Key Points
Question
Is ensartinib superior to crizotinib for patients with advanced anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have not been treated previously with an ALK inhibitor?
Findings
This randomized clinical phase 3 trial including 290 patients met the primary end point; the median progression-free survival was statistically significantly longer with ensartinib than with crizotinib (25.8 vs 12.7 months), and the confirmed intracranial response rate was 64% with ensartinib vs 21% with crizotinib for patients with brain metastases at baseline. Ensartinib had a favorable safety profile.
Meaning
Ensartinib represents a new first-line treatment option for patients with ALK-positive NSCLC.
Abstract
Importance
Ensartinib, an oral tyrosine kinase inhibitor of anaplastic lymphoma kinase (ALK), has shown systemic and central nervous system efficacy for patients with ALK-positive non–small cell lung cancer (NSCLC).
Objective
To compare ensartinib with crizotinib among patients with advanced ALK-positive NSCLC who had not received prior treatment with an ALK inhibitor.
Design, Setting, and Participants
This open-label, multicenter, randomized, phase 3 trial conducted in 120 centers in 21 countries enrolled 290 patients between July 25, 2016, and November 12, 2018. Eligible patients were 18 years of age or older and had advanced, recurrent, or metastatic ALK-positive NSCLC.
Interventions
Patients were randomized (1:1) to ensartinib, 225 mg once daily, or crizotinib, 250 mg twice daily.
Main Outcomes and Measures
The primary end point was blinded independent review committee–assessed progression-free survival (PFS). Secondary end points included systemic and intracranial response, time to central nervous system progression, and overall survival. Efficacy was evaluated in the intent-to-treat (ITT) population as well as a prespecified modified ITT (mITT) population consisting of patients with central laboratory–confirmed ALK-positive NSCLC.
Results
A total of 290 patients (149 men [51.4%]; median age, 54 years [range, 25-90 years]) were randomized. In the ITT population, the median PFS was significantly longer with ensartinib than with crizotinib (25.8 [range, 0.03-44.0 months] vs 12.7 months [range, 0.03-38.6 months]; hazard ratio, 0.51 [95% CI, 0.35-0.72]; log-rank P < .001), with a median follow-up of 23.8 months (range, 0-44 months) for the ensartinib group and 20.2 months (range, 0-38 months) for the crizotinib group. In the mITT population, the median PFS in the ensartinib group was not reached, and the median PFS in the crizotinib group was 12.7 months (95% CI, 8.9-16.6 months; hazard ratio, 0.45; 95% CI, 0.30-0.66; log-rank P < .001). The intracranial response rate confirmed by a blinded independent review committee was 63.6% (7 of 11) with ensartinib vs 21.1% (4 of 19) with crizotinib for patients with target brain metastases at baseline. Progression-free survival for patients without brain metastases was not reached with ensartinib vs 16.6 months with crizotinib as a result of a lower central nervous system progression rate (at 12 months: 4.2% with ensartinib vs 23.9% with crizotinib; cause-specific hazard ratio, 0.32; 95% CI, 0.16-0.63; P = .001). Frequencies of treatment-related serious adverse events (ensartinib: 11 [7.7%] vs crizotinib: 9 [6.1%]), dose reductions (ensartinib: 34 of 143 [23.8%] vs crizotinib: 29 of 146 [19.9%]), or drug discontinuations (ensartinib: 13 of 143 [9.1%] vs crizotinib: 10 of 146 [6.8%]) were similar, without any new safety signals.
Conclusions and Relevance
In this randomized clinical trial, ensartinib showed superior efficacy to crizotinib in both systemic and intracranial disease. Ensartinib represents a new first-line option for patients with ALK-positive NSCLC.
Trial Registration
ClinicalTrials.gov Identifier: NCT02767804
This randomized clinical trial compares ensartinib with crizotinib among patients with advanced anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who had not received prior treatment with an ALK inhibitor.
Introduction
Chromosomal rearrangements involving the anaplastic lymphoma kinase (ALK) gene (OMIM 105590) have been detected in 3% to 11% of non–small cell lung cancers (NSCLCs).1,2,3,4,5 The resultant ALK fusion protein is a validated target in NSCLC, and testing for the fusion protein is standard of care globally.6,7 Since the 2011 approval of crizotinib as a first-line treatment,8 ALK tyrosine kinase inhibitors have become standard of care for patients with ALK-positive NSCLC.9,10,11 Second-generation (ceritinib, alectinib, and brigatinib) and third-generation (lorlatinib) ALK tyrosine kinase inhibitors were developed to overcome crizotinib resistance and its poor brain penetration,12,13,14,15 and recent randomized clinical trials have shown that alectinib, brigatinib, and lorlatinib had superior efficacy compared with crizotinib for treatment-naive patients.16,17,18
Ensartinib is a second-generation small molecule that potently and selectively inhibits ALK.19 In the phase 1 to phase 2 eXalt2 trial, ensartinib, 225 mg once daily, the recommended phase 2 dose, was associated with high systemic and central nervous system (CNS) response rates in both ALK inhibitor−naive patients and those who received prior crizotinib and/or a second-generation ALK inhibitor.20 In addition, ensartinib’s safety profile is favorable, with rash and transaminitis as the most frequent and distinctive toxic effects, although they were mostly low grade and manageable.20
The eXalt3 study was designed to compare ensartinib vs crizotinib among patients with ALK-positive NSCLC who had not received prior treatment with an ALK inhibitor. Here we report the results of the interim analysis.
