Abstract
Background:
Nonalcoholic fatty liver disease is associated with cardiovascular risk factors in cross-sectional analyses. However, less is known about how changes in liver fat associate with the progression of cardiovascular risk factors.
Methods:
A substudy (n = 808) drawn from the Framingham Heart Study underwent serial computed tomography scans 6 years apart. We performed multivariable-adjusted regression to determine the association between changes in liver fat and progression of cardiovascular risk factors.
Results:
Each standard deviation increase in liver fat was associated with adverse progression of systolic blood pressure, diastolic blood pressure, fasting glucose, high-density lipoprotein and log triglycerides. After adjusting for baseline cardiovascular risk, baseline body mass index (BMI), and change in BMI, increasing liver fat was significantly associated with adverse changes in fasting glucose and triglycerides.
Conclusions:
In a longitudinal cohort, increasing liver fat over 6 years was associated with progression of cardiovascular risk factors, even after accounting for BMI changes.
Keywords: BMI, computed tomography, diabetes, metabolic syndrome, NAFLD
1 |. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) affects an estimated 25% of the globe’s adult population1 and is the most common chronic liver disease in industrialized countries.2 Individuals with NAFLD are at increased risk for all-cause and cardiovascular disease (CVD)-related mortality.3 We have previously shown that liver fat assessed by computed tomography (CT) at baseline is associated with incident cardiovascular risk factors, including diabetes, hypertension and impaired fasting glucose (IFG).4 Liver fat is a dynamic process that can regress or progress, even independent of weight loss.5 However, few observational studies have investigated how changes in liver fat content over time are associated with the progression CVD risk factors, after accounting for covariates, including changes in body mass index (BMI).
The aim of this study was to examine the longitudinal associations between changes in liver fat with the progression of multiple CVD risk factors over time. We hypothesized that those with increasing liver fat would have adverse progression of CVD risk factors over the 6-year follow-up period.
2 |. METHODS
The Framingham Heart Study is a large, prospective, multigenerational cohort study of CVD.6 Our study sample was drawn from Third Generation Cohort participants who underwent multidetector CT scans an average of 6 years apart (baseline exam 2002–2005; follow-up exam 2008–2011) (n = 1160), as a part of a substudy.7–9 We excluded participants who reported significant alcohol use, defined as >7 drinks per week for women and >14 drinks per week for men, at either baseline or follow-up (n = 248), were missing baseline covariate data (n = 1) or were missing evaluation of liver fat at either baseline or follow-up (n = 93). This resulted in a final sample size of 808 participants for the progression of CVD risk factor analyses. This study was approved by the institutional review boards of the Boston University Medical Center and Massachusetts General Hospital. All participants provided written informed consent.
2.1 |. Assessment of change in liver fat
The primary exposure was change in liver fat between CT scans. We quantified liver fat using the liver phantom ratio (LPR), which has been described previously.4,10 LPR was calculated from the mean liver fat attenuation, in Hounsfield units, from three areas of the liver compared to a standardized phantom present on all scans. A decrease in the LPR corresponds to an increase in liver fat. NAFLD is defined as an LPR of less than or equal to 0.33.
2.2 |. CVD risk factor assessment
CVD risk factors were measured at both the baseline and follow-up exams. Plasma glucose, total cholesterol, high-density lipoprotein(HDL) cholesterol and triglycerides were assessed from fasting blood samples.
2.3 |. Anthropometry and covariate assessment
Weight, height, smoking status and alcohol intake were recorded at both baseline and follow-up exam visits. Standing height was recorded, as was weight with light clothing, by trained technicians. Quantification of alcohol use and current smoking status was evaluated by a physician administered questionnaire. Participants were considered a current smoker if they smoked >1 cigarette in the year preceding the examination.
2.4 |. Statistical analysis
Multivariable-adjusted linear regression models were constructed to assess associations between changes in liver fat attenuation and changes in CVD risk factors. The β-coefficients for the linear regression models are presented per 0.05 unit decrease in LPR, which is approximately a one standard deviation decrease in liver fat.
