Abstract
The Human Epidermal Growth Receptor 2, or the HER2 is one of the highest expressed negative receptor that constitutes approximately 15-20% of malevolent breast cancerous tumors among women. The prevalence of HER2 has untimely and unfavorable consequences on breast cancer, and its underlying carcinomous cell processes, structures, and growth. Trastuzumab (TRZ), a humanized antibody that is rooted in relatively recent foundations, has been found operational in its construction of treatments against HER2-positive breast cancer. This drug is combined with radiotherapy or chemotherapy to deregulate HER2 genes in the body. However, patients who suffer from evolved tumors in advanced stages of cancer exhibit a good amount of tolerance towards singularly used TRZ treatment. Inversely, the factorization of Tumor Testing Fields (TTFields or TTFs) into cancer therapy revives the functions of a TRZ treatment plan, by sensitizing the HER2 genes to the drug. In turn, this facilitates TRZ to continue limiting cancerous cell multiplication and toxicity levels within the treatment. This research evaluates the aspects and effects of this pairing, both in vivo and in vitro through BT474 cells. The TTFields conduct an electromagnetic boundary, which generates sine-wave radiations to manipulate the HER2 gene structure. The methods followed in the research also examines the gene cell cultures and their viability through solutions like Tryptophan blue, or the Crystal violet which may or may not deliver certain testmants to the experiment. The Western Blot Test and the IHC confirm the presence of antibodies and negative receptors in the BT474 cells. These procedures contribute to the formulation of a treatment plan that overcomes the TRZ-resistant nature of the tumor, which is essentially the aim of the research. Thus, the paper substantiates that a healthy combination of TTF’s with TRZ can enhance the penetration of TRZ after inducing apoptosis due to TTFields therapy. The success of a TTField in undertaking this pursuit makes room for more utilization of it in future cancerous treatment ventures.
Keywords: TTF, trastuzumab, breast cancer
Introduction
One of the reasons for the increased mortality rate among US women is the ever-increasing breast cancer cases. According to the American Cancer Society’s 2015 census, around 60,290 breast carcinoma cases (in situ) were estimated to be identified. Further, of all the malignant tumors, breast cancer makes up for 7-10%, with around 3-4% yearly increase in China [1]. It has also been found that increased activation of human epidermal growth factor receptor 2 (HER2) tyrosine kinase receptor gene is a negative prognostic factor that is responsible for early node-positive breast cancer. Hence, an enhanced explanation of HER2 gene’s mechanism and action could lead to the reshaping of the classification, prognosis, as well as treatment of the disease [2]. Further, one finds a 15-20% highly expressed HER2 gene in various breast cancers which tends to modulate various cell processes including cell survival, proliferation, angiogenesis, invasion and metastasis [3]. Recently, a humanized monoclonal anti-ERBB2 antibody known as Trastuzumab (TRZ, Herceptin) has been discovered which significantly improves clinical outcome for early and advanced HER2-positive breast cancer [4]. TRZ affects HER2 by inhibiting its’ dimerization and hence affecting the growth signals which eventually shuts down the HER2 receptor gene’s expression. This drug also targets the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway and RAS/RAF/MEK/MAP kinase (MAPK) pathways [5]. Besides this, the Fc portion of TRZ participates in antibody-dependent cellular cytotoxicity (ADCC) function [6,7]. Though the antibody showed a high rate of initial effectiveness but the patients suffering from metastatic breast cancer stage developed a primary resistance against the antibody. So, this treatment failed to prove effective in this case [6,8]. Thus, this drawback poses a greater challenge in the treatment of HER2-positive breast cancer patients [2].
Tumor Treating Fields better abbreviated as TTFields is the therapy comprising of alternating electromagnetic field employed with low-intensity electrical fields to suppress cancer cell proliferation in the body [9,10]. This therapy targets to induce an electric field inside the human body to suppress the proliferation and invasion of cancerous cells [11]. This treatment induces apoptosis and hence shows an ability to annihilate the cancerous cells [12]. FDA has granted its approval for the application of TTFields to treat patients with recurrent GBM. This technique is found to increase the life expectancy of the patients. In the case of newly diagnosed GBM, this technique can be coupled with temozolomide preceding the surgery (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100034) (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100034S013) [13,14]. The American Society of Clinical Oncology (ASCO) regards this treatment as an advancement in cancer treatment due to its novel approach, effectiveness, and low toxicity profile [15]. The National Comprehensive Cancer Network (NCCN), recommends TTFields as a category 2A treatment for patients with newly diagnosed GBM and resulting in a good performance status (https://www.nccn.org/professionals/physician_gls/f_guidelines.asp). In contrast to the typically employed methods (chemotherapy and radiation), TTF doesn’t cause side effects like pain, nausea, fatigue or diarrhea [16]. Still, this technique is associated with some flaws noted in the trials which include topical skin rashes caused by prolonged electrode use. However, the effectiveness of TTF is considered to be similar to that of chemotherapy or radiotherapy. Subsequently, we studied TTF and TRZ’s combined antitumor effects on HER2-positive breast cancer representative cell lines alongside a tumor xenograft model. The results showed that the TTF therapy can enhance significantly the growth inhibition induce by TRZ.