Methods
Patients
Eligible patients were 18 years of age or older, had advanced or recurrent (stage IIIB not amenable for multimodality treatment) or metastatic (stage IV) NSCLC that was ALK positive as determined by local testing, and had measurable disease per Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1.21 After a protocol amendment, ALK testing was also performed at a central laboratory using the US Food and Drug Administration–approved Vysis fluorescence in situ hybridization assay (Abbott Laboratories). Patients with asymptomatic brain metastases were allowed to enroll. Patients may have received up to 1 prior chemotherapy regimen for metastatic disease. Patients were excluded if they had received cancer therapy within 4 weeks or radiotherapy within 14 days of study entry. Patients could not have received prior ALK tyrosine kinase inhibitors or programmed death 1 or programmed death ligand 1 therapy. Patients were not required to have progressive disease while receiving prior chemotherapy. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki22 and the International Conference on Harmonisation Guideline for Good Clinical Practice.23 All patients provided written informed consent prior to undergoing any research procedures, and the protocol and informed consent documents were approved by the governing ethical review boards. Complete inclusion and exclusion criteria are provided in the trial protocol (Supplement 1).
Trial Design
eXalt3 is a global, open-label, multicenter, randomized (1:1), phase 3 study conducted at 120 centers in 21 countries (NCT02767804). Patients were stratified by prior chemotherapy, Eastern Cooperative Oncology Group performance status (0 or 1 vs 2), CNS metastases at baseline, and geographic region (Asia vs rest of the world) and then randomized (centrally via an interactive voice response system using a permuted block randomization) to receive oral ensartinib, 225 mg once daily, or crizotinib, 250 mg twice daily. Patients were permitted to continue treatment until disease progression, unacceptable toxic effects, or withdrawal of consent. Crossover was not permitted. Disease assessments included imaging of the chest, abdomen, pelvis, head, and bone (if applicable) every 8 weeks for up to 19 months and then every 12 weeks. Responses were confirmed at least 4 weeks after the initial response. Patients who discontinued treatment without radiographic progression per RECIST, version 1.1 were followed up until radiographic progression or new anticancer therapy was started. Treatment beyond progression was allowed at the physician’s discretion. All adverse events (AEs) were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03.24
Outcomes
The primary end point for the study was progression-free survival (PFS) in the intent-to-treat (ITT) population as assessed by a blinded independent review committee according to RECIST, version 1.1. Key secondary end points included overall survival, CNS response rate, and CNS time to progression, as assessed by the blinded independent review committee.
Statistical Analysis
An O’Brien-Fleming Lan-DeMets25 alpha spending function was used to control the overall α level at .05 (2-sided). Assuming a median PFS (mPFS) of 10 months in the crizotinib group26 (based on the assumption that approximately two-thirds of the patients will be chemotherapy naive) with an improvement to 16 months in the ensartinib group, a sample size of 266 patients was determined to allow for detection of a 6-month improvement in PFS with a hazard ratio (HR) of 0.625 and 90% power. The final analyses were to be performed after 190 events of either progression or death. One interim analysis was planned at approximately 75% (143 events) of all 190 expected events (2-sided α of .019). Of the key secondary end points, only overall survival was tested formally at an α level of .05 (2-sided).
Efficacy was evaluated in the ITT population as well as in the modified ITT (mITT) population. Local ALK testing was allowed prior to protocol amendment 2.0, but ALK testing, as confirmed by central laboratory analysis using the Vysis fluorescence in situ hybridization test (Abbott Laboratories), was required after protocol amendment 2.0. All patients enrolled in the ITT population tested positive for ALK through local testing prior to protocol amendment 2.0. The mITT population included patients who were randomized into the trial based on positive local test results with additional central confirmation or positive central testing. Central testing was supportive of the required companion diagnostic development.
The primary end point was compared between the ensartinib and crizotinib groups by using a 2-sided log-rank test according to the factors used for randomization. For all time-to-event efficacy analyses, median values were estimated using Kaplan-Meier methods. Hazard ratios were estimated by using the Cox proportional hazards regression model. The primary end point of PFS was also analyzed in subgroups based on stratification variables as well as demographic and baseline patient characteristics. These results were considered exploratory because of the multiplicity issue and the smaller sample sizes that could not be prespecified. The safety population included patients who received at least 1 dose of either study drug.
Efficacy and safety data were reported as of July 1, 2020. Statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc). Statistical methods are described further in the statistical analysis plan included in Supplement 1.
