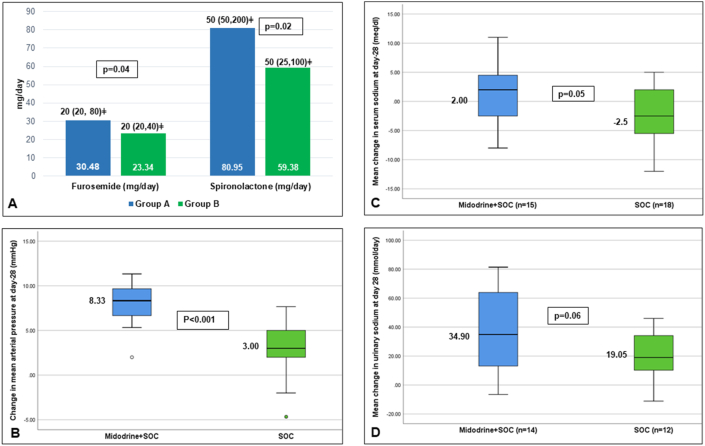
Patients in Gr. A tolerated higher dose of diuretics compared with Gr. B [Gr. A- Furosemide-20 (20, 80) mg/day; Spironolactone-50 (50–200) mg/day; Gr. B-Furosemide-20 (20–40) mg/day, p = 0.04; Spironolactone-50 (25–100) mg/day, p = 0.02] (Figure 2A). (Table 2) Diuretic-related complications developed in 54.2% of patients in Gr. B compared with only 23.8% in Gr. A (p = 0.03) at 1 month. In Gr. A, four patients developed AKI, and one patient developed AKI and hyperkalemia with hepatic encephalopathy. While in Gr. B, seven patients developed AKI, four developed hyponatremia, and two patients developed AKI and hyponatremia (one of whom also had hyperkalemia) (Table 3).
Figure 2.
A) Mean dose of furosemide and spironolactone (mg/day) in group A and B. B) Mean increase in mean arterial pressure (mmHg) in group A and B. C) Mean change in serum sodium (meq/dl) at 1 month D) Mean change in urinary sodium (mmol/day) at 1 month.
Table 2.
Therapy Received During the Period.
| Drugs | Gr. A | Gr. B | P-value |
|---|---|---|---|
| Furosemide (mg/day) | 20 (20,80)ǂ | 20 (20–40)ǂ | 0.04 |
| Spironolactone (mg/day) | 50 (50–200)ǂ | 50 (25–100)ǂ | 0.02′ |
| Beta-blocker | – | – | – |
| Albumin (g/month) | 85.71 ± 16.9 | 87.5 ± 24.18 | 0.77 |
ǂIs in median (minimum, maximum).
Table 3.
Clinical and Biochemical Variables at Baseline and 1 Month.
| Variables | Group A (n = 15) |
P-value | Group B (n = 18) |
P-value | P-value for difference between Gr. A and B at 1 month | ||
|---|---|---|---|---|---|---|---|
| Baseline | 1 month | Baseline | 1 month | ||||
| Heart rate (/minute) | 90.06 ± 10.01 | 88.06 ± 7.05 | 0.1 | 89.33 ± 7.89 | 88.12 ± 6.67 | 0.16 | 0.58 |
| MAP (mmHg) | 71.47 ± 4.14 | 79.46 ± 4.47 | <0.001 | 74.52 ± 4.77 | 77.33 ± 3.44 | 0.002 | <0.001 |
| Body weight (kg) | 78.36 ± 12.67 | 72.52 ± 12.05 | <0.001 | 74.08 ± 10.51 | 70.03 ± 10.49 | <0.001 | 0.08 |
| Urinary sodium (mmol/day) | 41.35 ± 18.24 | 80.52 ± 30.49 | <0.001 | 41.21 ± 17.49 | 61.54 ± 21.4 | 0.001 | 0.06 |
| Urine output (ml/day) | 1085 ± 107.47 | 1172.85 ± 181 | 0.058 | 1020.83 ± 126.88 | 1092.5 ± 173.84 | 0.07 | 0.77 |
| Serum creatinine (meq/dl) | 1.21 ± 0.44 | 1.13 ± 0.23 | 0.48 | 1.16 ± 0.4 | 1.22 ± 0.36 | 0.44 | 0.29 |
| Serum sodium (meq/dl) | 131.6 ± 5.22 | 132.8 ± 2.45 | 0.43 | 134.44 ± 4.64 | 131.91 ± 1.78 | 0.04 | 0.05 |
All the data are presented as mean ± standard deviation.
Group A—Midodrine + standard of care; Group B—Standard of care only; MAP—mean arterial pressure.
