Key Points
Question
What is the association of human papillomavirus (HPV) vaccination with future oropharynx cancer (OPC) incidence in the US?
Findings
This population-based age-period-cohort analysis estimates that HPV vaccination will have a modest association with OPC incidence in the next 25 years, with incidence reductions exclusively among young and middle-aged adults who are at lowest risk of OPC. Increasing incidence trends among those 70 years or older will remain unabated through 2045.
Meaning
These results suggest that it will take more than 25 additional years to slow an increasing incidence of OPC by current HPV vaccination rates because most disease will be among older individuals who have not yet been vaccinated.
Abstract
Importance
Oropharynx cancer (OPC) incidence has increased for several decades in the US. It is unclear when and how this trend will be affected by current HPV vaccination trends.
Objective
To assess the association of HPV vaccination with future OPC incidence in the US.
Design, Setting, and Participants
This population-based age-period-cohort analysis obtained OPC incidence data from the Surveillance, Epidemiology, and End Results program from 69 562 patients 34 to 83 years of age diagnosed with OPC. The HPV vaccination data were obtained from the National Immunization Survey–Teen (60 124 participants) and National Health Interview Survey (16 904 participants). Data were collected from January 1, 1992, to December 31, 2017. Age-period-cohort forecasting models projected expected 2018 to 2045 OPC incidence under a counterfactual scenario of no HPV vaccination and current levels of HPV vaccination, stratifying by sex. Data analyses were completed by December 2020.
Exposures
Age- and sex-specific cumulative prevalence of HPV vaccination in 2016 to 2017 projected forward.
Main Outcomes and Measures
Projected OPC incidence and number of OPC cases expected to be prevented by HPV vaccination.
Results
Under current HPV vaccination rates, between 2018 and 2045, OPC incidence is projected to decrease in younger individuals (36-45 years of age: from 1.4 to 0.8 per 100 000 population; 46-55 years of age: from 8.7 to 7.2 per 100 000 population) but continue to increase among older individuals (70-83 years of age: from 16.8 to 29.0 per 100 000 population). The association of HPV vaccination with overall OPC incidence through 2045 will remain modest (no vaccination vs vaccination: 14.3 vs 13.8 per 100 000 population in 2045). By 2045 HPV vaccination is projected to reduce OPC incidence among individuals 36 to 45 years of age (men: 48.1%; women: 42.5%) and 46 to 55 years of age (men: 9.0%; women: 22.6%), but among those 56 years or older, rates are not meaningfully reduced. Between 2018 and 2045, a total of 6334 OPC cases will be prevented by HPV vaccination, of which 88.8% of such cases occur in younger age (≤55 years) groups.
Conclusions and Relevance
According to the projections of this population-based age-period-cohort study, current HPV vaccination rates will have a limited association with overall OPC incidence through 2045 because older individuals who have not yet been vaccinated remain at high risk for OPC. However, reductions in OPC incidence should occur among young and middle-aged adults, the group at lowest risk of diagnosis. These findings forecast a continued shift in the landscape of OPC to an older population.
This age-period-cohort analysis examines whether human papillomavirus vaccination will alter the incidence of oropharynx cancer through 2045.
Introduction
Oropharynx cancer (OPC) incidence has been steadily increasing for the past several decades and is now the most common cancer caused by human papillomavirus (HPV) in the US.1,2,3 This increase has been observed among men and women of diverse ages and is most notable among those born in 1939 to 1955.1,4 Vaccination against HPV has been reported to effectively protect against new anogenital HPV infection and related malignant tumors5,6 and more recently to reduce prevalent oral HPV infection,7,8,9 the precursor to HPV-related OPC. Although the HPV vaccine was first licensed for cervical cancer prevention in 2006, only recently, in June 2020, did the US Food and Drug Administration expand the indication for HPV 9-valent vaccine for the prevention of OPC and other head and neck cancers caused by HPV.10 Because HPV vaccination is targeted to those 11 to 12 years of age, decades before the median age of OPC diagnosis (men: 61 years; women: 63 years11), the impact of HPV vaccination efforts on OPC incidence (as with other HPV-related cancers) will take time to be observed.9,12 Although recent modeling studies13,14 suggest substantial reductions in cervical cancer incidence will be achieved in the US in the next 2 decades, it is currently unclear when and how the incidence trends of OPC will be impacted by current HPV vaccination trends.
Forecasting the impact of HPV vaccination efforts is important to evaluate the projected timeline for OPC reduction and to inform cancer control efforts. We examined the magnitude and timing of the incidence reduction of OPC expected during the next 25 years in the US from current HPV vaccination rates (overall and by sex and age groups), using established age-period-cohort (APC) forecasting methods.
Methods
Data Sources
Multiple nationally representative data were used. The HPV vaccination data came from 2 sources. The National Immunization Survey–Teen is a cross-sectional telephone survey that provides practitioner-verified vaccination data for individuals 13 to 17 years of age.15 The National Health Interview Survey, a cross-sectional household interview, provides self-reported vaccination data for those 18 years or older.16 Data from January 1, 2016, to December 31, 2018, were used to quantify cumulative vaccination rates, age of HPV vaccination initiation, and annual trend in vaccine uptake by age and sex.
