This systematic review and meta-analysis assesses the efficacy and safety of neoadjuvant immunotherapy for resectable head and neck squamous cell carcinoma.
Key Points
Question
What is the efficacy and safety profile of neoadjuvant programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) inhibitors in patients with resectable head and neck cancer?
Findings
This systematic review and meta-analysis of 10 clinical trials of patients with resectable head and neck cancer revealed that neoadjuvant immunotherapy with PD-1/PD-L1 inhibitors had a favorable association with outcome measures of efficacy (pathological complete response) and safety (grade 3-4 adverse events and surgical delay).
Meaning
Neoadjuvant anti–PD-1/PD-L1 immunotherapy for resectable head and neck cancer appears to be well tolerated and therapeutically efficacious, as implied by pathological response.
Abstract
Importance
The emerging approach of neoadjuvant immunotherapy for solid cancers has set the ground for the integration of programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) inhibitors into the neoadjuvant setting of head and neck squamous cell carcinoma (HNSCC) treatment.
Objective
To assess the reported efficacy and safety of neoadjuvant immunotherapy for resectable HNSCC.
Data Sources and Study Selection
Electronic databases, including PubMed (MEDLINE), Embase, the Cochrane Library, and ClinicalTrials.gov were systematically searched for published and ongoing cohort studies and randomized clinical trials that evaluate neoadjuvant immunotherapy for resectable HNSCC. The search results generated studies from 2015 to July 2021.
Data Extraction and Synthesis
Two investigators (R.M. and L.K.) independently identified and extracted articles for potential inclusion. Random and fixed models were used to achieve pooled odds ratios. All results are presented with 95% CIs. Data quality was assessed by means of the Cochrane Collaboration’s risk of bias tool.
Main Outcomes and Measures
The primary outcomes were reported efficacy, evaluated by major pathological response and pathological complete response in the primary tumors and lymph nodes separately, and safety, assessed by preoperative grade 3 to 4 treatment-related adverse events and surgical delay rate.
Results
A total of 344 patients from 10 studies were included. In 8 studies, neoadjuvant immunotherapy only was administered, and the other 2 studies combined immunotherapy with neoadjuvant chemotherapy and/or radiotherapy. The overall major pathological response rate in the primary tumor sites from studies reporting on neoadjuvant immunotherapy only was 9.7% (95% CI, 3.1%-18.9%) and the pathological complete response rate was 2.9% (95% CI, 0%-9.5%). Preoperative grade 3 to 4 treatment-related adverse events were reported at a rate of 8.4% (95% CI, 0.2%-23.2%) and surgical delay at a rate of 0% (95% CI, 0%-0.9%). There was a favorable association of neoadjuvant immunotherapy with all outcome measures. The subgroup analyses did not find one specific anti–PD-1/PD-L1 agent to be superior to another, and the favorable association was demonstrated by either immunotherapy alone or in combination with anti–CTLA-4.
Conclusions and Relevance
In this systematic review and meta-analysis, neoadjuvant anti–PD-1/PD-L1 immunotherapy for resectable HNSCC was well tolerated and may confer therapeutic advantages implied by histopathological response. Long-term outcomes are awaited.
Introduction
The current standard of care for patients with locoregionally advanced head and neck squamous cell carcinoma (HNSCC) includes surgical resection with adjuvant therapy or definitive chemoradiotherapy. Despite this multimodal therapeutic approach, high rates of treatment failure and disease recurrence are responsible for poor survival outcomes.1,2 The past decade has witnessed the emergence of administration of immune checkpoint inhibitors as a promising approach in the treatment of metastatic or recurrent HNSCC. Use of pembrolizumab and nivolumab (anti–programmed cell death 1 [PD-1] antibodies) have demonstrated efficacy in platinum-refractory advanced HNSCC, leading to US Food and Drug Administration approval in this setting.3,4 Induction chemotherapy has been studied for advanced-stage oral cavity cancer compared with the same therapy administered in the adjuvant setting, and it failed to achieve any beneficial clinical outcome.5
A new approach of neoadjuvant administration of immunotherapy with a more favorable toxicity profile compared with chemotherapy cytotoxic agents has been introduced and has been shown to bestow a clinical benefit for metastatic or recurrent disease.6 The rationale for neoadjuvant immunotherapy derives from the early introduction of systemic therapy that can potentially reduce the risk of distant metastases and convert unresectable to resectable disease. Ultimately, it may modify the extent of surgery and reduce surgical morbidity. Tumor downstaging can also translate into reduced adjuvant therapy intensity.
The feasibility of neoadjuvant immunotherapy has already been evaluated in non–small cell lung cancer and melanoma.7,8 Several phase 2 trials of neoadjuvant anti–PD-1/PD-1 ligand 1 [PD-L1] administered several weeks prior to definitive resection in locoregionally advanced, resectable HNSCC are ongoing and have provided promising initial results. We performed a meta-analysis on the available data to evaluate the therapeutic benefit of the neoadjuvant approach while also assessing the potential associated limitations, such as toxic effects and delay of curative surgery.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines9 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.10 The detailed protocol is documented online in the International Prospective Register of Systematic Reviews registry (PROSPERO: CRD42021239707). Because this systematic review and meta-analysis did not use individualized patient data, no institutional review board approval was required.
Study Selection
We conducted a systematic search in PubMed (MEDLINE), Embase, the Cochrane Library, and ClinicalTrials.gov to identify published studies on neoadjuvant immunotherapy in patients with HNSCC reported before July 2021 (eMethods in the Supplement). We also searched unpublished reported data of ongoing clinical trials of neoadjuvant immunotherapy in patients with HNSCC presented at international oncological conferences (eTable 2 in the Supplement).