This paper aims to demonstrate the mechanism of TTFields on HER2-positive TRZ-resistant breast cancer cell lines in humans. An explanation of these mechanisms can enhance our understanding of breast cancer, thus helping in discovering novel treatment strategies.
Materials and methods
Experimental setup of the electric fields
The generation of TTFs took place via a pair of insulated wires linked to an amplifier of high voltage and a functional generator, creating sine-wave signals within the range of 0-800 V [17].
Cell culture
JIMT-1 and BT-474 (ACC-589) ductal carcinoma cells were derived from the American Type Tissue Culture Collection. The maintenance of BT-474 and JIMT-1 cells took place in DMEM-F12 supplemented via 2 mmol/L glutamine, 10% heat-inactivated fetal bovine serum (FBS), as well as 1% penicillin G-streptomycin. Maintenance of cells was done at a temperature of 37°C with 5% CO2.
Cell viability assay
Trypan blue exclusion assay determined the cell viability. Microscopy examined the percentage of viable cells and an equal volume of trypan blue reagent was added to a cell suspension. Assays were then performed in triplicate. To quantify cell viability, an equal volume of culture medium containing EZ-Cytox reagent (EZ3000, Daeillab Service, Chungcheongbuk-do, Republic of Korea) was added to the cells, and the mixture was incubated for 4 h. Cell viability was determined by measuring the absorbance at 450 nm using a Multiskan EX (Thermo Fisher Scientific; Waltham, MA, US).
Colony formation assay (CFA)
After being subjected to the TTFields 6 h following TRZ exposure at 5 μmol/L final concentration, cells were incubated for a period of 48 h. Colonies were stained with 0.4% Crystal Violet after 14-20 d (Sigma, St. Louis, MO, USA). The plating efficiency (PE) is indicative of the percentage of a particular cell line’s seeded cells that were generated under distinct conditions for culture. Expressed as an irradiation function, the survival fraction was calculated in the following manner: survival fraction = colonies counted/(cells seeded × PE/100).
Western blot analysis
The extraction of total proteins from OS cells took place using RIPA buffer (50 mM Tris-Cl, pH 7.4; 1% NP-40; 150 mM NaCl, and 1 mM EDTA), eventually complemented by protease inhibitors (1 μg/ml aprotinin, 1 mM PMSF, 1 mM Na3VO4, and 1 μg/ml leupeptin). The Bradford method was leveraged for quantification purposes. The protein samples (30 μg) were separated by SDS/polyacrylamide gel electrophoresis and were subsequently shifted to a nitrocellulose membrane. This was followed by blocking non-specific antibody binding sites, after which the incubation of membrane took place at 4°C overnight with mouse monoclonal antibodies. After incubation with peroxidase-conjugated secondary antibodies at 37°C for 1 h, the enhanced chemiluminescence reagent (GE Healthcare Biosciences, Pittsburgh, PA, USA) visualized the protein bands whereas detection was taken care of by Amersham Imager 680 (GE Healthcare Biosciences).
Immunohistochemistry
To undertake immunohistochemical assessment, 4-μm thick breast segments embedded with paraffin were mounted atop coated glass slides for the purpose of investigating the proteins. This was followed by blocking endogenous peroxidases, retrieving antigen, and performing nonspecific protein binding. Subsequently, the primary antibodies (bought from Cell Signaling Technology [Danvers, MA, USA]) were initially used for incubating the slide sections, after which secondary antibodies entailed the use of horseradish peroxidase. The development of all slides took place using 3,3’-diaminobenzidine. Their counterstaining took place via hematoxylin. This was followed by blind analysis.