The OPC incidence data for 1992 to 2017 came from the Surveillance, Epidemiology, and End Results (SEER) program: SEER 13 registries for 1992 to 1999, which cover 13% of the total US population, and SEER 18 registries for 2000 to 2017, which cover 28% of the total US population.17 Sex-stratified data were obtained by 2-year age groupings (34-35 years, 36-37 years, and so on until 82-83 years) and 2-year calendar periods (1992-1993, 1994-1995, and so on until 2016-2017) using SEER*Stat, version 8.3.6.18,19
Individuals with a diagnosis of oropharyngeal squamous cell carcinoma1 were identified using International Classification of Diseases for Oncology, Third Edition (ICD-O-3) (ICD-O-3 histology codes: 8050-8076, 8078, 8083-8084, and 8094), including base of tongue, lingual tonsil, soft palate, uvula, tonsil, oropharynx, and Waldeyer ring (ICD-O-3 topography codes: C019, C024, C051-C052, C090-C099, C100-C109, and C142).1
The proportion of OPC cases that are HPV-related was estimated by sex, using tumor HPV testing data from the SEER 18 registries custom data for head and neck in 2014 to 2016.20 Details of tumor HPV testing data were previously reported.21 Calculations were restricted to cases with known HPV status during this period (65%). The projected number of US men and women in 2018 to 2045 were obtained from the US Census Bureau.22 Because of its use of publicly available, deidentified data, this study was exempt from institutional review board approval.
Statistical Analysis
This was a population-based APC analysis using data from multiple sources over time. Primary analyses were sex stratified, and estimates were age standardized to the 2020 US population. Sex-specific estimates were also combined to calculate sex- and age-standardized rates in the total population.
Estimating Cumulative HPV Vaccination Rates, 2018-2055
Cumulative HPV vaccination rates in 2016 to 2017 (proportion who had ever received ≥1 dose) were derived by 2-year age groups, with appropriate weights to account for complex sampling design of the National Immunization Survey–Teen and the National Health Interview Survey. Calculations were restricted to individuals targeted by the US HPV vaccination program, including women who according to the 2016 to 2017 data were in the 12- to 13-year to 36- to 37-year age groups and men in the 12- to 13-year to 34- to 35-year age groups (because the vaccination started in 2006 for women and 2009 for men) (eFigure 1 in the Supplement). For example, a woman 37 years of age in 2017 was 26 years of age in 2006 and would have been within the recommended window for catch-up HPV vaccination when first licensed and thus included.
Observed vaccination rates were converted into birth cohort–specific rates and then projected from 2018 to 2055 by 2-year calendar periods (eTables 1 and 2 and eFigure 2 in the Supplement). For birth cohorts in the recommended age range for vaccination, vaccination rates were projected to increase annually until they reach the recommended age limit for vaccination (or 18 years of age for National Immunization Survey–Teen birth cohorts) and then remain constant through future periods (ie, conservatively assuming no vaccination after recommended ages targeted). This annual increase rate, adjusted for sex and race/ethnicity, was based on the observed increase in cumulative vaccination rates from 2017 to 2018 among age ranges recommended for HPV vaccination.
Estimating OPC Incidence, 2018-2045
Given the strong birth cohort and age effects,1,2,4,23,24 APC forecasting models were constructed to model OPC incidence, using previously published methods.1,25 The models used incidence rates in 1992 to 2017 to project expected OPC incidence in 2018 to 2045 by 2-year age groups (from 34-35 years to 82-83 years) and 2-year calendar periods. The forecasting methods assumed that previously observed age, period, and cohort effects would continue through 2045.
Estimating Population-Level Association of Current HPV Vaccination Rates With OPC Incidence
We projected future OPC incidence under the counterfactual scenario of no HPV vaccination (ie, assuming that observed contemporary trends of OPC incidence continue) compared with the model that used current levels of HPV vaccination to estimate their future impact. Adults 34 years or older in 2017 had low vaccine uptake (2.7%), so we conservatively assumed no vaccine association with observed OPC in these ages.
To estimate the OPC incidence under current HPV vaccination rates, we adapted the methods developed for cervical cancer by Bruni et al.26 Briefly, the projections of OPC incidence were based on expected OPC incidence assuming no vaccination and then applying vaccination coverage for each birth cohort. Estimates accounted for vaccine effectiveness at differing ages of vaccination. We assumed a 93.3% lifelong vaccine efficacy against oral HPV infection7 and that vaccine efficacy against HPV-positive OPC decreases by age of HPV vaccination initiation (<15 years of age: 93.3%; 15-21 years of age: 66.3%; 22-26 years of age: 35.6%), indicative of influences of age of acquiring causal infection according to a simulation study.27 eFigure 3 in the Supplement shows the age of HPV vaccination initiation for vaccinated birth cohorts. Sex-specific tumor HPV prevalence among OPC were then used to estimate the vaccine effectiveness against overall OPC. The annual number of OPC cases was calculated by multiplying the forecasted OPC rates with the projected age-, sex-, and period-specific US population size.22,25,28 The number of cases averted by HPV vaccination was estimated by comparing the projected number of OPC cases under 2 scenarios (no vaccination vs vaccination).
Additional analyses were performed stratified by race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic any race, and non-Hispanic other race) and restricted to the SEER 13 registries, both yielding similar results. Goodness of fit of the APC models was assessed visually (eFigures 4 and 5 in the Supplement). The dispersion parameter had negligible overdispersion.
Full details on model assumptions and calculation are described in the eMethods in the Supplement. Analyses were conducted using R, version 3.6.3 (R Foundation for Statistical Computing) and SAS University Edition (SAS Institute Inc).