We performed two multivariable models to account for potential confounding variables. The first multivariable model adjusted for age, sex, baseline LPR, current smoking, baseline drinks per week and baseline continuous CVD risk factors including: diastolic and systolic blood pressure, fasting glucose, total cholesterol, HDL, triglycerides and treatment variables at baseline and follow-up. The second model added adjustments for baseline BMI and change in BMI. We tested for interaction by sex and serum aminotransferase levels. We performed a sensitivity analysis by adding adjustment for baseline CT-derived visceral fat and change in visceral fat to the multivariable model.
We also stratified participants into groups according to quartiles of LPR change and examined CVD risk factor progression across groups. The top quartile of LPR change (most negative quartile of change in LPR) was defined as increasing liver fat and the bottom quartile of LPR change (most positive quartile of change in LPR) was defined as decreasing liver fat. The middle two quartiles were combined and defined as stable liver fat. We used linear regression to assess for a trend in outcomes across categories.
A 2-tailed P < .05 was considered statistically significant. To account for multiple testing, we used the Benjamini-Hochberg procedure11 with a false discovery rate threshold of 0.01 or 0.05. We used SAS software version 9.3 (SAS Institute, Cary, North Carolina) to perform all statistical analyses.
3 |. RESULTS
3.1 |. Participant characteristics
The baseline characteristics of the study sample are described in Table 1. The mean age of the participants was 45 ± 6 years. Participants were followed for a mean of 6 years, to a mean age of 51 ± 6 years, at which point the mean BMI had increased from 27.2 ± 5.1 to 28.2 ± 5.4 kg/m2 and the prevalence of NAFLD increased from 14% to 24%.
TABLE 1.
Baseline characteristics of the participants
| Characteristics | Overall Participants (n = 808) |
|
|---|---|---|
| Baseline | Follow-up | |
| Age, y | 45.2 ± 6.3 | 51.3 ± 6.3 |
| Women, n (%) | 379 (46.9%) | |
| Smoking, n (%)a | 80 (9.9%) | 58 (7.2%) |
| Drinks per week | 2.8 ± 3.3 | 3.0 ± 3.3 |
| Weight (kg) | 79.9 ± 17.1 | 82.2 ± 18.0 |
| Body mass index, kg/m2 | 27.2 ± 5.1 | 28.2 ± 5.4 |
| Waist circumference, cm | 94.9 ± 14.1 | 98.3 ± 14.1 |
| Liver phantom ratio | 0.36 ± 0.05 | 0.35 ± 0.07 |
| Systolic blood pressure, mmHg | 118 ± 14 | 117 ± 13 |
| Diastolic blood pressure, mmHg | 76 ± 9 | 75 ± 8 |
| Fasting glucose, mg/dL | 97 ± 20 | 98 ± 19 |
| HDL cholesterol, mg/dL | 53 ± 16 | 58 ± 18 |
| Triglycerides, mg/dL, median (25% to 75%) | 97 (67–142) | 96 (70–134) |
| NAFLD (liver phantom ratio ≤0.33) | 112 (13.9%) | 193 (23.8%) |
Note: Data are represented as mean ± standard deviation or n, % unless otherwise noted.
Abbreviation: HDL, high-density lipoprotein.
Defined as smoking >1 cigarette per day over the past year.
3.2 |. Association between change in continuous liver fat and change in CVD risk factors
One standard deviation increase in liver fat from baseline to follow-up was associated with an increase in SBP (β 1.84; 95% confidence interval (CI) 1.18–2.50), DBP (β 0.93; 95% CI 0.47–1.39), glucose (β 2.69; 95% CI 1.74–3.64), triglycerides (β 0.06; 95% CI 0.04–0.08) and a decrease in HDL cholesterol (β −1.27; 95% CI −1.85 to −0.69) in multivariable-adjusted models. For HDL cholesterol, we did observe a significant sex interaction (P = .001) with results overall stronger for women compared to men (β −1.74; 95% CI −2.77 to −0.71 for women and β −0.93; 95% CI −1.59 to −0.27 for men). However, after additionally accounting for baseline and change in BMI, increases in liver fat only remained associated with increases in glucose (β 2.01; 95% CI 0.94–3.07) and increases in triglycerides (β 0.03; 95% CI 0.01–0.06), but not associated with SBP (β 0.46; 95% CI −0.24 to 1.15), DBP (β 0.12; 95% CI −0.37 to 0.61), total cholesterol (β −0.04; 95% CI −2.10 to 2.02) and HDL (β −0.44, 95% CI −1.07 to 0.19). In the sensitivity analysis, replacing baseline and change in CT-derived visceral fat with BMI in the multivariable model did not meaningfully change the results (data not shown). After using the Benjamini-Hochberg procedure to evaluate for multiple testing, all of the models remained significant.