Results
Patients
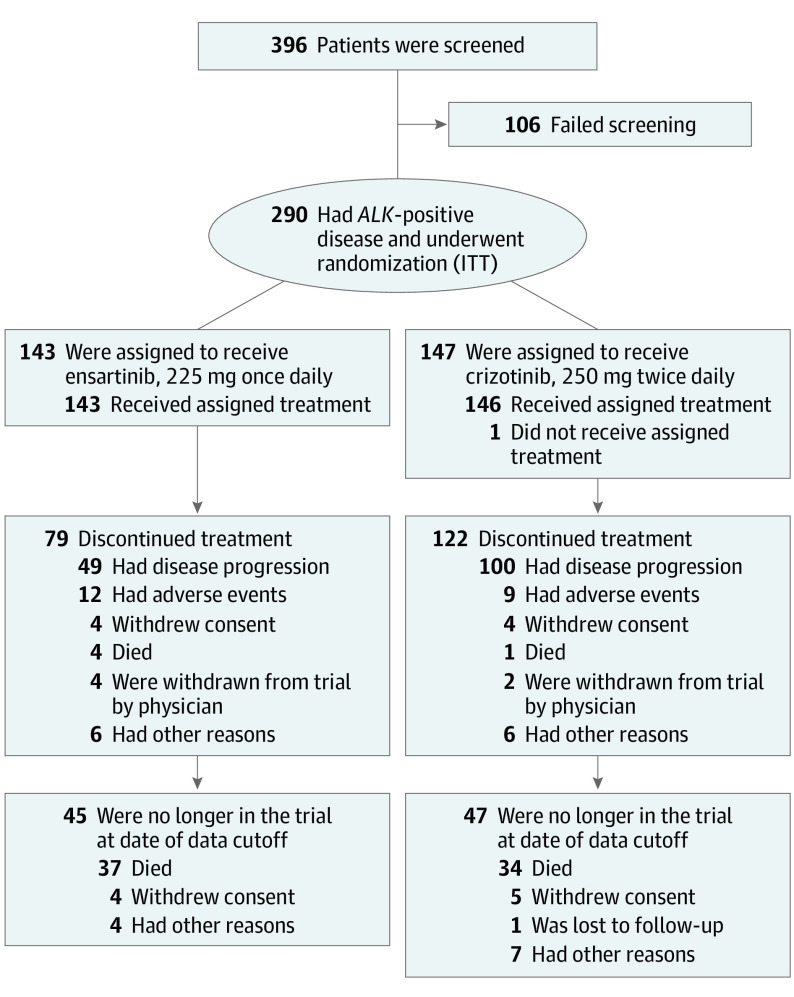
Between July 25, 2016, and November 12, 2018, 396 patients were screened; 290 patients (149 men [51.4%]; median age, 54 years [range, 25-90 years]) were randomized (ITT; ensartinib, 143 patients; crizotinib, 147 patients; Figure 1). The baseline characteristics were balanced (Table); 47 patients (32.9%) in the ensartinib group and 57 (38.8%) in the crizotinib group had brain metastases at baseline, with similar rates of prior brain radiotherapy (ensartinib, 7 patients [4.9%]; crizotinib, 7 patients [4.8%]). There were imbalances in the baseline characteristics between Asian and non-Asian patients (non-Asian patients included White [ensartinib, 63 (44.1%); crizotinib, 57 (38.8%)], Black or African American [ensartinib, 1 (0.7%); crizotinib, 3 (2.0%)], and other [ensartinib, 3 (2.1%); crizotinib, 4 (2.7%)]); Asian patients had more brain metastases (64 of 157 [40.8%] vs 40 of 133 [30.1%]) and were younger, with fewer patients older than 65 years (19 of 157 [12.1%] vs 28 of 133 [21.1%]). Of 76 patients who had received prior chemotherapy, 55.3% (42; 22 in the ensartinib group and 20 in the crizotinib group) showed evidence of progressive disease during or after chemotherapy.
Figure 1. Screening, Enrollment, Randomization, and Follow-up.
Data reported as of the cutoff for the first interim analysis (July 1, 2020) are shown. In the ensartinib group, 49 patients had documented disease progression according to the Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1. In the crizotinib group, 100 patients had documented disease progression according to RECIST, version 1.1. ALK indicates anaplastic lymphoma kinase gene; ITT, intent to treat.
Table. Baseline Characteristics of Patients in the ITT Population.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Ensartinib (n = 143) | Crizotinib (n = 147) | |
| Age, median (range), y | 54 (25-86) | 53 (26-90) |
| >65 y | 24 (16.8) | 23 (15.6) |
| Sex | ||
| Male | 72 (50.3) | 77 (52.4) |
| Female | 71 (49.7) | 70 (47.6) |
| Race and ethnicity | ||
| Non-Asiana | 66 (46.2) | 63 (42.9) |
| Asian | 77 (53.8) | 84 (57.1) |
| ECOG performance status | ||
| 0 or 1 | 136 (95.1) | 139/146 (95.2) |
| 2 | 7 (4.9) | 7/146 (4.8) |
| History of tobacco use | ||
| Never smoked | 85 (59.4) | 94 (63.9) |
| Current or former smoker | 58 (40.6) | 53 (36.1) |
| Stage of disease | ||
| IIIB | 13 (9.1) | 10 (6.8) |
| IV | 130 (90.9) | 137 (93.2) |
| ALK status assessed centrallyb | 121 (84.6) | 126 (85.7) |
| Brain metastases at baselinec | 47 (32.9) | 57 (38.8) |
| Previous radiotherapy to brain | 7 (4.9) | 7 (4.8) |
| Previous chemotherapy | 34 (23.8) | 42 (28.6) |
Abbreviations: ALK, anaplastic lymphoma kinase gene; ECOG, Eastern Cooperative Oncology Group; ITT, intent-to-treat.
Non-Asian patients included White (ensartinib, 63 [44.1%]; crizotinib, 57 [38.8%]), Black or African American (ensartinib, 1 [0.7%]; crizotinib, 3 [2.0%]), and other (ensartinib, 3 [2.1%]; crizotinib, 4 [2.7%]).
ALK positivity assessed by the Abbott fluorescence in situ hybridization assay.
The presence of brain metastases was assessed by a blinded independent review committee.
The mITT population included 247 patients randomly assigned to the trial based on positive results of local testing for ALK with subsequent central confirmation (n = 112) or positive central testing for ALK (n = 135), as shown in eFigure 1 in Supplement 2. Of the patients who were randomly assigned prior to protocol amendment 2.0, 43 were excluded from the mITT population because they had insufficient samples available (n = 27), uninterpretable findings (n = 7), or had negative test results on central confirmation of a local positive ALK test (n = 9). The mITT population ultimately included 121 patients in the ensartinib group and 126 in the crizotinib group.
As of July 1, 2020, 64 patients (44.8%) in the ensartinib group and 25 (17.0%) in the crizotinib group still received treatment, with a median follow-up of 23.8 months (range, 0-44 months) in the ensartinib group and 20.2 months (range, 0-38 months) in the crizotinib group.