Results
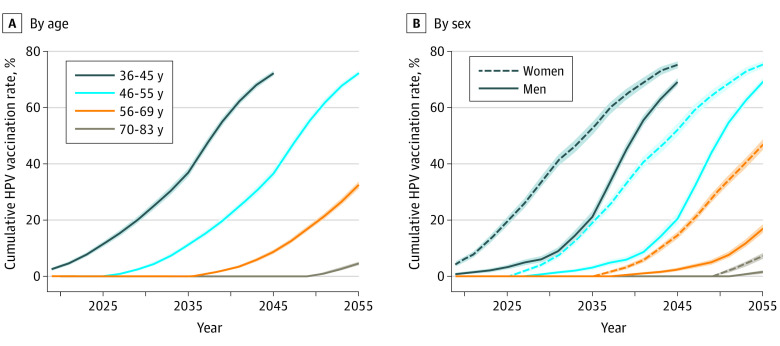
Cumulative HPV vaccination rates among those 36 years or older are projected to increase markedly between 2018 and 2045 as children and young adults who have been vaccinated age into older adulthood (Figure 1A). By 2045, an estimated 72.2% (95% CI, 71.4%-72.9%) of individuals 36 to 45 years will have been vaccinated. The proportion of older individuals who will have had the HPV vaccine by 2045 is lower, estimated at 36.6% (95% CI, 35.2%-37.9%) of those 46 to 55 years of age, 8.7% (95% CI, 8.1%- 9.3%) for those 56 to 69 years of age, and 0% (95% CI, 0%-0%) of those 70 to 83 years of age.
Figure 1. Projected Age-Standardized Proportion of Each Age Group That Will Have Been Vaccinated With at Least 1 Dose of Human Papillomavirus (HPV) Vaccine for 2018 to 2055.
Data are censored for the 36- to 45-year age group after 2045 when birth cohorts 2012 and later start to enter the age group; these birth cohorts were not included in the study population for the analyses. Shaded areas represent 95% CIs for the estimates of cumulative HPV vaccination rates.
The earlier licensing and uptake of HPV vaccine among girls than boys resulted in a consistently higher level of HPV vaccination among women than men of the same age group (Figure 1B). However, these discrepancies will attenuate over time. Among those 36 to 45 years of age, an estimated 19.5% (95% CI, 18.1%-20.9%) of women and 3.3% (95% CI, 2.6%-3.9%) of men will have been vaccinated by 2025, but by 2045, 75.2% (95% CI, 74.1%-76.3%) of women and 69.1% (95% CI, 68.0%-70.2%) of men will have been vaccinated.
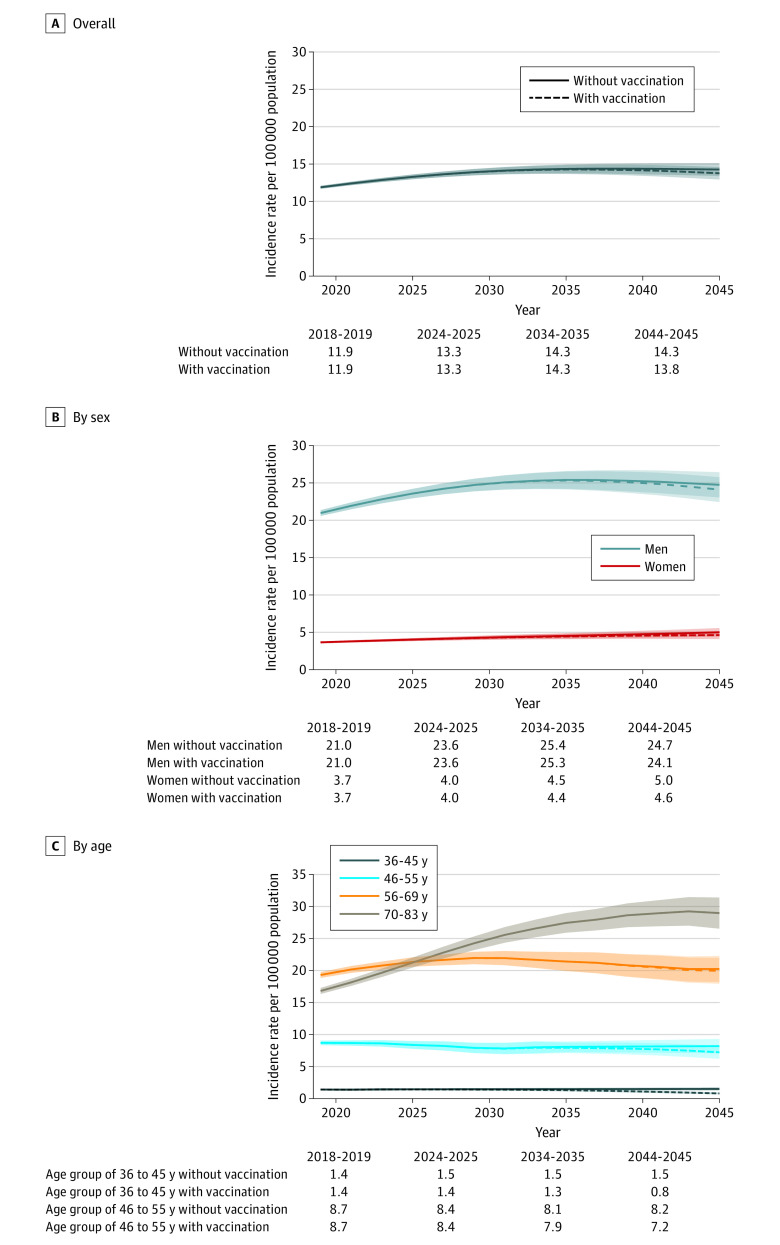
The expected association of these HPV vaccination rates with OPC incidence was next modeled (eTable 3 in the Supplement). Figure 2 shows the forecasted OPC incidence between 2018 and 2045 in the counterfactual scenario of no HPV vaccination compared with the forecasted OPC incidence under current HPV vaccination rates. In the scenario of no vaccination, overall OPC incidence is projected to increase from 11.9 (95% CI, 11.7-12.1) to 14.3 (95% CI, 13.4-15.1) per 100 000 population, with moderate increases projected among both men (from 21.0 to 24.7 per 100 000 population) and women (from 3.7 to 5.0 per 100 000 population).