Mortality at day 30
A total of 12 patients expired at day 30 in the whole cohort. Mortality at day 30 was similar in both the groups (Gr. A-28.57%, Gr. B-25%; p = 0.85). Patients who had presented with AVB (3 in Gr. A and 4 in Gr. B) had 100% mortality at 4 weeks. Of these seven patients, four patients also had proven bacterial infection. Another five patients (3 in Gr. A and 2 patients in Gr. B) developed sepsis with multiorgan failure and succumbed at day 30. In total, nine patients had sepsis. The source of sepsis was pneumonia in 55.56% patients, spontaneous bacterial peritonitis in 22.22%, and urinary tract infection in 22.22%.
Hemodynamics and laboratory changes at day 28
The addition of midodrine significantly increased the MAP without any change in the heart rate (Table 4). There was an increase in MAP in Gr. B (2.81 ± 3.24) as well but less compared with Gr. A (8 ± 2.41) (Figure 2B). There was a significant increase in urine output in Gr. A than Gr. B, but the change was not statistically significant when compared among the groups. Further, there was a significant decrease in serum sodium in Gr. B, but not in Gr. A at day 28 (Figure 2C). The mean increase in urine sodium was significantly more in Gr. A than Gr. B (p = 0.06) (Figure 2D). Weight loss was more in Gr. A patients than Gr. B at 1 month (Table 3).
Table 4.
Number of Patients with Diuretic-Induced Complications in Group A and B.
| Group A | Group B | p-value | |
|---|---|---|---|
| Total number of patients with diuretic-related complications (n) | 5 | 13 | 0.03 |
| AKI (n) | 5¶ | 9#¥ | 0.32 |
| Hyponatremia (n) | 0 | 6#¥ | 0.01 |
| Hyperkalaemia (n) | 1¶ | 1# | 0.46 |
| Hypokalaemia (n) | 0 | 0 | |
| Hepatic encephalopathy (n) | 1¶ | 0 |
¶1 patient had concomitant AKI, hyperkalemia, and hepatic encephalopathy in group A. #1 patient had concomitant AKI, hyponatremia, and hyperkalemia, and ¥another patient had AKI and hyponatremia in group B.
Group A—Midodrine + standard of care; Group B—Standard of care only.
AKI—acute kidney injury.
ǂMedian (minimum, maximum).
Discussion
This distinctive study is the first to report the use of midodrine in ACLF patients. Midodrine for short duration improved the ascites control and diuretic tolerability by increasing the MAP and urinary sodium excretion. Midodrine for 7 days has been shown to raise the urinary sodium excretion and improve the systemic hemodynamics in patients with/without ascites.10 Midodrine is used in hepatorenal syndrome (with octreotide), refractory ascites, and hepatic hydrothorax and for the prevention of paracentesis-induced circulatory dysfunction.5,11, 12, 13 However, midodrine has not been evaluated in ACLF patients.
The tolerability of diuretics is poorer in ACLF patients, which can be improved by the addition of midodrine. ACLF patients have circulatory dysfunction due to acute worsening of portal hypertension, a rapid rise in intrahepatic resistance, cardiac dysfunction, reduced systemic vascular resistance, and an increase in serum bile acids, which may predispose ACLF patients to kidney injury and dyselectrolytemia.2
Previously, two studies have evaluated midodrine in refractory ascites.5,14 Singh et al. demonstrated that midodrine at a fixed dose of 7.5 mg thrice daily significantly increased the MAP, decreased the plasma renin activity with better control of ascites after 1 month in 40 cirrhosis patients with refractory/recurrent ascites. Alcohol was the most common cause of cirrhosis, and the mean MELD was 14. There was a significant decrease in the incidence of hyponatremia in the midodrine group, and midodrine significantly prolonged survival. However, the study did not mention the maximum dose of diuretics tolerated after the addition of midodrine.
In another study of 78 cirrhosis patients, midodrine at a dose of 12.5 ± 2.5 mg/day (based on tolerability) improved the MAP, increased the urinary volume, and led to better control of ascites at 1 month. Viral hepatitis was the predominant cause of cirrhosis, and the mean MELD score was 15. The time to addition of midodrine was 4 years after the diagnosis of cirrhosis. The mean dose of loop diuretics and anti-mineralocorticoid was similar in both the control and midodrine group.
While in our study, we recruited newly diagnosed ACLF patients satisfying the APASL criteria with a mean MELD NA score of 28. Midodrine at a dose of 22.5 (7.5–22.5) mg/day for a mean duration of 22.29 ± 8.75 days improved the MAP, urine output, diuretic tolerability and thereby aided in better control of ascites. A lesser number of patients developed diuretic-induced complications with the addition of midodrine.