Efficacy
ITT Population
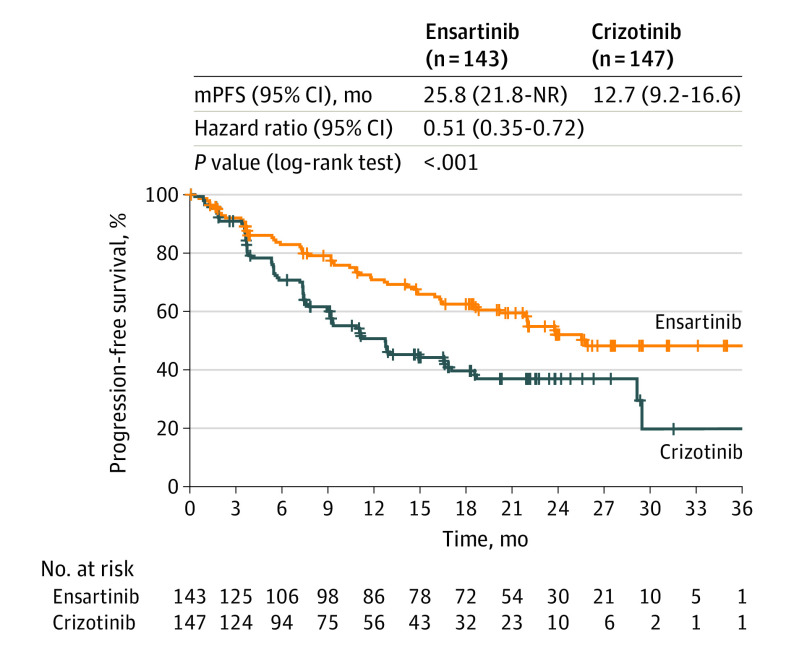
As of July 1, 2020, 139 PFS events (73.2% of 190 expected total PFS events) for the primary end point had occurred in the ITT population. The mPFS in the ensartinib group was statistically superior to that in the crizotinib group (25.8 months [range, 0.03-44.0 months] vs 12.7 months months [range, 0.03-38.6 months]; HR, 0.51; 95% CI, 0.35-0.72; log-rank P < .001) (Figure 2).
Figure 2. Efficacy of Ensartinib and Crizotinib Among Patients With Non–Small Cell Lung Cancer.
Kaplan-Meier estimates of progression-free survival by blinded independent review committee among patients in the intent-to-treat population. Tick marks indicate censored data. mPFS indicates median progression-free survival; NR, not reached.
The confirmed objective response rate was 74% (95% CI, 66%-81%) in the ensartinib group and 67% (95% CI, 58%-74%) in the crizotinib group (eTable 1 in Supplement 2). The median duration of response among patients with complete or partial response was not reached (95% CI, 22.0 months to not reached) in the ensartinib group and was 27.3 months (95% CI, 12.9 months to not reached) in the crizotinib group.
mITT Population
The mITT population was used to describe the efficacy among patients with central laboratory–confirmed ALK-positive status. The baseline characteristics of the mITT population were similar to those of the ITT population and were balanced by group. In the mITT population, 119 PFS events for the primary end point had occurred as of July 1, 2020 (62.6% of the 190 expected PFS events). The mPFS in the ensartinib group was not reached, with 54.2% (95% CI, 43.4%-63.7%) of PFS probability at 24 months, compared with 36.4% (95% CI, 27.1%-45.9%) of PFS probability at 24 months in the crizotinib group (Figure 3A). The median PFS in the ensartinib group was not reached (95% CI, 20.2 months to not reached), and the median PFS in the crizotinib group was 12.7 months (95 CI%, 8.9-16.6 months; HR, 0.45; 95% CI, 0.30-0.66; log-rank P < .001).
Figure 3. Efficacy of Ensartinib and Crizotinib Among Patients With Centrally Confirmed Anaplastic Lymphoma Kinase−Positive Non–Small Cell Lung Cancer.