Figure 2. Projected Age-Standardized Oropharynx Cancer Incidence Rates per 100 000 Population Overall, by Sex, and by Age Group Under Current Human Papillomavirus (HPV) Vaccination Rates Compared With the Scenario of Expected Incidence With No Vaccination for 2018 to 2045.
Solid lines represent the projected age-standardized oropharynx cancer incidence rates in the scenario of no vaccination. Dashed lines represent the projected age-standardized oropharynx incidence rates under current HPV vaccination rates. Shaded areas represent the 95% CIs for the estimates of oropharynx cancer incidence rates.
Under current HPV vaccination rates, overall OPC incidence is also projected to continue to increase between 2018 and 2045 from 11.9 (95% CI, 11.7-12.1) to 13.8 (95% CI, 12.9-14.6) per 100 000 population. This increase is associated with increasing incidence among older individuals in whom HPV vaccination is projected to have minimal (56-69 years of age) or no (≥70 years of age) impact on OPC incidence. Among individuals 70 years or older, OPC incidence is projected to increase from 16.8 (95% CI, 16.3-17.3) to 29.0 (95% CI, 26.5-31.4) per 100 000 population. Of note, OPC incidence is projected to substantially decrease among young adults (36-45 years of age: from 1.4 [95% CI, 1.3-1.6] to 0.8 [95% CI, 0.7-0.9] per 100 000 population) and middle-aged adults (46-55 years of age: from 8.7 [95% CI, 8.4-9.1] to 7.2 [95% CI, 6.2-8.2] per 100 000 population) (Figure 2C). However, given that the bulk of OPC will occur among individuals 56 years or older who are not currently targeted for vaccination (representing 84% of incident OPC cases in 2045), the reduction in overall OPC incidence by HPV vaccination remains modest through 2045 (no vaccination vs vaccination: 14.3 [95% CI, 13.4-15.1] vs 13.8 [95% CI, 12.9-14.6] per 100 000 population in 2045) (Figure 2A).
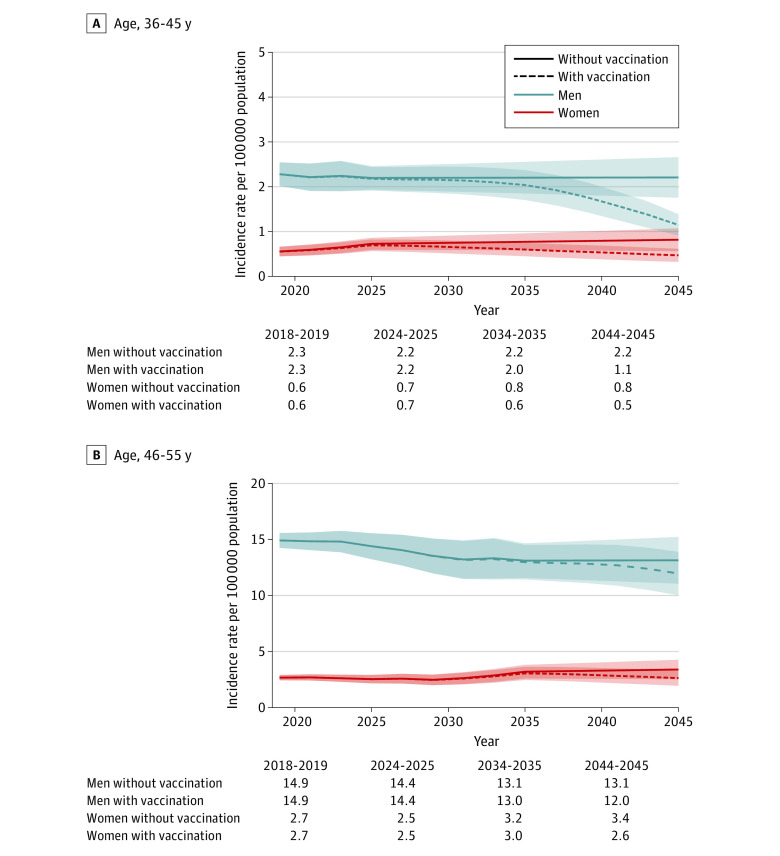
We next projected OPC incidence by age groups separately in men and women (Figure 3 and eTable 3 in the Supplement). Among those 36 to 45 years of age in 2045, HPV vaccination is projected to reduce OPC incidence by 48.1% in men (no vaccination vs vaccination: 2.2 vs 1.1 per 100 000 population) and 42.5% in women (0.8 vs 0.5 per 100 000 population) (eFigure 6 in the Supplement). Among those 46 to 55 years of age, HPV vaccination will reduce OPC incidence from 13.1 to 12.0 per 100 000 population in men, representing a 9.0% reduction, and from 3.4 to 2.6 per 100 000 population in women, representing a 22.6% reduction (eFigure 6 in the Supplement).
Figure 3. Projected Age-Standardized Oropharynx Cancer Incidence Rates per 100 000 Population by Sex in Individuals 36 to 45 Years of Age and 46-55 Years of Age Under Current Human Papillomavirus (HPV) Vaccination Rates Compared With the Scenario of Expected Incidence With No Vaccination for 2018 to 2045.
Solid lines represent the projected age-standardized oropharynx cancer incidence rates in the scenario of no vaccination. Dashed lines represent the projected age-standardized oropharynx incidence rates under current HPV vaccination rates. Shaded areas represent 95% CIs for the estimates of oropharynx cancer incidence rates.