Data Extraction
Two investigators (R.M. and L.K.) independently identified and extracted articles for potential inclusion. Disagreements were resolved by referral to a third reviewer (N.M.). The full texts of the resulting articles were then retrieved and analyzed. A summary of the characteristics of the included studies is provided in the Table.11,12,13,14,15,16,17,18,19,20 Additional details of the included studies are provided in eTable 1 in the Supplement.
Table. Characteristics of Studies of Neoadjuvant Immunotherapy in Head and Neck Cancer.
| Source | ClinicalTrials.gov identifier | Study phase | Intervention model | Masking | Study type | Randomization method | Main inclusion criteria | Article type | Outcome reported |
|---|---|---|---|---|---|---|---|---|---|
| Wise-Draper et al,11 2021 | NCT02641093 | Phase 2 | Single arm | Open label | Cohort study | NA | Clinically high risk (T3 or T4 and/or ≥2 LNs) in resectable HNSCC | Conference abstract |
|
| Uppaluri et al,12 2020 | NCT02296684 | Phase 2 | Sequential assignment, dual arm | Open label | Cohort study | Nonrandomized | Surgically resectable stage III and IV head and neck cancer | Full text |
|
| IMCISION trial,13 2020 | NCT03003637 | Phase 1B/2 | Single arm | Open label | Cohort study | NA | Advanced (T3-4, N0-3, M0) HNSCC eligible for curative-intent surgical resection | Conference abstract |
|
| Horton et al,14 2019 | NCT03021993 | Phase 2 | Single arm | Open label | Cohort study | NA | Stage II-IVA OCSSC | Conference abstract |
|
| CIAO study,15 2019 | NCT03144778 | Phase 1 | Parallel assignment, dual arm | Open label | RCT | Randomized | Stage II-IVA oropharyngeal SCC | Full text |
|
| CheckMate 358 study,16 2021 | NCT02488759 | Phase 1/2 | Single arm | Open label | Cohort study | Randomized | Resectable SCC of the oral cavity, pharynx, or larynx with ≥T1 primary lesions and ≥N1 nodal disease | Full text |
|
| Schoenfeld et al,17 2020 | NCT02919683 | Phase 2 | Parallel assignment, dual arm | Open label | RCT | Randomized | ≥T2 and/or evidence of regional nodal involvement OCSCC | Full text |
|
| Kim et al,18 2021 | NCT03737968 | Phase 2 | Single arm | Open label | Cohort study | Randomized | Surgically resectable head and neck cancer | Conference abstract |
|
| Neoadjuvant immunotherapy in combination with chemotherapy or radiation therapy | |||||||||
| Leidner et al,19 2021 | NCT03247712 | Phase 1/2 | Single arm | Open label | Cohort study | Nonrandomized | Locally advanced HPV-associated oropharyngeal HNSCC | Full text |
|
| Zinner et al,20 2020 | NCT03342911 | Phase 2 | Single arm | Open label | Cohort study | NA | Stage II-III HPV + SCCHN without distant metastasis and candidates for surgery | Conference abstract |
|
Abbreviations: HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; LN, lymph node; MPR, major pathological response; NA, not applicable; OCSSC, oral cavity squamous cell carcinoma, pCR, pathological complete response; RCT, randomized clinical trial; SCC, squamous cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; TRAEs, treatment-related adverse event.
Quality Assessment and Risk of Bias
The selected studies were assessed with the Cochrane Collaboration’s risk of bias tool, which is used to assign a rating of high, low, or unclear risk of bias for the domains of selection, performance, detection, attrition, and reporting. Summary assessment of the risk of bias (high, low, or unclear) was derived for each outcome in each trial. Two reviewers (R.M. and L.K.) independently assessed the risk of bias. Disagreements were resolved through consensus or referral to a third reviewer (N.M.) (eFigures 8 and 9 in the Supplement).
Data Synthesis and Statistical Analysis
The meta-analysis was performed by means of noncomparative binary data in RevMan software, version 5.4 (Cochrane Collaboration), because most of the studies were 1-arm clinical trials. The odds ratios (ORs) and 95% CIs were the effect measures.21,22 The Freeman-Tukey double-arcsine transformation was performed for raw incidence rates. Result heterogeneity among the studies was quantified with the heterogeneity index (I2). Subgroup analyses were performed for specific anti–PD-1/PD-L1 or for combined use with other therapies. A P < .05 was considered statistically significant. For more information, see eMethods in the Supplement.
Results
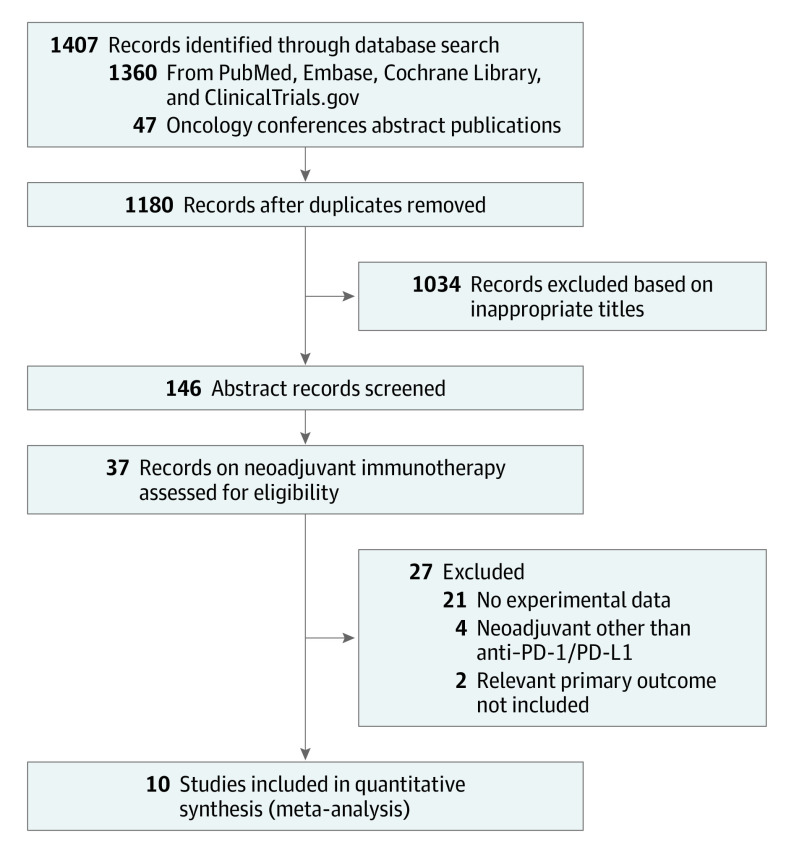
The search strategy identified 1407 citations. After screening the abstracts and reviewing the available full texts, 10 studies met the inclusion criteria, yielding a total number of 344 patients for inclusion in this meta-analysis. Five of those 10 studies were ongoing trials for which only the abstracts were available, and the remaining 5 were published as full-length articles. The selection process is illustrated in Figure 1.