3.3 |. Association between categories of liver fat change and change in CVD risk factors
We observed a significant linear adverse trend across liver fat change categories (decreasing liver fat, stable liver fat and increasing liver fat) for the change in the least-square mean SBP, DBP, glucose and HDL cholesterol (Figure 1). Change in total cholesterol and change in triglycerides showed no significant difference across the groups.
FIGURE 1.
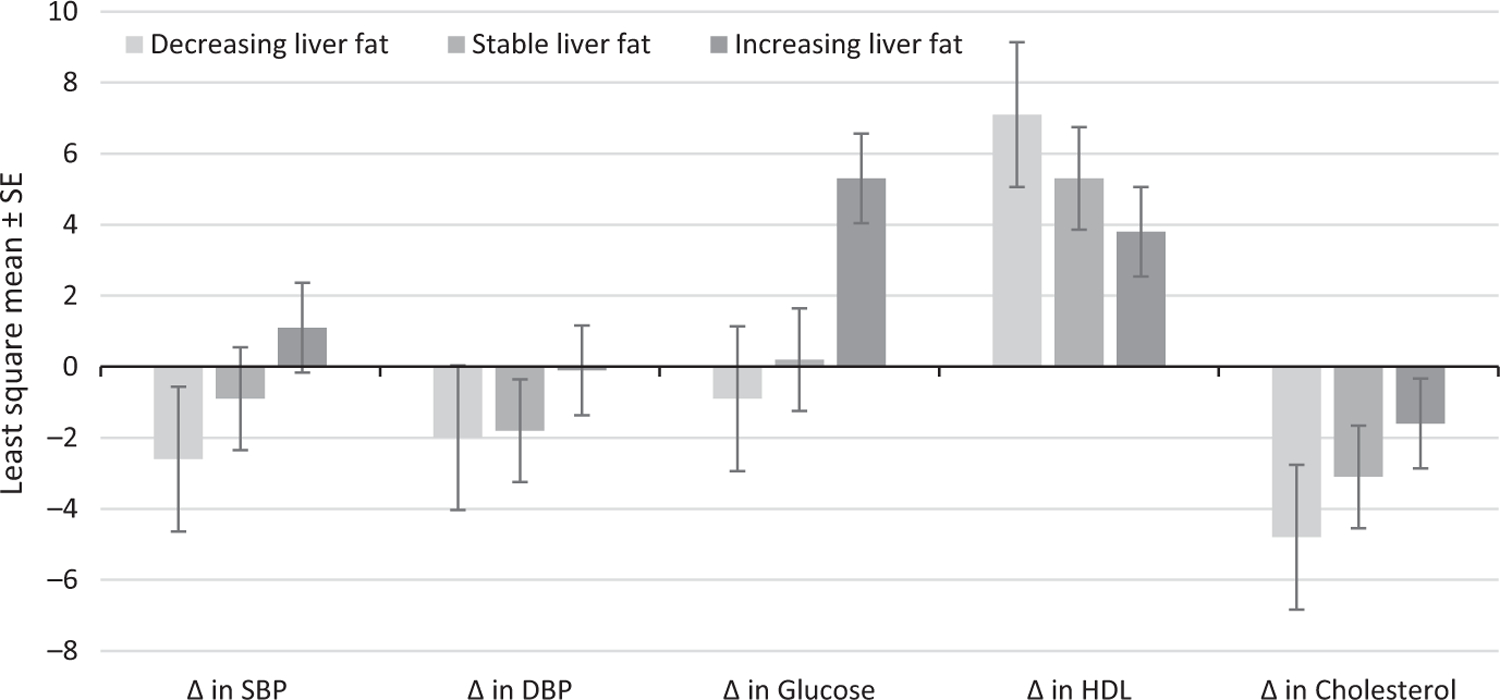
Multivariable-adjusted least-square means of the change in cardiovascular disease risk factors by categories of liver fat change. The categories of liver fat change were defined as decreasing liver fat (bottom quartile of liver phantom ratio change; n = 202), stable liver fat (middle two quartiles of liver phantom ratio change; n = 404), and increasing liver fat (top quartile of liver phantom ratio change; n = 202). P values are for the test for trend. Models were adjusted for baseline age, sex, baseline smoking status, baseline drinks per week, baseline liver phantom ratio, baseline outcome variables and treatment variables at baseline and follow-up
4 |. DISCUSSION
In our longitudinal, observational cohort study, we characterized the association between changes in liver fat and several cardiometabolic risk factors. We observed a general increase in liver fat, along with BMI, over the approximately 6-year long follow-up period, as well as the adverse progression of several CVD risk factors. After accounting for increasing BMI over the follow-up period, increases in glucose and triglycerides remained significantly associated with an adverse progression of liver fat. Among both sexes, changes in other CVD risk factors, including SBP, DBP and HDL did not remain significant after adjusting for change in BMI, perhaps indicating that progression of these variables may be accounted for by increasing BMI.