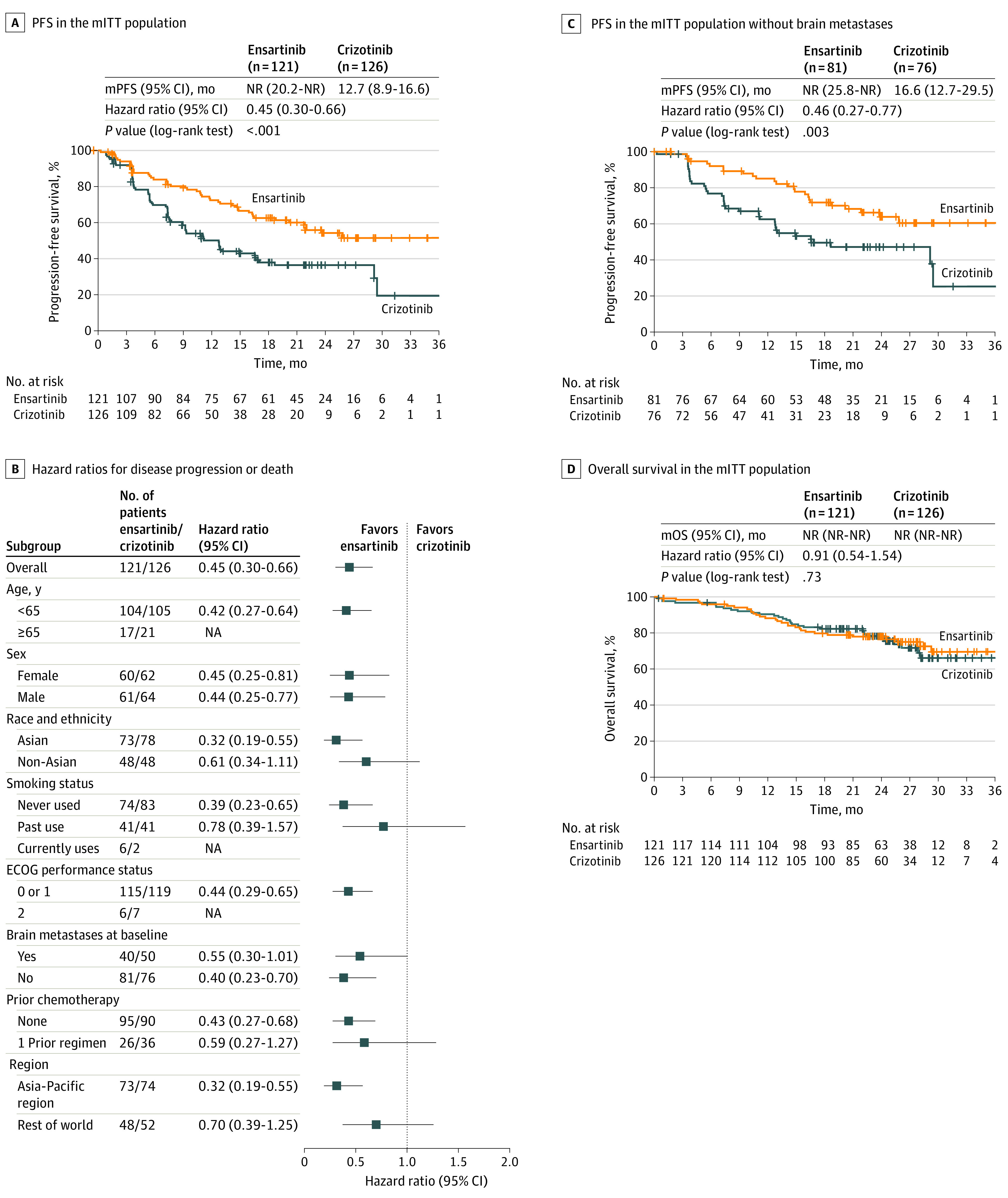
A, Kaplan-Meier estimates of progression-free survival (PFS) by blinded independent review committee in the modified intent-to-treat (mITT) population. B, The presence of brain metastases at baseline was assessed by the investigator. C, Kaplan-Meier estimates of PFS by blinded independent review committee. D, Overall survival in the mITT population. ECOG indicates Eastern Cooperative Oncology Group; mITT, modified intent-to-treat; mOS, median overall survival; mPFS, median progression-free survival; NA, not applicable; and NR, not reached.
Subgroup analyses (Figure 3B) were not powered to provide significant comparisons because of small sample sizes. However, several differences were observed, including a nonsignificantly greater benefit for Asian patients. Among patients without prior chemotherapy, the mPFS was not reached (95% CI, 20.2 months to not reached) in the ensartinib group and was 11.1 months (95% CI, 7.8-16.6 months) in the crizotinib group. Among patients who had received prior chemotherapy, the mPFS was 22.0 months (95% CI, 10.9 months to not reached) in the ensartinib group and 12.8 months (95% CI, 5.5 months to not reached) in the crizotinib group. The best systemic change from baseline in target lesions is shown in eFigure 2 in Supplement 2. The confirmed objective response rate in the mITT population was 75% (95% CI, 66.5%-82.6%) in the ensartinib group and 68% (95% CI, 58.5%-75.5%) in the crizotinib group. Complete response rates were more than 2-fold higher with ensartinib (14% [95% CI, 8.4%-21.5%]) compared with crizotinib (6% [95% CI, 2.3%-11.1%]). The median duration of response in this population was not reached in the ensartinib group, with 59% (95% CI, 45%-70%) of patients having durable responses lasting 36 months or more.
Eleven patients receiving ensartinib and 19 patients receiving crizotinib had measurable brain metastases at baseline and a postbaseline CNS evaluation. If the target lesion had disappeared, the measurement was recorded as 0 mm. The best change from baseline in target brain lesions was 63.6% in the ensartinib group (n = 7) vs 21.1% in the crizotinib group (n = 4). The intracranial best change from baseline for these patients is shown in eFigure 3 in Supplement 2. Progression-free survival for patients without brain metastases was not reached with ensartinib vs 16.6 months with crizotinib. A significantly lower percentage of patients without brain metastases at baseline in the ensartinib group vs the crizotinib group developed brain metastases at 12 months (4.2% vs 23.9%; cause-specific HR, 0.32; 95% CI, 0.16-0.63; P = .001) (eFigure 4 in Supplement 2). The mPFS among patients without baseline brain metastases receiving ensartinib was not reached (Figure 3C), with 61% of patients disease free at 36 months vs 25% of patients who received crizotinib. The mPFS among patients with brain metastases at baseline was 11.8 months (95% CI, 5.5 months to not reached) in the ensartinib group and 7.5 months (95% CI, 5.5-9.3 months) in the crizotinib group (HR, 0.55; 95% CI, 0.30-1.01; P = .05) (eFigure 5 in Supplement 2). In the ensartinib and crizotinib groups, 21 of 47 (44.7%) and 35 of 57 (61.4%) PFS events, respectively, involved the CNS.
Sixty-two patients in the mITT population died (30 of 121 [24.8%] in the ensartinib group and 32 of 126 [25.4%] in the crizotinib group [HR, 0.91; 95% CI, 0.54-1.54]) with a 2-year overall survival rate of 78% (95% CI, 69%-84%) in the ensartinib group and 78% (95% CI, 70%-85%) in the crizotinib group. The median overall survival was not reached in either group (Figure 3D).