Figure 1. PRISMA Flow Diagram.
PD-1/PD-L1 indicates programmed cell death 1/programmed cell death 1 ligand 1.
Evaluation of Efficacy Outcomes
Pathological complete response (pCR) and major pathologic response (MPR) were distinguished in the primary tumor site (Figure 211,12,13,14,15,16,17,18) vs pCR and MPR in regional lymph nodes (eFigure 4 in the Supplement). The rates of pCR at the primary tumor ranged from 0% to 16.7%. The mean pCR was 2.9% (95% CI, 0%-9.5%) (eFigure 1A in the Supplement). Pooled results from the trials using neoadjuvant immunotherapy showed a statistically significant benefit (OR, 0.07; 95% CI, 0.03-0.18; Figure 2A). The heterogeneity of the results was low (I2 = 34%). Significant favorable association was observed in nodal pCR (eFigure 4C in the Supplement). Zinner et al20 and Leidner et al19 reported that neoadjuvant immunotherapy combined with chemotherapy and/or radiation prior to surgery were analyzed separately with overall pCR of 53% (eFigure 3A in the Supplement).
Figure 2. Efficacy Evaluation of Neoadjuvant Immunotherapy for Resectable Head and Neck Cancer.
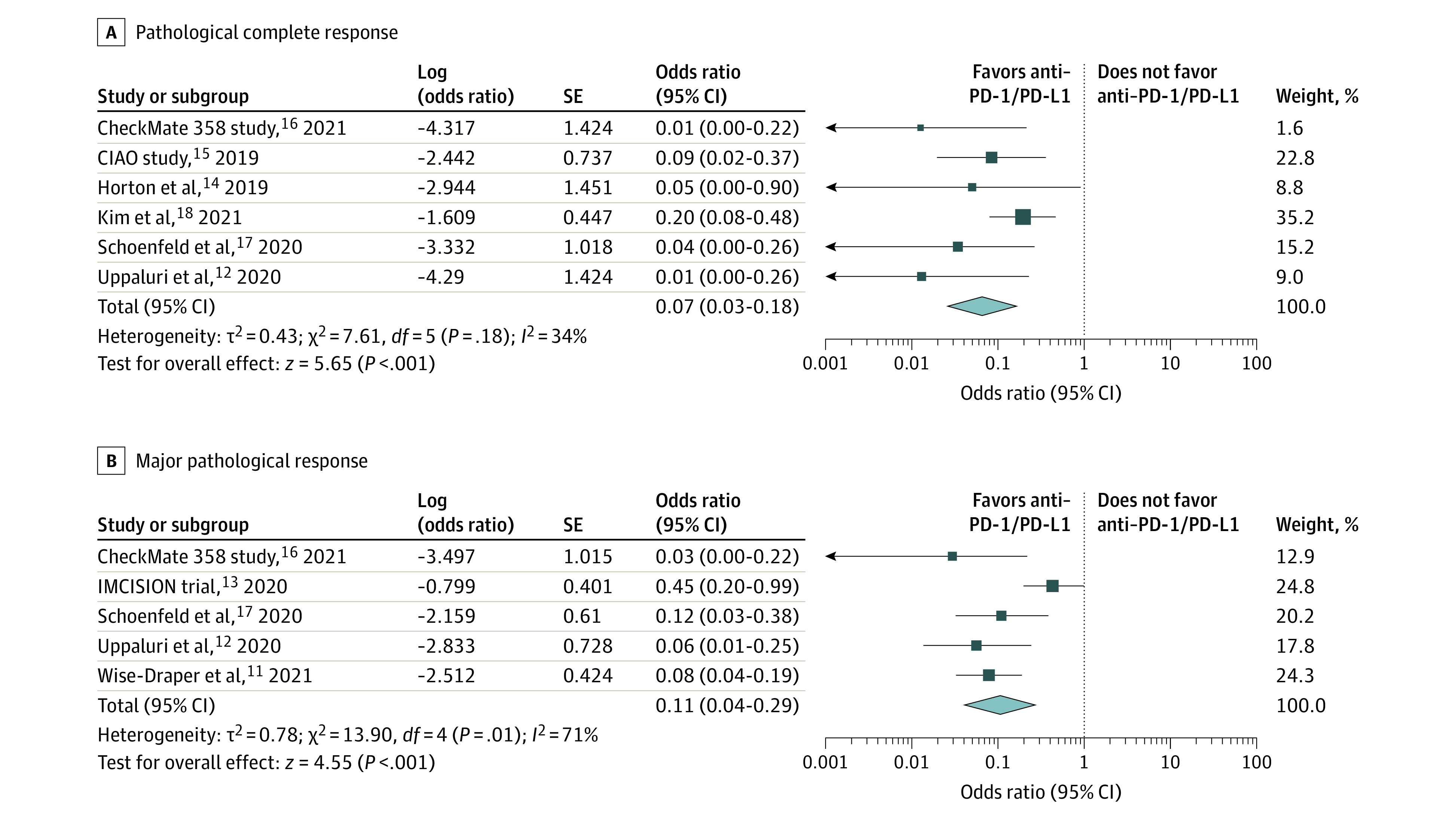
Pathological complete response (A) and major pathological response (B) at the primary tumor sites in clinical trials. PD-1/PD-L1 indicates programmed cell death 1/programmed cell death 1 ligand 1.