Conjugation of Alexa Fluor 488 to trastuzumab
DMSO was used to prepare a solution of Alexa 488-NHS Ester (Invitrogen, Waltham, MA, USA). This comprised 1% acetic acid and was dissolved in 500 μL TRZ (10 mg/mL) in a solution of 1 M sodium bicarbonate n with a pH of 8.4. The reaction derived was incubated at a room temperature of 1 h, subsequent to which it was purified via size exclusion PD-10 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) before being linked with an ultra-violet/visible detector set at 517-nm maximum wavelength. The measurement of an aliquot (100 μg/μL, PBS, pH 7.2) was done along with conjugated Alexa 488-TRZ via Nano Drop Spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). The Alexa 488 molecules surrounding TRZ were estimated via a high-performance liquid chromatography profile by making a comparison with the peak intensity between Alexa 488 free eluted solution and conjugated Alexa 488-TRZ.
In vivo antibody penetration studies
Through subcutaneous injection, the administration of BT-474 cells (5 × 106) took place into male BALB/nude mice (n = 6 per group). A digital caliper was used to measure the tumor size. To calculate the volume, the formula of width 2 × length × 0.5 was applied. For all mice-related experiments (5-6 weeks old; weighing 18-20 g), the protocol of Institutional Animal Care and Use Committee (IACUC) (number KIRAMS 2018-0016; date of approval: 15 May 2018) of Korea Institute of Radiological and Medical Sciences (KIRAMS) was followed.
When the size of tumor attained ~200 mm3, 150 μg of Alexa 488-TRZ was injected intravenously, following which TTFied (0.9 V/cm) was exposed for five days. Thereafter, the mice were exsanguinated by cardiac puncture before being dissected. The isolated tumors were immediately fixed with paraformaldehyde (4%) before being kept overnight at 4°C. Subsequently, the tumor tissues got embedded within the optimum cutting temperature (OCT) compound before being frozen until further usage at -70°C. Divided into 8 µm-thick portions with a Leica CM 1850 cryostat (Leica microsystems, USA), the frozen tissue samples were rehydrated through the use of PBS, stained using DAPI, and were placed under observation in a fluorescent microscope (In cell analyzer 2200, GE Healthcare, USA). The Click-iT® TUNEL Alexa Fluor® 647 Imaging Assay kit (Invitrogen, Carlsbad, CA, USA) was used to stain TUNEL-positive cells. Further, TUNEL assay was used to calculate the apoptotic cells.
Histological image acquisition
Given that the In cell analyzer is customized with a mosaic stitching software (In cell developer toolbox, GE Healthcare, USA) and fluorescent microscope, fluorescent images were derived with 10 × objective lenses using three independent channels. DAPI was used for Rhodamine lectin, nuclei (shown in blue), and Alexa 488-TRZ (green) to trace blood vessel (red) images. Autofocus was used to determine Offset.
Image analysis of Alexa 488 trastuzumab accumulation in tumor
An in-house program written in MIPAV (NIH, USA) and MATLAB (Math Works, Natick, MA, USA), ZEN (Carl Zeiss Microscopy, Jena, Germany) was utilized to undertake image analysis. The export of individual channels took place in a TIFF file from ZEN (Carl Zeiss Microscopy, Jena, Germany). The reading of derived graph, depicting line intensity versus line distance, was done via the in-house MATLAB program and MIPAV application. The signal intensity of TRZ was observed to be 60 μm from the tumor vessel. Meanwhile, line profiling was used for piloting the TRZ penetration from the tumor vessel by measuring each tumor’s intensity in various ROI from the vessels situated in peripheral and central regions. In addition, the calculation of area underneath the curve was done from 10 sections in three tumors for each one of them in two groups.
Statistical analysis
Statistical significance was determined using ANOVA and Student’s t-test. Differences were considered significant if the P value was less than 0.05, 0.01 or 0.001. (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Inhibited cell proliferation in trastuzumab-resistant HER2-positive human breast cancer cell in Vitro after TTF treatment
To ascertain TTF’s ability to control TRZ resistance, two HER2-positive (Jimt1 and BT474) cell lines were treated for 48 h with 1-100 µg (4-fold dilution).
The acquired data indicated that cells display dose-dependent sensitivity to TRZ (Supplementary Figure 1A). Moreover, treatment with TRZ inhibited cell growth significantly in the TRZ-resistant cells (Supplementary Figure 1B) and both cancer cell lines had decreased cell viability, depending on the various dosages, with approximately 10% viability inhibition observed at 100 μg.