Safety
The most common (≥10% of patients) ensartinib-related AEs were rash (67.8% [97 of 143]), elevated transaminase levels (aspartate aminotransferase, 37.8% [54 of 143]; alanine aminotransferase, 48.3% [69 of 143]), pruritus (26.6% [38 of 143]), nausea (22.4% [32 of 143]), constipation (20.3% [29 of 143]), edema (21.0% [30 of 143]), anemia (14.0% [20 of 143]), vomiting (11.9% [17 of 143]), blood alkaline phosphatase increase (13.3% [19 of 143]), blood creatinine increase (14.0% [20 of 143]), γ-glutamyltransferase increase (13.3% [19 of 143]), and decreased appetite (11.2% [16 of 143]), most of which were grade 2 or less (Figure 4). Grade 3 rash was reported in 11.2% of patients (16 of 143) and was managed by withholding and reducing the dose; the median duration of grade 3 rash was 18 days. The treatment-emergent AEs occurring with both ensartinib and crizotinib are listed in eTable 2 in Supplement 2.
Figure 4. Treatment-Related Adverse Events Reported in 10% or More of All Patients.
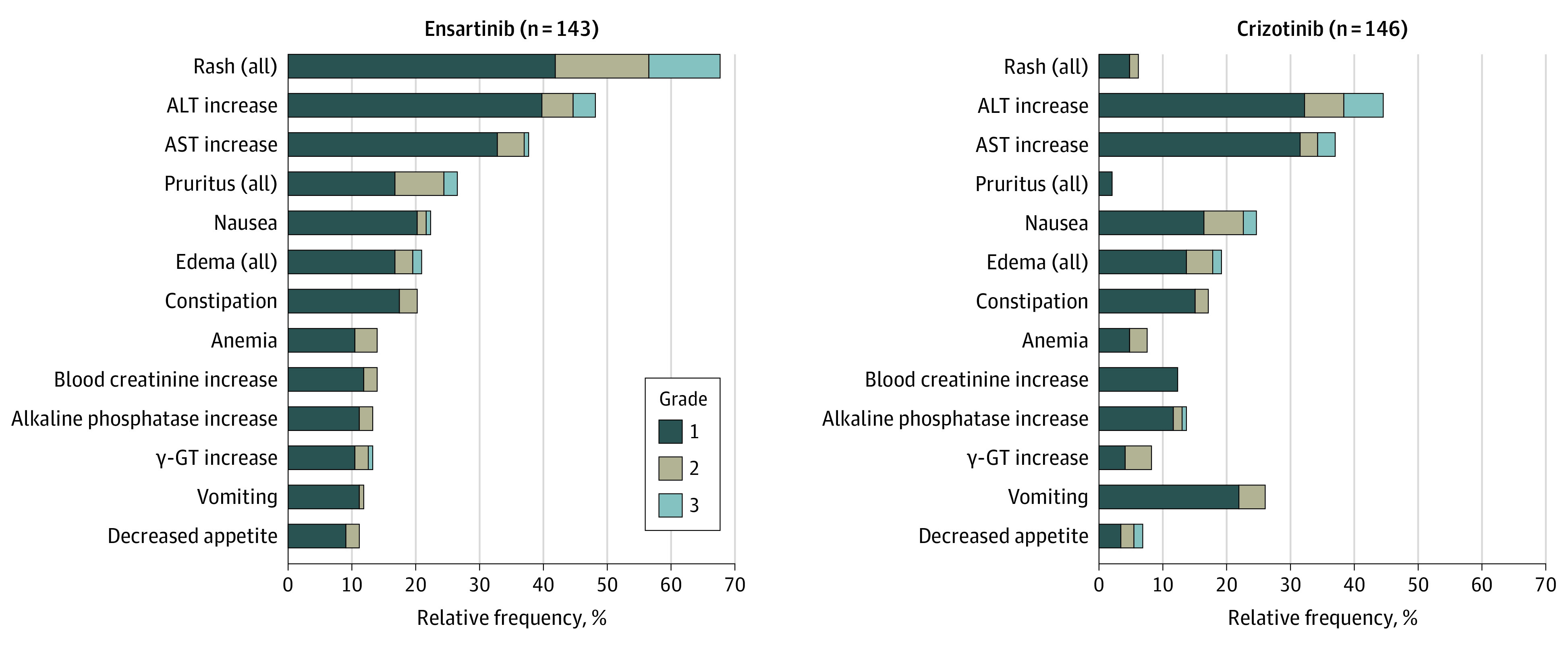
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; and γ-GT, γ-glutamyltransferase.
Only 3 grade 4 AEs associated with ensartinib were observed (increased bilirubin level, increased creatine phosphokinase level, and hyponatremia). The most frequent AEs associated with crizotinib were liver toxic effects, nausea, edema, and constipation. The frequency of treatment-related serious AEs (ensartinib, 11 patients [7.7%]; crizotinib, 9 patients [6.1%]) was low and, for ensartinib, consisted mainly of rash (n = 3) and liver toxic effects (n = 3). The treatment-related AEs associated with dose reduction (ensartinib, 34 of 143 [23.8%]; crizotinib, 29 of 146 [19.9%]) and dose discontinuation (ensartinib, 13 of 143 [9.1%]; crizotinib, 10 of 146 [6.8%]) were similar. Adverse effects associated with treatment discontinuation of ensartinib included increased liver enzymes (n = 12), pneumonitis (n = 2), chest pain (n = 1), pyrexia (n = 1), abnormal hepatic function (n = 1), vomiting (n = 1), dry skin (n = 1), hyponatremia (n = 1), cerebral infarction (n = 1), interstitial lung disease (n = 1), and rash (n = 1), whereas most dose reductions were associated with fatigue, rash, transaminase elevation, constipation, edema, and sinus bradycardia. No treatment-related deaths were reported in either group.