Our longitudinal study builds on prior cross-sectional studies, which show an association between NAFLD and cardiometabolic risk factors including obesity, diabetes,12 dyslipidaemia10 and hypertension.13 Additionally, a history of fatty liver at baseline is a risk factor for incident CVD risk factors.4 Few prior observational studies correlate changes in liver fat with changes in CVD risk factors. One large Korean-based study utilized ultrasound to define liver fat, which is not quantitative, so they were not able to investigate the association between increasing liver fat and CVD risk factors.14 Those with resolution of liver fat over 4.9 years of follow-up had significant improvements in SBP, DBP, total cholesterol, HDL and triglycerides, even after adjusting for baseline BMI and change in BMI,14 which is in contrast to our study. There are a number of possible explanations for these discrepant results. In the Korean study, those with known baseline diabetes, hypertension, CVD or dyslipidaemia were excluded.14 Our sample included those with cardiometabolic disease at baseline so it is possible that improvements in liver fat are not associated with progression of CVD risk factors among a sample of participants with established disease. It is also possible that the improvements in liver fat in our study were not substantial enough to confer a benefit on CVD risk factors, or that ethnic differences in the underlying populations could explain this discrepancy.
Many studies assess the relationship between changes in liver fat and CVD risk factors in the context of an intervention, such as exercise or dietary modification. However, these studies tend to have limited follow-up, evaluate select populations, such as individuals with obesity15 or diabetes,16,17 and may not adjust for changes in weight or BMI. A small study of 28 participants with diabetes who were randomized to an exercise programme showed improvement in liver fat measured by magnetic resonance (MR) spectroscopy at 12 weeks, along with significant improvement in glycosylated haemoglobin, but not in total cholesterol, SBP, DBP or fasting glucose compared to controls, though this study did not account for changes in BMI.16 Other small intervention studies with short-term follow-up, that have resulted in decreased or increased MR spectroscopy-defined liver fat, have not observed changes in CVD risk factors.15 In the Fatty Liver Ancillary Study of the Look AHEAD (Action for Health in Diabetes) clinical trial, lifestyle modification among 96 participants with diabetes reduced NAFLD at 12 months, and changes in liver fat were significantly associated with improvements in various cardometabolic risk factors; however, the results were no longer significant after accounting for changes in weight or BMI.17 Our study adds to the existing literature by evaluating the natural history of liver fat change in a large community-based cohort carefully phenotyped for CVD risk factors over a relatively long follow-up interval of 6 years.
We have previously shown that increasing abdominal fat volumes, including both CT-derived subcutaneous and visceral adipose tissue, are associated with incident hypertension, hypertriglyceridaemia and metabolic syndrome, but not incident diabetes, even after accounting for changes in BMI.18 In contrast, in this study, we noted a strong association with change in liver fat and increased glucose, but no association with change in SBP or DBP after accounting for BMI change. We have previously demonstrated that visceral fat and hepatic fat contribute more to CVD risk compared to other ectopic fat depots.19 Taken together with our prior work, our study supports the hypothesis that ectopic fat depots may contribute differently to CVD risk,20 though additional studies are needed.