To translate the projected vaccination impact, we calculated the number of OPC cases that would be prevented by current HPV vaccination rates (eTable 4 and eFigure 7 in the Supplement). Between 2018 and 2045, the number of projected incident OPC cases is 736 518 in the scenario of no HPV vaccination, and HPV vaccination is projected to lead to a reduction of only 6334 OPC cases, of which 88.8% are in younger age groups (≤55 years of age). Our projections suggest that beginning in approximately 2033, nearly 100 cases will be prevented each year, and by 2045 this will increase 10-fold to 998 cases prevented. Notably, under current HPV vaccination, despite decreasing incidence among younger ages, the annual number of incident OPC cases overall is projected to continue increasing between 2018 and 2045 from 19 690 to 28 670.
Discussion
Although the OPC incidence among young and middle-aged individuals will start to decrease, the modeling in this study suggests that the association of HPV vaccination with overall OPC incidence is limited in magnitude through 2045. Thereafter, a steadily increasing reduction in new OPC cancers overall is expected as vaccinated individuals reach ages of higher cancer incidence. To our knowledge, this is the first study to estimate the population-level association of HPV vaccination with contemporary OPC incidence in the US.
Vaccination against HPV has the promise of altering the forecasted epidemic of HPV-related OPC; however, previous OPC trend models have not included HPV vaccination.1,2,29 Given that the risk of OPC among younger individuals is low, the reduction in overall OPC incidence from current HPV vaccination will remain negligible through 2045. Vaccinated birth cohorts will first approach the median age at OPC diagnosis (61-63 years of age11,30) in approximately 2060, so we expect to realize a substantial reduction in overall OPC incidence associated with HPV vaccination by then.
These observations are important for several reasons. First, the overall incidence of OPC is expected to continue to increase for the foreseeable future, assuming the increasing incidence trends in older adults continue unabated. This increase, in combination with a reduction in OPC in younger cohorts, will result in an increase in the age at OPC diagnosis. Hence, we forecast a continuing shift in the landscape of OPC to an older population during the next 25 years. Although it has recently been appreciated that HPV-related OPC is no longer a disease of younger patients,30,31,32,33 the trend will be amplified in the future. This finding has several important consequences and treatment implications. Comorbidities of older patients with OPC are high,34,35 which confers significant medical costs and indirect costs attributable to lost productivity and morbidity.36,37,38,39 In addition, older individuals have not been eligible for initial clinical trials of deintensification, yet this may be an ideally suited population for such studies. The priorities of an older patient population differ from younger patients.40,41,42 Clinical trials are warranted to identify treatment paradigms optimized for the expected older patients with OPC with specific consideration to integration of geriatric assessments, goals of care, health-related quality of life, and quality-adjusted survival.43,44,45,46 The survival benefit conferred by HPV-positive tumors appears to be attenuated among older patients with OPC30,31,33; therefore, health-related quality-adjusted survival may be most relevant in this patient population.
Second, before the full impact of the HPV vaccination is achieved, the OPC epidemic will continue to pose a significant health and economic burden. This analysis can inform plans for future allocation of health care and economic resources for the projected burden of OPC in the US. Third, our findings have important prevention implications. Despite recent improvements in HPV vaccine uptake,47,48 current HPV vaccination rates are well below the Healthy People 2020 target of 80% coverage, and a notable portion of those vaccinated in the first 10 years of the program initiated vaccination at 15 years or older. Continued efforts are needed to increase HPV vaccine uptake at younger ages among men and women to achieve population-level vaccine benefits. Our estimates of HPV vaccination impact assumed a 93.3% lifelong vaccine efficacy against oral HPV infection and that vaccination benefits for reducing OPC risk decrease by age of HPV vaccination initiation. Surveillance data suggest comparable effectiveness of HPV vaccine for reducing oral HPV prevalence (88.2%).9 An ongoing clinical trial49 investigating HPV vaccine efficacy against oral HPV persistence in men will provide additional data to refine estimates of the impact of the HPV vaccination program and inform prevention efforts for OPC.
Prior HPV vaccine modeling studies13,50,51 have included some estimates of the aggregate noncervical HPV-related cancer impact and found that it would remain modest, although continue increasing, during the next 3 decades. Our analysis suggests that timing in OPC reductions will be later than that projected for cervical cancer. Under the current recommendation for cervical cancer screening and HPV vaccination, cervical cancer incidence is projected to decrease in the US to 4 cases or fewer per 100 000 women-years by 2038 to 2046, a proposed threshold for cervical cancer elimination.14 The later timing of cancer reduction for OPC than cervical cancer may be because reduction in cervical cancer incidence reflects combined primary (HPV vaccination) and secondary (screening) prevention efforts, whereas there are currently no screening methods for OPC,24,52,53 and premalignant OPC lesions remain to be identified. In addition, the median age at OPC diagnosis is more than 10 years higher than the age of cervical cancer diagnosis11 and has been increasing.30,31,32 Third, the earlier licensing of HPV vaccines for girls and consequent earlier uptake of vaccine among girls than boys 54 might contribute to a delay in vaccination impact among men than women. Indeed, our analyses project that cumulative HPV vaccination rates will be consistently higher among women than men of the same age group between 2018 and 2045.
Limitations
Our study has several limitations. Our estimations of the association of vaccine with OPC incidence represent a simplified realization of the direct vaccine benefits. We did not include the possible protection for unvaccinated individuals from herd immunity within the community, so our estimates of vaccine impact may be conservative. In a study55 of the National Health and Nutrition Examination Survey, a decrease in the prevalence of vaccine-type oral HPV from 2.7% to 1.6% was observed among unvaccinated men between 2009 and 2016, suggesting potential herd immunity. Among young adults where high vaccination rates are now being observed, it is possible we will begin to see an effect of herd immunity; however, because OPC rates are low among those younger than 46 years and vaccination rates among older adults are not at a level for herd immunity, it is unlikely to affect our estimates for the overall OPC incidence.