However, there was no significant mortality benefit due to sicker group patients in both the groups (MELD NA 28), small sample size, and shorter duration of follow-up. And beta-blockers could not be added due to the low MAP.15 Whether midodrine led to a sudden rise in MAP and variceal rupture is largely speculative. However, the cause of mortality was similar in both groups.
ACLF patients presenting with AVB have high mortality.15 There is a lack of data on esophageal variceal classification and management in ACLF patients. Beta-blockers are safe and effective in lower grades of ACLF; however, whether midodrine with non-selective beta-blockers improves mortality in ACLF patients with low MAP needs evaluation.15,16
Our study's major limitations are the retrospective analysis and small sample size, which may lead to bias in selection and type 1 error. The second limitation is the short duration of follow-up. We did not assess the plasma renin activity, norepinephrine levels, or pro-BNP levels, which would have made the study more robust. However, midodrine for a short duration is safe and effective in ACLF patients. Midodrine addition in the early stage of acute portal hypertension improves the hemodynamics, tolerability of diuretics, and ascites control. This proof of concept study's promising results need to be validated in large randomized controlled trials with hemodynamic assessments such as right heart catheterization and hepatic venous pressure gradient measurement.
CRediT authorship contribution statement
Anand V. Kulkarni: Conceptualization, Software, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Pramod Kumar: Methodology, Writing - original draft. Mithun Sharma: Writing - review & editing. Sowmya T. Ravikumar: Data curation, Investigation. Harshvardhan Tevethia: Formal analysis, Writing - review & editing. Samragni Vasireddy: Data curation, Investigation. Duvvuru N. Reddy: Project administration, Resources, Validation, Visualization. Padaki N. Rao: Conceptualization, Resources, Project administration, Supervision.
Conflicts of interest
The authors have none to declare.
Acknowledgments
None.
Financial disclosures
None.
Grant support
None.
Ethics approval
Yes.
References
- 1.Sarin S.K., Choudhury A., Sharma M.K. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport A., Sheikh M.F., Lamb E., Agarwal B., Jalan R. Acute kidney injury in acute-on-chronic liver failure: where does hepatorenal syndrome fit? Kidney Int. 2017;92:1058–1070. doi: 10.1016/j.kint.2017.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A., Das K., Sharma P., Mehta V., Sharma B.C., Sarin S.K. Hemodynamic studies in acute-on-chronic liver failure. Dig Dis Sci. 2009;54:869–878. doi: 10.1007/s10620-008-0421-9. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni A.V.R.P., Reddy B., Choudhury A.K., Sarin S.K. Ascites or encephalopathy in acute-on-chronic liver failure—which is more ominous? Hepatology International. APASL. 2020;14:S392. [Google Scholar]
- 5.Singh V., Dhungana S.P., Singh B. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol. 2012;56:348–354. doi: 10.1016/j.jhep.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Angeli P., Fasolato S., Mazza E. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98–104. doi: 10.1136/gut.2008.176495. [DOI] [PubMed] [Google Scholar]
- 7.Santos J., Planas R., Pardo A. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192. doi: 10.1016/s0168-8278(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 8.Fimiani B., Guardia D.D., Puoti C. The use of terlipressin in cirrhotic patients with refractory ascites and normal renal function: a multicentric study. Eur J Intern Med. 2011;22:587–590. doi: 10.1016/j.ejim.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 9.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Kalambokis G., Fotopoulos A., Economou M., Pappas K., Tsianos E.V. Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites. J Hepatol. 2007;46:213–221. doi: 10.1016/j.jhep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni A.V., Kumar P., Singh S. Prevention of paracentesis induced circulatory dysfunction-A systematic review and network meta-analysis. GastroHep. 2020;(2):92–101. doi: 10.1002/ygh2.395. [DOI] [Google Scholar]
- 12.Angeli P., Volpin R., Gerunda G. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–1697. doi: 10.1002/hep.510290629. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni A.V., Sharma M., Kumar P., Gupta R., Rao P.N. Midodrine for hepatic hydrothorax. Hepatology. 2020 doi: 10.1002/hep.31513. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Obiedallah A.A., Abdelmohsen E., Kelani A.I., Mousa M. Effect of midodrine in patients with liver cirrhosis and refractory ascites. Am J Intern Med. 2017;5:12–17. [Google Scholar]
- 15.Kumar M., Kainth S., Choudhury A. Treatment with carvedilol improves survival of patients with acute-on-chronic liver failure: a randomized controlled trial. Hepatol Int. 2019;13:800–813. doi: 10.1007/s12072-019-09986-9. [DOI] [PubMed] [Google Scholar]
- 16.Mookerjee R.P., Pavesi M., Thomsen K.L. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64:574–582. doi: 10.1016/j.jhep.2015.10.018. [DOI] [PubMed] [Google Scholar]