The MPR to neoadjuvant immunotherapy, defined as 10% or less residual viable tumor, was reported in 5 studies, and it ranged from 2.9% to 31.0%. The mean MPR rate was 9.7% (95% CI, 3.1%-18.9%; eFigure 1B in the Supplement). Neoadjuvant immunotherapy had a statistically significantly beneficial association in terms of MPR (OR, 0.11; 95% CI, 0.04-0.29; Figure 2B). There was a high level of heterogeneity, but after a sensitivity analysis was performed and the IMCISION trial,13 which had the highest weight in the analysis, was excluded (eTable 3 in the Supplement), the heterogeneity decreased. The remaining trials indicated a favorable association of anti–PD-1/PD-L1 with MPR (OR, 0.08; 95% CI, 0.04-0.14). Nodal MPR reported in 3 studies has also shown statistically significant favorability of neoadjuvant immunotherapy (eFigure 4B in the Supplement). The studies by Zinner et al20 and Leidner et al19 were analyzed separately with overall pCR of 75.3% (eFigure 3B in the Supplement).
Safety of Neoadjuvant Immunotherapy
Treatment-related adverse events (TRAEs), assessed by the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0, are associated with neoadjuvant immunotherapy safety. Preoperative grade 3 to 4 neoadjuvant immunotherapy–related adverse events include autoimmune colitis, duodenal hemorrhage, mucositis, nausea, vomiting, and syncope. The incidence of preoperative grade 3 to 4 TRAEs was 8.4% (95% CI, 0.2%-23.2%; eFigure 2 in the Supplement). Neoadjuvant immunotherapy was administered with an acceptable safety profile, supported by all 6 included studies12,13,14,16,17,18 (OR, 0.17; 95% CI, 0.07-0.41; Figure 3A). Exclusion of the IMCISION trial,13 which had the highest weight in the analysis, demonstrated a decrease in heterogeneity (eTable 4 in Supplement). As for surgical delay rate, the pooled OR was supportive of neoadjuvant immunotherapy (OR, 0.02; 95% CI, 0.01-0.05; Figure 3B12,13,14,15,16,17,18).
Figure 3. Safety of Neoadjuvant Immunotherapy for Resectable Head and Neck Cancer.
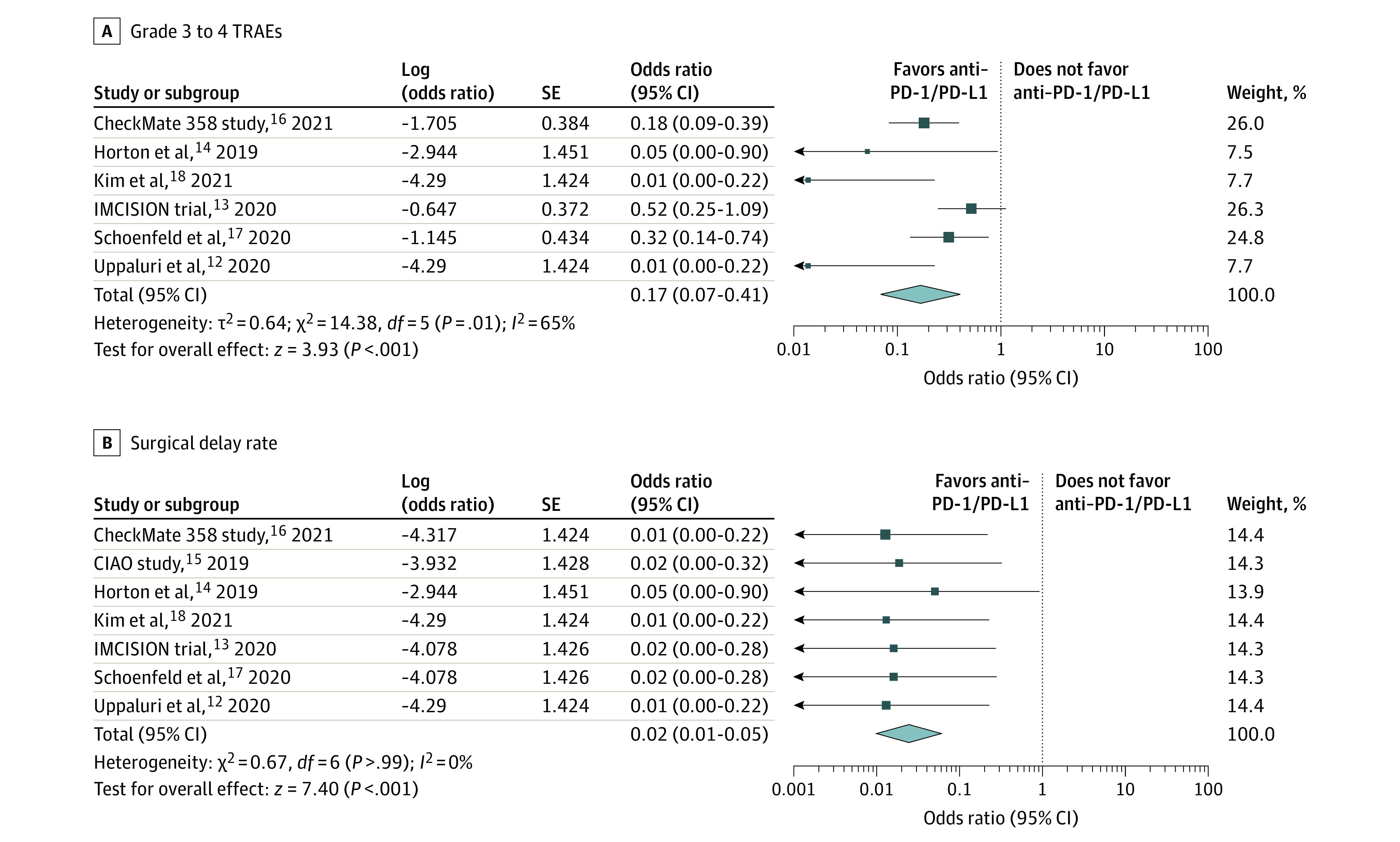
Preoperative grade 3 to 4 treatment-related adverse events (TRAEs) (A) and surgical delay rates (B) in clinical trials. PD-1/PD-L1 indicates programmed cell death 1/programmed cell death 1 ligand 1.