Next, Jimt1 cells was treated with fixed dose of TRZ to evaluate its effects on breast cancer cells in cell morphology and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays and colony forming assay (Figure 1A-C). Jimt1 cells were treated with TTFs (0.9 V/cm), TRZ, or a combination of the two and the changes related to morphology were observed using a phase-contrast microscope in combined treatment group. Also, the cell growth was noted to be significantly inhibited after a 48-h treatment at 100-μg TRZ. These data indicated that TTF increased TRZ sensitivity in TRZ-resistant HER2-positive human breast cancer cell.
Figure 1.
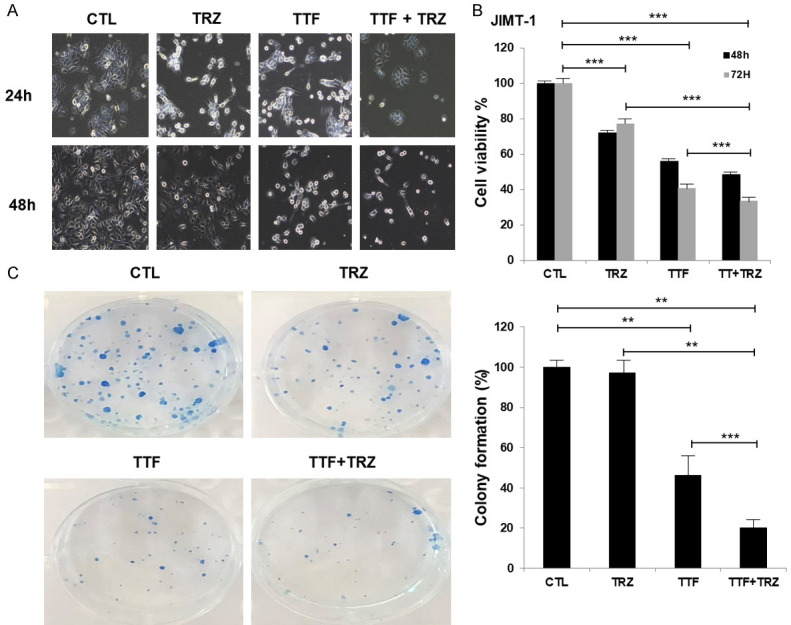
Effects of Trastuzumab-or TTF on cell viability in Trastuzumab-resistant HER2-positive human breast cancer cell. (A) TTF Inhibited Cell Proliferation in Trastuzumab-resistant HER2-positive human breast cancer cell in vitro after TTF Treatment (B) Cell viability (%) using MTT assay (C) colony forming assay. The survival fraction, which was expressed as a function of the irradiation dose, was calculated as follows: survival fraction = colonies counted/(cells seeded × plating efficiency/100). **P < 0.01, ***P < 0.001.
Trastuzumab promotes TTFields sensitivity in vivo
To study TTFs and TRZ’s combined effect on growth of breast cancer in vivo, we utilized a subcutaneous HER2-positive breast cancer model that was achieved by injecting mice with human BT474 cells. As shown in Figure 2A, the xenografts treated with TTF and TRZ combined displayed growth reduction, as compared to the control group and the groups that received either of the treatments. Thus, mono-treated groups’ tumors were markedly larger than those in the combinatorial treatment group. At the same time, the volume of the tumor was reduced in combinatorial treatment mice, compared to the volume in mice that received either of the treatments (Figure 2A).
Figure 2.
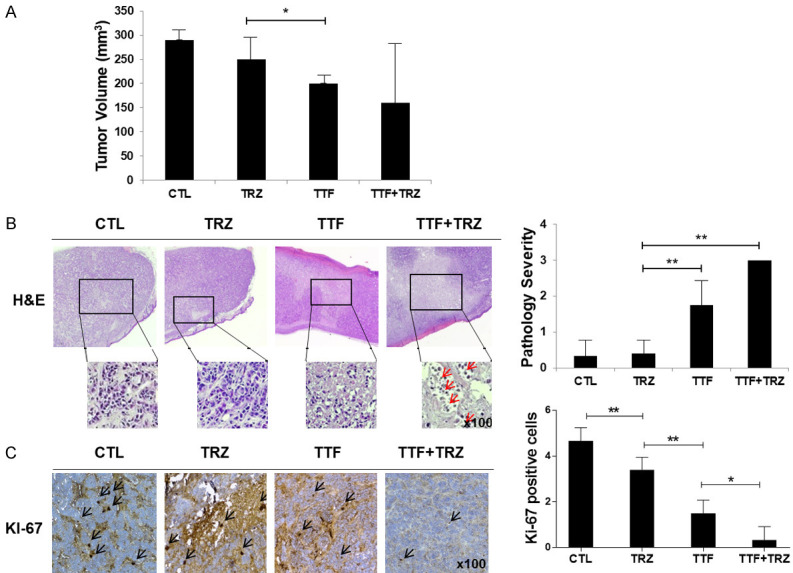
Anti-trastuzumab-resistant tumor effects of TTF in a BT474-cell line human breast cancer xenograft model. (A) Nude mice bearing BT474R cells as xenografts were treated using control (saline), trastuzumab, TTF, combination. Treatment effects on tumor volume (B) Representative hematoxylin and eosin (H&E) staining (C) Representative of Ki-67. Data are presented as the mean ± standard error of the mean (n = 5), *P < 0.05, **P < 0.01.