Discussion
In the eXalt3 study, ensartinib showed superior efficacy compared with crizotinib against both systemic and intracranial disease among patients with ALK-positive NSCLC who had not received any prior ALK inhibitor. At the planned 75% interim analysis, the prespecified threshold for significance for the primary end point of mPFS as assessed by the blinded independent review committee was met. With a median follow-up of 23.8 months in the ensartinib group and 20.2 months in the crizotinib group, ensartinib was associated with a 49% lower risk of disease progression or death (HR, 0.51; log-rank P < .001). In addition, the blinded independent review committee–assessed mPFS with ensartinib was 25.8 months, which is consistent with the ALTA-1L27 (24.0 months) and ALEX17 (25.7 months) studies, which explored brigatinib vs crizotinib and alectinib vs crizotinib, respectively, also in the same first-line setting with an open-label design and independent radiologic assessment. (In the CROWN study,18 PFS was not reached with lorlatinib.) Cross-trial comparisons should be interpreted with caution owing to differences in enrolled populations and different performances of the control groups.
The prespecified, centrally tested ALK mITT population is unique to this study because previous phase 3 studies either mandated central testing from the study start (ALEX17) or accepted local testing (CROWN18 and ALTA-1L27). In the mITT population, the mPFS with ensartinib was not reached and was potentially trending toward longer PFS with further follow-up of ongoing patients and an anticipated rate of less than 1 event per month in the experimental group. This data point seems comparable to the one recently reported in the CROWN study,18 although the median follow-up time was 6 months shorter for lorlatinib than ensartinib and the populations differed owing to prior chemotherapy not being allowed. A notable earlier separation of the PFS curves (at approximately 3 vs 6 months) than seen in other second-generation ALK inhibitor studies with alectinib and brigatinib17,28 suggests higher efficacy with ensartinib for patients with primary resistance, despite differences in study populations, and warrants further investigation to identify the molecular basis behind such primary resistance. Primary and acquired resistance patterns to first-line ALK inhibitors will be the determinants to select an agent of choice and the sequencing of next-generation ALK inhibitors. Reports of exploratory biomarker data from all 4 recent phase 3 studies should expand our understanding of the dynamic evolution of resistance patterns and may lead to the choice of an optimal first-line strategy.29,30
Consistent with previous results,20 ensartinib continued to demonstrate efficacy in controlling intracranial disease. In the small group of 11 patients treated with ensartinib who had measurable brain metastases at baseline and a valid postbaseline assessment, the confirmed objective response rate was 64%, with a disease control rate of 100%. Such activity against brain metastases mirrors what has been observed in previous reports of ensartinib in phase 1 and 2 data sets that showed consistent waterfall plots of very high disease control rates of target brain metastases.20,31 The cohorts of patients with target brain lesions in the other 3 available randomized clinical trials16,17,18 with ALK inhibitor vs crizotinib consist of 17 to 21 patients and are therefore too small to draw relevant comparisons, especially considering other factors, such as prior therapies (eg, brain radiotherapy or chemotherapy) that could also account for differences across studies. However, all these agents represent a step toward controlling intracranial disease in the brain compared with first-generation ALK inhibitors. More importantly, on the clinical management side, the incidence of intracranial failure with ensartinib was significantly lower than that with crizotinib (4.2% vs 23.9% at 12 months) among patients without brain metastases, the larger proportion of patients with newly diagnosed ALK-positive NSCLC; consequently, the mPFS among patients without brain metastases at baseline was not reached with ensartinib, suggesting durable protection against disease spreading to the brain. Overall survival data for this trial are currently too immature to draw definitive conclusions.
The safety profiles of ensartinib and crizotinib were consistent with those in previous studies.20,26 The number of dose reductions or discontinuations owing to treatment-related AEs was similar between treatment groups, as was the frequency of serious AEs. Consistent with findings from the eXalt2 study,32 rash and other skin toxic effects (eg, pruritus) continue to be the most frequently observed AEs with ensartinib, together with transaminitis and edema, which were mostly low grade and asymptomatic. Of patients who experienced grade 3 rash (11.2%), all instances were successfully managed by withholding or reducing the dose. Gastrointestinal (eg, diarrhea and vomiting), ocular, and cardiac toxic effects were less frequent with ensartinib; thus, it compares favorably with other second-generation ALK inhibitors, such as alectinib17 or brigatinib,28 in terms of overall safety profile. The distinct safety profile of this class of agents possibly stems from a different pattern of off-target effects resulting from their kinase profiles.
Limitations
This interim analysis has some limitations. The overall survival data are immature and potentially confounded by subsequent use of other ALK tyrosine kinase inhibitors at progression in both study groups outside the current protocol. With further follow-up, data in both groups will mature and help to better contextualize the role of ensartinib compared with other next-generation ALK inhibitors. Another limitation is that the study included patients whose ALK status was not centrally confirmed, thus possibly impacting efficacy outcomes in the ITT population.
Conclusions
Ensartinib represents a new first-line treatment option for patients with ALK-positive NSCLC. In this randomized clinical trial, ensartinib showed superior systemic and intracranial efficacy compared with crizotinib and an overall favorable safety profile that is distinct from that of other agents in this class. A more precise understanding of primary and acquired resistance mechanisms is warranted to establish the optimal sequence of ensartinib and other available second- and third-generation ALK inhibitors in the first-line setting.