Subgroup Analyses
The subgroup analyses did not find any specific anti–PD-1/PD-L1 agent to be superior to another (eFigure 6 in the Supplement). Subgroup analyses of safety and efficacy outcomes (pCR, MPR, and adverse events) did not demonstrate any difference in the efficacy and safety of any neoadjuvant anti–PD-1/PD-L1 agent administered alone or in combination with CTLA-4 blockage (eFigure 7 in the Supplement). For preoperative grade 3 to 4 TRAEs, high heterogeneity was detected in studies with combined anti–CTLA-4 and anti–PD-1/PD-L1 treatments (IMCISION trial13 and HR Kim et al18), which contributed the most to the heterogeneity of the overall grade 3 to 4 TRAEs results.
Discussion
To the best of our knowledge, this is the first meta-analysis that evaluated the efficacy and safety of neoadjuvant immunotherapy for patients with resectable HNSCC. Results demonstrated favorable outcomes and acceptable tolerance of the administration of neoadjuvant PD-1\PD-L1 inhibitors. The neoadjuvant approach has been evaluated by pCR and MPR as indicators of treatment efficacy.23 Several studies distinguished between these measures of pathologic response in the primary tumor and in lymph node metastases, while others reported these outcomes in primary tumor alone. Concordant MPR and pCR in the tumor and metastasis were consistently observed, except for higher MPR in lymph nodes than in the primary tumor site, as reported in the CIAO study.15 Overall, we found a favorable association of neoadjuvant immunotherapy, both in primary site and in nodal disease.
Combinations of neoadjuvant immunotherapy with chemotherapy or radiotherapy are being evaluated in clinical trials.24,25,26,27 Whether these combinations have synergistic effects or provide any therapeutic benefit compared with single-agent therapy is still under investigation. It has been hypothesized that chemotherapy preceding the administration of neoadjuvant immunotherapy may increase antigen presentation by dendritic cells and enhance immune activation against the tumor, which can potentially increase therapeutic efficacy.28,29,30,31 In this meta-analysis, we analyzed the therapeutic efficacy of the combined approach (neoadjuvant immunotherapy with chemotherapy and/or radiation), as reported by Zinner et al20 and Leidner et al19 separately. Both Zinner et al20 and Leidner et al19 reported higher MPR and pCR than the rest of the trials included in this meta-analysis. Zinner et al20 reported on 26 patients who received neoadjuvant carboplatin and paclitaxel in addition to nivolumab and achieved a pCR rate of 42% and MPR rate of 65%. The remarkable pCR rate of 66.6% and MPR rate of 86.6% observed by Leidner et al19 could be attributed to the fact that most of the patients included in the study had tested positive for human papillomavirus (HPV). These patients greatly responded to irradiation therapy alone, as demonstrated by an 83% rate of MPR. These results suggest that the superiority of combinatorial neoadjuvant therapy should be considered with caution owing to the heterogeneity of patients’ inclusion criteria (such as HPV status). Other ongoing trials are NCT03721757,32 NCT03635164,33 and NCT03618134,34 which are investigating the efficacy and safety outcomes of neoadjuvant immunoradiotherapy and will submit data in the near future that can help assess the individual contribution of each modality. Further research is required to determine the optimal doses, timing, and combinations for best short- and long-term outcomes.
The present study’s subgroup analyses showed no superiority of immunotherapy alone vs immunotherapy combined with other immune checkpoint inhibitors (CTLA-4 blockade). There was no statistically significant difference between the various anti–PD-1/PD-L1 agents in terms of efficacy and safety. It has been suggested that downstaging may decrease the need or the intensity of adjuvant postoperative radiation and/or chemotherapy in patients undergoing surgery.6,35 Downstaging was reported in 3 studies in this analysis (eFigure 5 in the Supplement). The CIAO study15 even reported reduced intensity of adjuvant radiotherapy. The risk of severe TRAEs that may delay curative surgery is a limitation of any neoadjuvant immunotherapy approach that must be considered. In the present study, neoadjuvant immunotherapy resulted in almost no TRAEs that caused surgical delay events.
The use of neoadjuvant chemotherapy prior to surgery in patients with HNSCC is controversial. Neoadjuvant chemotherapy has shown little benefit in a number of reports, with the pCR rate ranging between 13% and 27%.36,37,38,39,40,41,42 In one of the reports, MPR was observed with neoadjuvant chemotherapy in 27% of patients.36 Similar rates were reported in neoadjuvant anti–PD-1/PD-L1 combined with CTLA-4 inhibitors,13,17 and higher rates were measured when neoadjuvant chemotherapy was combined with immunotherapy. In terms of safety, the mean rate of grade 3 to 4 adverse effects in this meta-analysis was 8.4%, indicating better tolerability compared with higher rates reported with neoadjuvant chemotherapy (37%).38 Moreover, the death rate with neoadjuvant chemotherapy was reported to be 3%38 compared with no deaths with neoadjuvant immunotherapy. This provides further evidence for the safety of immunotherapy in the neoadjuvant setting.