Mathematical simulation models, such as dynamic transmission models, coupled with microsimulation models have been used to model the vaccination impact and the uncertainty surrounding the predictions in some studies of anogenital HPV and related outcomes.56,57 However, these models rely on accurate parameterization, which cannot be fully achieved in the setting of oral HPV given fewer data on oral HPV transmission and natural history of HPV-related OPC. Second, our projections did not consider any potential interventions other than vaccination that could affect future OPC incidence. Models also assumed that contemporary birth cohort trends, believed to reflect differences in sexual behaviors,58 would continue. This assumption appears logical given that previous studies59,60,61 found no association of HPV vaccination with sexual risk behaviors. Third, we did not consider the number of HPV vaccine doses. However, a single dose of HPV vaccine was found to provide protection against HPV infection for more than a decade, supporting possible efficacy of single-dose HPV vaccination.62 Furthermore, most individuals vaccinated are expected to have completed the vaccination series. Recent national data indicate 76% of teenagers who initiated HPV vaccination have received the recommended vaccine doses.48
Conclusions
These findings forecast a continued shift in the landscape of OPC to an older population. The results of this study suggest that the association of current US HPV vaccination trends with OPC will be modest during the next 25 years because the reduction in OPC incidence associated with HPV vaccination among older adults, who have the highest incidence of disease, will take longer.
eMethods. Supplemental Methods
eTable 1. Observed and Projected Cumulative HPV Vaccination Rates (≥1 Dose) Between 2016-2055 by Age Groups and Calendar Periods for Men
eTable 2. Observed and Projected Cumulative HPV Vaccination Rates (≥1 Dose) Between 2016-2055 by Age Groups and Calendar Periods for Women
eTable 3. Projected Age-Standardized Oropharynx Cancer Incidence Rates per 100,000 Population Between 2018-2045 Under Current HPV Vaccination Rates Compared to the Scenario of Expected Incidence With No Vaccination, Overall, by Sex, by Age Groups, and by Sex and Age Groups
eTable 4. Projected Annual Number of Incident Oropharynx Cancer Cases in 2018, 2025, 2035 and 2045, and the Total Number of Projected Cases Between 2018-2045 Under Current HPV Vaccination Rates Compared to the Scenario of Expected Number of Cases With No Vaccination, Overall, by Sex, by Age Groups, and by Sex and Age Groups
eFigure 1. HPV Advisory Committee on Immunization Practices (ACIP) Vaccine Recommendation Update Timeline
eFigure 2. Relationship Between Age Groups, Calendar Periods and Birth Cohorts Included in the Study
eFigure 3. Proportion of Those Vaccinated Who Initiated HPV Vaccination <15, 15-21, and 22-26 Years of Age, by Birth Cohort and Sex
eFigure 4. Cohort Rate Ratios for Oropharynx Cancer and the Fitted Joinpoint Models Among Men and Women of Birth Cohorts 1910-1982
eFigure 5. Deviance Residuals of Age-Period-Cohort Models for Men and Women
eFigure 6. Relative Reduction of Projected Age-Standardized Oropharynx Cancer Incidence Rates Under Current HPV Vaccination Rates Compared to the Scenario of Expected Incidence With No Vaccination Between 2018-2045, by Sex in Individuals Aged 36-45 Years and 46-55 Years
eFigure 7. Projected Number of Oropharynx Cancers Each Year That Will Be Averted Because of Current HPV Vaccination Rates by Age Groups in Men and Women Between 2018-2045
References
- 1.Tota JE, Best AF, Zumsteg ZS, Gillison ML, Rosenberg PS, Chaturvedi AK. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538-1546. doi: 10.1200/JCO.19.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus-associated cancers—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918-924. doi: 10.15585/mmwr.mm6733a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tota JE, Anderson WF, Coffey C, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973-2012. Oral Oncol. 2017;67:146-152. doi: 10.1016/j.oraloncology.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 5.Petrosky E, Bocchini JA Jr, Hariri S, et al. ; Centers for Disease Control and Prevention (CDC) . Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300-304. [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5):F123-F138. doi: 10.1016/j.vaccine.2012.04.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrero R, Quint W, Hildesheim A, et al. ; CVT Vaccine Group . Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329. doi: 10.1371/journal.pone.0068329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirth JM, Chang M, Resto VA, Guo F, Berenson AB; HPV Study Group . Prevalence of oral human papillomavirus by vaccination status among young adults (18-30 years old). Vaccine. 2017;35(27):3446-3451. doi: 10.1016/j.vaccine.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262-267. doi: 10.1200/JCO.2017.75.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA Approves Merck’s GARDASIL 9 for the Prevention of Certain HPV-Related Head and Neck Cancers. Accessed October 3, 2020. https://www.merck.com/news/fda-approves-mercks-gardasil-9-for-the-prevention-of-certain-hpv-related-head-and-neck-cancers/
- 11.Centers for Disease Control and Prevention (CDC) . HPV-associated cancer diagnosis by age. Accessed November 28, 2020. https://www.cdc.gov/cancer/hpv/statistics/age.htm
- 12.Schmeler KM, Sturgis EM. Expanding the benefits of HPV vaccination to boys and men. Lancet. 2016;387(10030):1798-1799. doi: 10.1016/S0140-6736(16)30314-2 [DOI] [PubMed] [Google Scholar]
- 13.Laprise JF, Chesson HW, Markowitz LE, et al. Effectiveness and cost-effectiveness of human papillomavirus vaccination through age 45 years in the United States. Ann Intern Med. 2020;172(1):22-29. doi: 10.7326/M19-1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger EA, Smith MA, Killen J, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):e213-e222. doi: 10.1016/S2468-2667(20)30006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Immunization Surveys-Teen (NIS-Teen), 2016-2018. Accessed July 2, 2020. https://www.cdc.gov/vaccines/imz-managers/nis/datasets-teen.html