Trial Protocol and Statistical Analysis Plan
eTable 1. Rates of Confirmed Objective Response by BIRC
eTable 2. Treatment-Emergent Adverse Events in ≥10% of the Safety Population
eTable 3. List of Investigators
eFigure 1. Randomization Schema for ITT and mITT Populations
eFigure 2. BIRC-Assessed Best Systemic Change from Baseline in the mITT Population
eFigure 3. BIRC-Assessed Intracranial Best Change From Baseline in Patients With Measurable and Evaluable Brain Metastases in the mITT Population
eFigure 4. BIRC-Assessed Time to Progression in the Brain in Patients Without Brain Metastases at Baseline in the Modified Intention-to-Treat Population
eFigure 5. Median Progression-Free Survival (mPFS) in Patients With Brain Metastases at Baseline in mITT
Data Sharing Statement
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561-566. doi: 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 2.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275-4283. doi: 10.1158/1078-0432.CCR-08-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998-2006. doi: 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15(9):3143-3149. doi: 10.1158/1078-0432.CCR-08-3248 [DOI] [PubMed] [Google Scholar]
- 5.Lin E, Li L, Guan Y, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res. 2009;7(9):1466-1476. doi: 10.1158/1541-7786.MCR-08-0522 [DOI] [PubMed] [Google Scholar]
- 6.Chuang JC, Neal JW. Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Transl Lung Cancer Res. 2015;4(5):639-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network . NCCN Clinical practice guidelines in oncology: non-small cell lung cancer. Accessed October 5, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 8.Xalkori (crizotinib). Prescribing information. Pfizer; 2011. Accessed October 5, 2020. http://labeling.pfizer.com/showlabeling.aspx?id=676
- 9.McCusker MG, Russo A, Scilla KA, Mehra R, Rolfo C. How I treat ALK-positive non-small cell lung cancer. ESMO Open. 2019;4(suppl 2):e000524. doi: 10.1136/esmoopen-2019-000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881-1888. doi: 10.1200/JCO.2014.59.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443-e445. doi: 10.1200/JCO.2010.34.1313 [DOI] [PubMed] [Google Scholar]
- 12.Rothenstein JM, Chooback N. ALK inhibitors, resistance development, clinical trials. Curr Oncol. 2018;25(suppl 1):S59-S67. doi: 10.3747/co.25.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118-1133. doi: 10.1158/2159-8290.CD-16-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw AT, Solomon BJ, Besse B, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37(16):1370-1379. doi: 10.1200/JCO.18.02236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20(12):1691-1701. doi: 10.1016/S1470-2045(19)30655-2 [DOI] [PubMed] [Google Scholar]
- 16.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol. 2020;38(31):3592-3603. doi: 10.1200/JCO.20.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters S, Camidge DR, Shaw AT, et al. ; ALEX Trial Investigators . Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377(9):829-838. doi: 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, Bauer TM, de Marinis F, et al; CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018-2029. doi: 10.1056/NEJMoa2027187 [DOI] [PubMed] [Google Scholar]
- 19.Lovly CM, Heuckmann JM, de Stanchina E, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 2011;71(14):4920-4931. doi: 10.1158/0008-5472.CAN-10-3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-positive non–small cell lung cancer: results from a first-in-human phase I/II, multicenter study. Clin Cancer Res. 2018;24(12):2771-2779. doi: 10.1158/1078-0432.CCR-17-2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Dixon JR Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Common terminology criteria for adverse events: version 4.03. Accessed July 29, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx
- 25.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13(13-14):1341-1352. doi: 10.1002/sim.4780131308 [DOI] [PubMed] [Google Scholar]
- 26.Solomon BJ, Mok T, Kim DW, et al. ; PROFILE 1014 Investigators . First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167-2177. doi: 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 27.Camidge DR, Kim H, Ahn M, et al. Brigatinib vs crizotinib in patients with ALK inhibitor–naive advanced ALK+ NSCLC: updated results from the phase III ALTA-1L trial. Ann Oncol. 2019;30(suppl 9):ix195-ix196. [Google Scholar]
- 28.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med. 2018;379(21):2027-2039. doi: 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- 29.Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36(12):1199-1206. doi: 10.1200/JCO.2017.76.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou S-HI, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non–small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34(7):661-668. doi: 10.1200/JCO.2015.63.9443 [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Zhou J, Zhou J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med. 2020;8(1):45-53. doi: 10.1016/S2213-2600(19)30252-8 [DOI] [PubMed] [Google Scholar]
- 32.Wakelee H, Reckamp K, Leal T, et al. Rash and efficacy in anaplastic lymphoma kinase positive (ALK+) non-small cell lung cancer patients treated with ensartinib. J Thoracic Oncol. 2019;14(10S):S566. Abstract P1.14-32. doi: 10.1016/j.jtho.2019.08.1183 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Rates of Confirmed Objective Response by BIRC
eTable 2. Treatment-Emergent Adverse Events in ≥10% of the Safety Population
eTable 3. List of Investigators
eFigure 1. Randomization Schema for ITT and mITT Populations
eFigure 2. BIRC-Assessed Best Systemic Change from Baseline in the mITT Population
eFigure 3. BIRC-Assessed Intracranial Best Change From Baseline in Patients With Measurable and Evaluable Brain Metastases in the mITT Population
eFigure 4. BIRC-Assessed Time to Progression in the Brain in Patients Without Brain Metastases at Baseline in the Modified Intention-to-Treat Population
eFigure 5. Median Progression-Free Survival (mPFS) in Patients With Brain Metastases at Baseline in mITT
Data Sharing Statement