Early systemic therapy may reduce the risk of distant metastatic spread and may increase overall survival (OS).6 Neoadjuvant chemotherapy did not demonstrate survival benefit in a phase 3 randomized clinical trial with 11.5 years of follow-up.37 As for neoadjuvant immunotherapy, long-term data are still pending. Schoenfeld et al17 reported an 89% OS rate with 14.2 months of follow-up. At 24 months postsurgery, the CheckMate 358 study16 has reported 88.2% and 54.2% recurrence-free survival (RFS) rates for the HPV-positive and HPV-negative cohorts, respectively. Association between pathologic response and long-term outcome was assessed by Wise-Draper et al,11 who demonstrated considerably improved 1-year disease-free survival (DFS) in patients with MPR compared with patients with no pathologic response. Similar association was reported in the IMCISION trial,13 with considerably better RFS in patients with more than 90% pathologic response than patients with less than 90% pathologic response. In the Checkmate 358 study,16 however, there were no clear associations between pathologic response and RFS or OS. It should be noted that these studies have administered different combinatorial adjuvant therapies and the individual contribution to each modality in optimizing the long-term outcome should be considered. Other ongoing clinical trials will report survival data in the upcoming years. One of these trials is the IMSTAR-HN,43 a phase 3 clinical trial assessing neoadjuvant nivolumab with and without ipilimumab as first-line treatment with curative intent for HNSCC. It will report DFS, OS, and progression-free survival outcomes.
Another potential advantage of the neoadjuvant setting is the “window” phase that allows the investigation of biomarkers that may be associated with treatment response to neoadjuvant immunotherapy and optimize patient selection.44,45,46,47 Potential prognostic biomarkers of HNSCC disease response, such as PD-L1 expression, tumor immune infiltration, tumor mutational burden, circulating tumor cells or tumor DNA, and other markers, are currently under evaluation.48,49 Data from phase 3 randomized trials investigating pembrolizumab in the recurrent or metastatic setting (KEYNOTE-048 and KEYNOTE-040) showed considerably increased survival in PD-L1–positive patients,48,49,50 suggesting that PD-L1 may be a potential biomarker. This association in the neoadjuvant immunotherapy setting, however, is controversial. Both Uppaluri et al12 and Wise-Draper et al11 have confirmed an association between PD-L1 expression and improved pathological response after neoadjuvant pembrolizumab treatment. However, PD-L1 expression was not associated with improved pathological response in the trials of Schoenfeld et al,17 Horton et al,14 or the CIAO study,15 suggesting that PD-L1 expression alone is not sufficient to predict treatment response. Further studies are warranted to validate potential predictive biomarkers.
Limitations
There are several limitations to this meta-analysis. Half of the included data were derived from ongoing trials or reported in conference abstracts, while the results of only 5 of the 10 included trials were published in full-text articles. The relatively small number of patients included and the scarcity of randomized clinical trials are major limitations. Furthermore, the variations in study design, treatment protocols, different immunotherapeutic agents, HPV status, and patient characteristics all contribute to heterogeneity and limit the strength of these findings. Lastly, long-term outcomes, such as DFS and OS, that better indicate treatment efficacy have not yet been reported.
Conclusions
Results of this systematic review and meta-analysis show that neoadjuvant immunotherapy demonstrated a therapeutic benefit and that it was well tolerated. Conclusive evidence of its use awaits more data from trials on neoadjuvant immunotherapy in patients with resectable HNSCC.
eMethods. Supplementary methods
eTable 1. Data of the studies included in the meta-analysis
eTable 2. Ongoing neoadjuvant PD-1/PD-L1 inhibitors clinical trials - unpublished abstracts eTable 3. Sensitivity analysis for MPR outcome
eTable 4. Sensitivity analysis for Grade 3-4 AEs outcomes
eFigure 1. Meta-analysis of the efficacy of neoadjuvant immunotherapy in HNSCC patients. A and B represent proportions of pCR and MPR in primary tumor
eFigure 2. Meta-analysis of the safety of neoadjuvant immunotherapy in HNSCC patients. A and B represent proportions of Grade 3, 4 TRAEs and surgical delay
eFigure 3. Clinical Trials of the effect of neoadjuvant immunotherapy combined with
chemo/radiotherapy in HNSCC patients (A) pCR and (B) MPR
eFigure 4. Meta-analysis and incidence of (A,B) nodal MPR (C) and nodal pCR in HNSCC patients receiving neoadjuvant immunotherapy
eFigure 5. Meta-analysis of the effect of neoadjuvant immunotherapy on downstaging
eFigure 6. Subgroup analysis based on anti-PD1/PD-L1 drug type for (A) pCR and (B) MPR
eFigure 7. Subgroup analysis based upon neoadjuvant immune checkpoint inhibitor combinations for (A)MPR (B) pCR (C) Grade3-4 TRAEs
eFigure 8. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies
eFigure 9. Risk of bias summary: review authors' judgements about each risk of bias item for each included study
eReferences.