- 16.Centers for Disease Control and Prevention. National Health Interview Survey, 2016-2018. Accessed July 2, 2020. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- 17.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Number of persons by race and Hispanic ethnicity for SEER participants (2010. Census data). Accessed August 13, 2020. https://seer.cancer.gov/registries/data.html
- 18.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER*Stat Software (version 8.3.6). Accessed July 2, 2020. https://seer.cancer.gov/seerstat/
- 19.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER Incidence Data. November 2019.. Accessed July 2, 2020. https://seer.cancer.gov/data/
- 20.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Head and Neck with HPV Status Database. November 2018. Accessed July 2, 2020. https://seer.cancer.gov/seerstat/databases/hpv/index.html
- 21.Mahal BA, Catalano PJ, Haddad RI, et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660-1667. doi: 10.1158/1055-9965.EPI-19-0038 [DOI] [PubMed] [Google Scholar]
- 22.United States Census Bureau. National Population Projections Datasets (2018-2045). 2017.. Accessed July 13, 2020. https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html
- 23.Brouwer AF, Eisenberg MC, Meza R. Age effects and temporal trends in HPV-related and HPV-unrelated oral cancer in the United States: a multistage carcinogenesis modeling analysis. PLoS One. 2016;11(3):e0151098. doi: 10.1371/journal.pone.0151098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235-3242. doi: 10.1200/JCO.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best AF, Haozous EA, de Gonzalez AB, et al. Premature mortality projections in the USA through 2030: a modelling study. Lancet Public Health. 2018;3(8):e374-e384. doi: 10.1016/S2468-2667(18)30114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453-e463. doi: 10.1016/S2214-109X(16)30099-7 [DOI] [PubMed] [Google Scholar]
- 27.Burger EA, Kim JJ, Sy S, Castle PE. Age of acquiring causal human papillomavirus (HPV) infections: leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin Infect Dis. 2017;65(6):893-899. doi: 10.1093/cid/cix475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg PS, Barker KA, Anderson WF. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J Natl Cancer Inst. 2015;107(9):1-7. doi: 10.1093/jnci/djv159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Dahlstrom KR, Lairson DR, Sturgis EM. Projected oropharyngeal carcinoma incidence among middle-aged US men. Head Neck. 2019;41(9):3226-3234. doi: 10.1002/hed.25810 [DOI] [PubMed] [Google Scholar]
- 30.Rettig EM, Zaidi M, Faraji F, et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of Human Papillomavirus is attenuated among older patients: analysis of the National Cancer Database. Oral Oncol. 2018;83(June):147-153. doi: 10.1016/j.oraloncology.2018.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Windon MJ, D’Souza G, Rettig EM, et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer. 2018;124(14):2993-2999. doi: 10.1002/cncr.31385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2(12):1617-1623. doi: 10.1001/jamaoncol.2016.1804 [DOI] [PubMed] [Google Scholar]
- 33.Lu DJ, Luu M, Mita A, et al. Human papillomavirus-associated oropharyngeal cancer among patients aged 70 and older: dramatically increased prevalence and clinical implications. Eur J Cancer. 2018;103:195-204. doi: 10.1016/j.ejca.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 34.Bigelow EO, Blackford AL, Eytan DF, Eisele DW, Fakhry C. Burden of comorbidities is higher among elderly survivors of oropharyngeal cancer compared with controls. Cancer. 2020;126(8):1793-1803. doi: 10.1002/cncr.32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of comorbidities among older head and neck cancer survivors in the United States. Otolaryngol Head Neck Surg. 2019;160(1):85-92. doi: 10.1177/0194599818796163 [DOI] [PubMed] [Google Scholar]
- 36.Jacobson JJ, Epstein JB, Eichmiller FC, et al. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012;4(1):15. doi: 10.1186/1758-3284-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CF, Lairson DR, Dahlstrom KR, et al. Lifetime health care costs of oropharyngeal cancer for commercially insured patients in the United States. Head Neck. 2020;42(9):2321-2329. doi: 10.1002/hed.26201 [DOI] [PubMed] [Google Scholar]
- 38.Lairson DR, Wu CF, Chan W, Dahlstrom KR, Tam S, Sturgis EM. Medical care cost of oropharyngeal cancer among Texas patients. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1443-1449. doi: 10.1158/1055-9965.EPI-17-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollenbeak CS, Kulaylat AN, Mackley H, Koch W, Schaefer EW, Goldenberg D. Determinants of Medicare costs for elderly patients with oral cavity and pharyngeal cancers. JAMA Otolaryngol Head Neck Surg. 2015;141(7):628-635. doi: 10.1001/jamaoto.2015.0940 [DOI] [PubMed] [Google Scholar]