Our study has several clinically relevant implications. First, those with progression of liver fat had an increasing fasting glucose and triglycerides, which may contribute to CVD. Additionally, many of the associations between increasing liver fat and progression of CVD risk factors can be explained by increasing BMI; however, the associations between increasing liver fat and change in glucose and triglycerides remained after accounting for change in BMI. Future studies may add additional information about whether further prognostic information is gained from liver fat quantification versus BMI measurement alone.
Strengths of our work include the 6-year follow-up period and sample size, which has facilitated an improved understanding of the natural history of liver fat and its relationship with several CVD risk factors. Some limitations are worth noting. First, our study is observational and can show associations but not causation. The relationship between changes in liver fat and changes in CVD risk is complex and may be bi-directional. Second, we used a reliable, noninvasive, method of quantifying changes in liver fat attenuation over time as it would be impractical and unethical to perform liver biopsies on a large scale. CT imaging is more specific for fatty liver than blood-based tests; however, CT imaging does not differentiate between simple steatosis versus nonalcoholic steatohepatitis or hepatic fibrosis. We do not know if those with increasing liver fat in our study developed steatohepatitis. Also, as hepatic fibrosis progresses, liver fat may regress, so those characterized as improving liver fat attenuation may include individuals with truly improving disease and those with progressive fibrosis, which may have biased our results towards the null. However, given that our sample was community-based, we expect the proportion with advanced fibrosis to be relatively low. We also do not have information on viral hepatitis status or other secondary causes of NAFLD which may have caused misclassification and biased our results towards the null. Finally, the Framingham cohort is primarily Caucasian-American so our results may not be generalizable to other racial or ethnic groups.
5 |. CONCLUSION
Our findings highlight the dynamic nature of hepatic fat and the importance of quantitative assessment of liver fat to further our understanding of cardiometabolic risk. Additional studies to confirm our findings and to elucidate possible causal mechanisms are needed.
Key points.
Fat in the liver is associated with poor cardiovascular health but less is known about how changes in liver fat impact health.
Increasing fat in the liver over time is linked to adverse changes in fasting blood sugar and triglyceride levels
Funding information
Dr Brunner is supported in part by the Boston University Clinical and Translational Science Institute (grant UL1-TR000157). Dr Long is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252, Gilead Sciences, the Doris Duke Charitable Foundation and the Boston University School of Medicine Department of Medicine Career Investment Award. Dr Benjamin is supported by R01HL128914; 2R01 HL092577; 2U54HL120163; American Heart Association, 18SFRN34110082; The Framingham Heart Study is funded by HHSN268201500001I; N01-HC 25195.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- CT
Computed tomography
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- LPR
liver phantom ratio
- MR
magnetic resonance
- MV
multivariable
- NAFLD
nonalcoholic liver disease
- OR
odds ratio
Footnotes
CONFLICTS OF INTEREST
Alison Pedley is an employee of Merck. The other authors have no conflicts to report.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‒meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. [DOI] [PubMed] [Google Scholar]
- 4.Ma J, Hwang S-J, Pedley A, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol 2017;66:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism 2017;68:119–132. [DOI] [PubMed] [Google Scholar]
- 6.Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 9.Roseman DA, Hwang S-J, Manders ES, et al. Renal artery calcium, cardiovascular risk factors, and indexes of renal function. Am J Cardiol 2014;113:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology 2010;51:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995;57:289–300. [Google Scholar]
- 12.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–285. [DOI] [PubMed] [Google Scholar]
- 13.Juneja A Non-alcoholic fatty liver disease (NAFLD)‒the hepatic component of metabolic syndrome. JAPI 2009;57:201. [PubMed] [Google Scholar]
- 14.Sung K-C, Lee M-Y, Lee J-Y, et al. Resolution of fatty liver and weight loss: Independent associations with changes in serum lipids and apolipoproteins. Atherosclerosis 2018;272:47–53. [DOI] [PubMed] [Google Scholar]
- 15.Westerbacka J, Lammi K, Häkkinen A-M, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab 2005;90:2804–2809. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy S, Thoma C, Hallsworth K, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2016;59:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol 2016;68:1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Levy D, Long MT. Visceral and intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: the Framingham heart study. Am J Med 2018;131(684–692):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol 2013;169:166–176. [DOI] [PubMed] [Google Scholar]