References
- 1.Shin DM, Khuri FR. Advances in the management of recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck. 2013;35(3):443-453. doi: 10.1002/hed.21910 [DOI] [PubMed] [Google Scholar]
- 2.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257-264. doi: 10.1016/S1470-2045(13)70011-1 [DOI] [PubMed] [Google Scholar]
- 3.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad R, Seiwert T, Pfister DG, et al. Pembrolizumab after progression on platinum and cetuximab in head and neck squamous cell carcinoma (HNSCC): results from KEYNOTE-055. Ann Oncol. 2016;27(suppl 6):vi330. doi: 10.1093/annonc/mdw376.09 [DOI] [Google Scholar]
- 5.Lau A, Li K-Y, Yang W-F, Su Y-X. Induction chemotherapy for squamous cell carcinomas of the oral cavity: a cumulative meta-analysis. Oral Oncol. 2016;61:104-114. doi: 10.1016/j.oraloncology.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 6.Hanna GJ, Adkins DR, Zolkind P, Uppaluri R. Rationale for neoadjuvant immunotherapy in head and neck squamous cell carcinoma. Oral Oncol. 2017;73:65-69. doi: 10.1016/j.oraloncology.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 7.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976-1986. doi: 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181-193. doi: 10.1016/S1470-2045(18)30015-9 [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 11.Wise-Draper TM, Takiar V, Mierzwa ML, et al. Association of pathological response to neoadjuvant pembrolizumab with tumor PD-L1 expression and high disease-free survival (DFS) in patients with resectable, local-regionally advanced, head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2021;39(suppl 15):6006. doi: 10.1200/JCO.2021.39.15_suppl.6006 [DOI] [Google Scholar]
- 12.Uppaluri R, Campbell KM, Egloff AM, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin Cancer Res. 2020;26(19):5140-5152. doi: 10.1158/1078-0432.CCR-20-1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuur L, Vos JL, Elbers JB, et al. LBA40 Neoadjuvant nivolumab and nivolumab plus ipilimumab induce (near-) complete responses in patients with head and neck squamous cell carcinoma: the IMCISION trial. Ann Oncol. 2020;31(suppl 4):S1169. doi: 10.1016/j.annonc.2020.08.2270 [DOI] [Google Scholar]
- 14.Horton JD, Knochelmann H, Armeson K, Kaczmar JM, Paulos C, Neskey D. Neoadjuvant presurgical PD-1 inhibition in oral cavity squamous cell carcinoma. J Clin Oncol. 2019;37(suppl 15):2574. doi: 10.1200/JCO.2019.37.15_suppl.2574 [DOI] [Google Scholar]
- 15.Ferrarotto R, Bell D, Rubin ML, et al. Checkpoint inhibitors assessment in oropharynx cancer (CIAO): safety and interim results. J Clin Oncol. 2019;37(suppl 15):6008. doi: 10.1200/JCO.2019.37.15_suppl.6008 [DOI] [Google Scholar]
- 16.Ferris RL, Spanos WC, Leidner R, et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Immunother Cancer. 2021;9(6):e002568. doi: 10.1136/jitc-2021-002568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol. 2020;6(10):1563-1570. doi: 10.1001/jamaoncol.2020.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HR, Kim CG, Hong MH, et al. Interim analysis for window of opportunity trial of single dose preoperative durvalumab (D) with or without tremelimumab (T) for operable head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2021;39(suppl 15):e18043. doi: 10.1200/JCO.2021.39.15_suppl.e18043 [DOI] [Google Scholar]
- 19.Leidner R, Crittenden M, Young K, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Immunother Cancer. 2021;9(5):e002485. doi: 10.1136/jitc-2021-002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinner R, Johnson JM, Tuluc M, et al. 968P Neoadjuvant nivolumab (N) plus weekly carboplatin (C) and paclitaxel (P) outcomes in HPV(-) resectable locally advanced head and neck cancer. Ann Oncol. 2020;31(suppl 4):S682. doi: 10.1016/j.annonc.2020.08.1083 [DOI] [Google Scholar]
- 21.Chen Y-H, Du L, Geng X-Y, Liu G-J. Implement meta-analysis with non-comparative binary data in RevMan software. Chin J Evidence-Based Med. 2014;14(7):889-896. [Google Scholar]
- 22.Jia XH, Xu H, Geng LY, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: a meta-analysis. Lung Cancer. 2020;147:143-153. doi: 10.1016/j.lungcan.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 23.Melero I, Berraondo P, Rodríguez-Ruiz ME, Pérez-Gracia JL. Making the most of cancer surgery with neoadjuvant immunotherapy. Cancer Discov. 2016;6(12):1312-1314. doi: 10.1158/2159-8290.CD-16-1109 [DOI] [PubMed] [Google Scholar]
- 24.Stafford M, Kaczmar J. The neoadjuvant paradigm reinvigorated: a review of pre-surgical immunotherapy in HNSCC. Cancers Head Neck. 2020;5:4. doi: 10.1186/s41199-020-00052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiegand S, Wichmann G, Dietz A. Perspectives of induction with chemo and/or immune check point inhibition in head and neck organ preservation treatment. Front Oncol. 2019;9:191. doi: 10.3389/fonc.2019.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plavc G, Strojan P. Combining radiotherapy and immunotherapy in definitive treatment of head and neck squamous cell carcinoma: review of current clinical trials. Radiol Oncol. 2020;54(4):377-393. doi: 10.2478/raon-2020-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajewski TF. Fast forward—neoadjuvant cancer immunotherapy. N Engl J Med. 2018;378(21):2034-2035. doi: 10.1056/NEJMe1803923 [DOI] [PubMed] [Google Scholar]
- 28.Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102(1):115-123. doi: 10.1038/sj.bjc.6605465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013;62(3):405-410. doi: 10.1007/s00262-012-1390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455-465. doi: 10.1038/nrclinonc.2010.82 [DOI] [PubMed] [Google Scholar]
- 31.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183(1):137-144. doi: 10.4049/jimmunol.0900734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CA209-891: Neoadjuvant and adjuvant nivolumab as immune checkpoint inhibition in oral cavity cancer (NICO). ClinicalTrials.gov identifier: NCT03721757. Updated January 5, 2021. Accessed July 22, 2021. https://clinicaltrials.gov/ct2/show/NCT03721757