- 40.Naik AD, Martin LA, Moye J, Karel MJ. Health values and treatment goals of older, multimorbid adults facing life-threatening illness. J Am Geriatr Soc. 2016;64(3):625-631. doi: 10.1111/jgs.14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Windon MJ, D’Souza G, Faraji F, et al. Priorities, concerns, and regret among patients with head and neck cancer. Cancer. 2019;125(8):1281-1289. doi: 10.1002/cncr.31920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Windon MJ, Fakhry C, Faraji F, et al. Priorities of human papillomavirus-associated oropharyngeal cancer patients at diagnosis and after treatment. Oral Oncol. 2019;95(June):11-15. doi: 10.1016/j.oraloncology.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szturz P, Vermorken JB. Treatment of elderly patients with squamous cell carcinoma of the head and neck. Front Oncol. 2016;6(AUG):199. doi: 10.3389/fonc.2016.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mady LJ, Nilsen ML, Johnson JT. Head and neck cancer in the elderly: frailty, shared decisions, and avoidance of low value care. Clin Geriatr Med. 2018;34(2):233-244. doi: 10.1016/j.cger.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 45.Klein J, Livergant J, Ringash J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: a systematic review. Oral Oncol. 2014;50(4):254-262. doi: 10.1016/j.oraloncology.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 46.VanderWalde NA, Fleming M, Weiss J, Chera BS. Treatment of older patients with head and neck cancer: a review. Oncologist. 2013;18(5):568-578. doi: 10.1634/theoncologist.2012-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18-26, 2013-2018. NCHS Data Brief. 2020;(354):1-8. [PubMed] [Google Scholar]
- 48.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109-1116. doi: 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Efficacy Against Oral Persistent Infection, Immunogenicity and Safety of the 9-valent Human Papillomavirus Vaccine (9vHPV) in Men Aged 20-45 Years (V503-049). ClinicalTrials.gov identifier: NCT04199689. Accessed July 24, 2021. https://clinicaltrials.gov/ct2/show/NCT04199689
- 50.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858-6867. doi: 10.1016/j.vaccine.2010.08.030 [DOI] [PubMed] [Google Scholar]
- 51.Smith MA, Lew JB, Walker RJ, Brotherton JM, Nickson C, Canfell K. The predicted impact of HPV vaccination on male infections and male HPV-related cancers in Australia. Vaccine. 2011;29(48):9112-9122. doi: 10.1016/j.vaccine.2011.02.091 [DOI] [PubMed] [Google Scholar]
- 52.Kreimer AR, Shiels MS, Fakhry C, et al. Screening for human papillomavirus-driven oropharyngeal cancer: considerations for feasibility and strategies for research. Cancer. 2018;124(9):1859-1866. doi: 10.1002/cncr.31256 [DOI] [PubMed] [Google Scholar]
- 53.D’Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017;28(12):3065-3069. doi: 10.1093/annonc/mdx535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for Disease Control and Prevention (CDC) . Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30. [PubMed] [Google Scholar]
- 55.Chaturvedi AK, Graubard BI, Broutian T, et al. Prevalence of oral HPV infection in unvaccinated men and women in the United States, 2009-2016. JAMA. 2019;322(10):977-979. doi: 10.1001/jama.2019.10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8-e17. doi: 10.1016/S2468-2667(16)30001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burger EA, de Kok IMCM, Groene E, et al. Estimating the natural history of cervical carcinogenesis using simulation models: a CISNET comparative analysis. J Natl Cancer Inst. 2020;112(9):955-963. doi: 10.1093/jnci/djz227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Souza G, Cullen K, Bowie J, Thorpe R, Fakhry C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One. 2014;9(1):e86023. doi: 10.1371/journal.pone.0086023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook EE, Venkataramani AS, Kim JJ, Tamimi RM, Holmes MD. Legislation to increase uptake of HPV vaccination and adolescent sexual behaviors. Pediatrics. 2018;142(3):e20180458. doi: 10.1542/peds.2018-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brouwer AF, Delinger RL, Eisenberg MC, et al. HPV vaccination has not increased sexual activity or accelerated sexual debut in a college-aged cohort of men and women. BMC Public Health. 2019;19(1):821. doi: 10.1186/s12889-019-7134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madhivanan P, Pierre-Victor D, Mukherjee S, et al. Human papillomavirus vaccination and sexual disinhibition in females: a systematic review. Am J Prev Med. 2016;51(3):373-383. doi: 10.1016/j.amepre.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 62.Kreimer AR, Sampson JN, Porras C, et al. ; Costa Rica HPV Vaccine Trial (CVT) Group . Evaluation of durability of a single dose of the bivalent HPV Vaccine: the CVT Trial. J Natl Cancer Inst. 2020;112(10):1038-1046. doi: 10.1093/jnci/djaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Observed and Projected Cumulative HPV Vaccination Rates (≥1 Dose) Between 2016-2055 by Age Groups and Calendar Periods for Men
eTable 2. Observed and Projected Cumulative HPV Vaccination Rates (≥1 Dose) Between 2016-2055 by Age Groups and Calendar Periods for Women
eTable 3. Projected Age-Standardized Oropharynx Cancer Incidence Rates per 100,000 Population Between 2018-2045 Under Current HPV Vaccination Rates Compared to the Scenario of Expected Incidence With No Vaccination, Overall, by Sex, by Age Groups, and by Sex and Age Groups
eTable 4. Projected Annual Number of Incident Oropharynx Cancer Cases in 2018, 2025, 2035 and 2045, and the Total Number of Projected Cases Between 2018-2045 Under Current HPV Vaccination Rates Compared to the Scenario of Expected Number of Cases With No Vaccination, Overall, by Sex, by Age Groups, and by Sex and Age Groups
eFigure 1. HPV Advisory Committee on Immunization Practices (ACIP) Vaccine Recommendation Update Timeline
eFigure 2. Relationship Between Age Groups, Calendar Periods and Birth Cohorts Included in the Study
eFigure 3. Proportion of Those Vaccinated Who Initiated HPV Vaccination <15, 15-21, and 22-26 Years of Age, by Birth Cohort and Sex
eFigure 4. Cohort Rate Ratios for Oropharynx Cancer and the Fitted Joinpoint Models Among Men and Women of Birth Cohorts 1910-1982
eFigure 5. Deviance Residuals of Age-Period-Cohort Models for Men and Women
eFigure 6. Relative Reduction of Projected Age-Standardized Oropharynx Cancer Incidence Rates Under Current HPV Vaccination Rates Compared to the Scenario of Expected Incidence With No Vaccination Between 2018-2045, by Sex in Individuals Aged 36-45 Years and 46-55 Years
eFigure 7. Projected Number of Oropharynx Cancers Each Year That Will Be Averted Because of Current HPV Vaccination Rates by Age Groups in Men and Women Between 2018-2045