- 33.Radiotherapy with durvalumab prior to surgical resection for HPV negative squamous cell carcinoma. ClinicalTrials.gov identifier: NCT03635164. Updated May 20, 2021. Accessed July 22, 2021. https://clinicaltrials.gov/ct2/show/NCT03635164
- 34.Stereotactic body radiation therapy and durvalumab with or without tremelimumab before surgery in treating participants with human papillomavirus positive oropharyngeal squamous cell cancer. ClinicalTrials.gov identifier: NCT03618134. Updated December 8, 2020. Accessed March 11, 2021. https://clinicaltrials.gov/ct2/show/NCT03618134
- 35.Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382-1399. doi: 10.1158/2159-8290.CD-16-0577 [DOI] [PubMed] [Google Scholar]
- 36.Zhong LP, Zhang CP, Ren GX, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31(6):744-751. doi: 10.1200/JCO.2012.43.8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bossi P, Lo Vullo S, Guzzo M, et al. Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Ann Oncol. 2014;25(2):462-466. doi: 10.1093/annonc/mdt555 [DOI] [PubMed] [Google Scholar]
- 38.Licitra L, Grandi C, Guzzo M, et al. Primary chemotherapy in resectable oral cavity squamous cell cancer: a randomized controlled trial. J Clin Oncol. 2003;21(2):327-333. doi: 10.1200/JCO.2003.06.146 [DOI] [PubMed] [Google Scholar]
- 39.Marta GN, Riera R, Bossi P, et al. Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: systematic review and meta-analysis. Eur J Cancer. 2015;51(17):2596-2603. doi: 10.1016/j.ejca.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Yi J, Huang X, et al. Prognostic impact of pathological complete remission after preoperative irradiation in patients with locally advanced head and neck squamous cell carcinoma: re-analysis of a phase 3 clinical study. Radiat Oncol. 2019;14(1):225. doi: 10.1186/s13014-019-1428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang XR, Liu ZM, Liu XK, et al. Influence of pathologic complete response to neoadjuvant chemotherapy on long-term survival of patients with advanced head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(2):218-223. doi: 10.1016/j.oooo.2012.09.084 [DOI] [PubMed] [Google Scholar]
- 42.Kies MS, Boatright DH, Li G, et al. Phase II trial of induction chemotherapy followed by surgery for squamous cell carcinoma of the oral tongue in young adults. Head Neck. 2012;34(9):1255-1262. doi: 10.1002/hed.21906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zech HB, Moeckelmann N, Boettcher A, et al. Phase III study of nivolumab alone or combined with ipilimumab as immunotherapy versus standard of care in resectable head and neck squamous cell carcinoma. Future Oncol. 2020;16(36):3035-3043. doi: 10.2217/fon-2020-0595 [DOI] [PubMed] [Google Scholar]
- 44.Benitez JC, Remon J, Besse B. Current panorama and challenges for neoadjuvant cancer immunotherapy. Clin Cancer Res. 2020;26(19):5068-5077. doi: 10.1158/1078-0432.CCR-19-3255 [DOI] [PubMed] [Google Scholar]
- 45.Kao H-F, Lou P-J. Immune checkpoint inhibitors for head and neck squamous cell carcinoma: current landscape and future directions. Head Neck. 2019;41(suppl 1):4-18. doi: 10.1002/hed.25930 [DOI] [PubMed] [Google Scholar]
- 46.Wang H-C, Yeh T-J, Chan L-P, Hsu C-M, Cho S-F. Exploration of feasible immune biomarkers for immune checkpoint inhibitors in head and neck squamous cell carcinoma treatment in real world clinical practice. Int J Mol Sci. 2020;21(20):E7621. doi: 10.3390/ijms21207621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliva M, Spreafico A, Taberna M, et al. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol. 2019;30(1):57-67. doi: 10.1093/annonc/mdy507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soulieres D, Cohen E, Le Tourneau C, et al. Updated survival results of the KEYNOTE-040 study of pembrolizumab vs standard-of-care chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma. Am Assoc Cancer Res. 2018;78(13). doi: 10.1158/1538-7445.AM2018-CT115 [DOI] [Google Scholar]
- 49.Cohen EEW, Soulières D, Le Tourneau C, et al. ; KEYNOTE-040 investigators . Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156-167. doi: 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 50.Burtness B, Harrington KJ, Greil R, et al. KEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann Oncol. 2018;29(suppl 8):viii729. doi: 10.1093/annonc/mdy424.045 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary methods
eTable 1. Data of the studies included in the meta-analysis
eTable 2. Ongoing neoadjuvant PD-1/PD-L1 inhibitors clinical trials - unpublished abstracts eTable 3. Sensitivity analysis for MPR outcome
eTable 4. Sensitivity analysis for Grade 3-4 AEs outcomes
eFigure 1. Meta-analysis of the efficacy of neoadjuvant immunotherapy in HNSCC patients. A and B represent proportions of pCR and MPR in primary tumor
eFigure 2. Meta-analysis of the safety of neoadjuvant immunotherapy in HNSCC patients. A and B represent proportions of Grade 3, 4 TRAEs and surgical delay
eFigure 3. Clinical Trials of the effect of neoadjuvant immunotherapy combined with
chemo/radiotherapy in HNSCC patients (A) pCR and (B) MPR
eFigure 4. Meta-analysis and incidence of (A,B) nodal MPR (C) and nodal pCR in HNSCC patients receiving neoadjuvant immunotherapy
eFigure 5. Meta-analysis of the effect of neoadjuvant immunotherapy on downstaging
eFigure 6. Subgroup analysis based on anti-PD1/PD-L1 drug type for (A) pCR and (B) MPR
eFigure 7. Subgroup analysis based upon neoadjuvant immune checkpoint inhibitor combinations for (A)MPR (B) pCR (C) Grade3-4 TRAEs
eFigure 8. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies
eFigure 9. Risk of bias summary: review authors' judgements about each risk of bias item for each included study
eReferences.