Xenografts of mice that received either of the treatments depicted stronger proliferation marker Ki-67 staining than that in combinatorial-treated mice (Figure 2B, 2C). Together, the data suggest that TTF combined with TRZ slows down breast cancer growth in vivo.
Treatment with TTF plus TRZ inhibits P-HER2 expression and increases apoptosis marker levels in vitro
p-HER2’s expression levels and apoptosis marker cleaved PARP as well as anti-apoptosis marker Bcl-2 were studied by the western (or protein immunoblot) blotting technique. The expression levels of p-HER2 and Bcl-2 were downregulated and cleaved PARP was upregulated in Jimt1 cell line following 24 h treatment with TTF+TRZ (Figure 3A). To calculate combinatorial therapy’s ability to induce apoptosis in vivo, the apoptotic rate was evaluated through a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Figure 3B). TTF increased apoptotic region were stained by using Click-iT® TUNEL Alexa Fluor® 647 Imaging Assay kit. Apoptotic cells are shown in red (Figure 3C). Notably, upon combinatorial treatment, the cell death in apoptotic cells went up.
Figure 3.
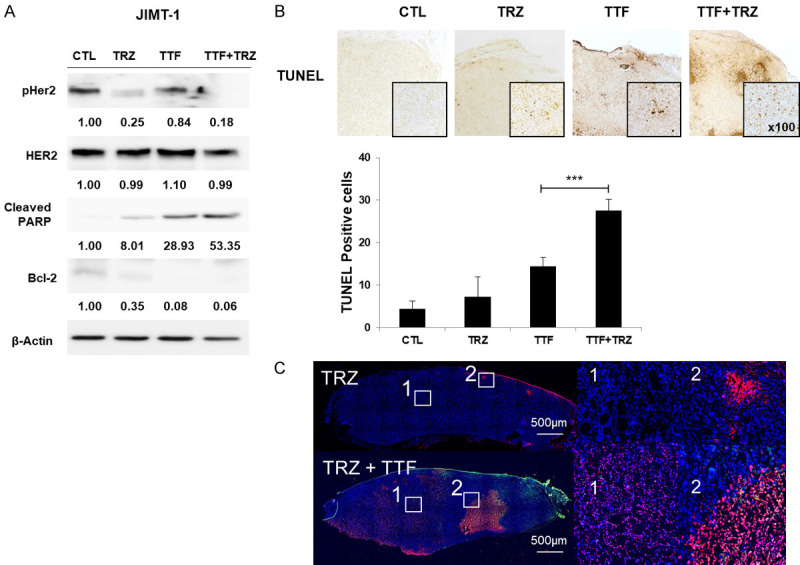
Combination treatment induces cell apoptosis in trastuzumab-resistant HER2-positive cell line. A. Jimt1 cells were treated with DMSO, TRZ (5 µg/ml), TTF, combination for 48 h. Immunoblotting was performed on cell lysates (30 µg) with antibodies against pHER2, HER2, Cleaved PARP, Bcl-2, and β-actin. B. Representative of TUNEL. Data are presented as per the mean ± standard error of the mean (n = 10), *P < 0.05. C. TUNEL-positive cells were stained and imaged with 10 × objective lenses using in cell analyzer. Apoptotic cells are shown in red.
Treatment with TTF inhibits p-HER2 and its downstream mediators AKT and MAPK expression levels in vivo
Histopathological studies showed that tumors that were treated with TTF expressed low HER2 levels, as compared with the control group. Moreover, tumors treated using TTF combined with TRZ expressed lower levels of pAKT, pERK, and pHER2 in comparison to the control group (Figure 4).
Figure 4.
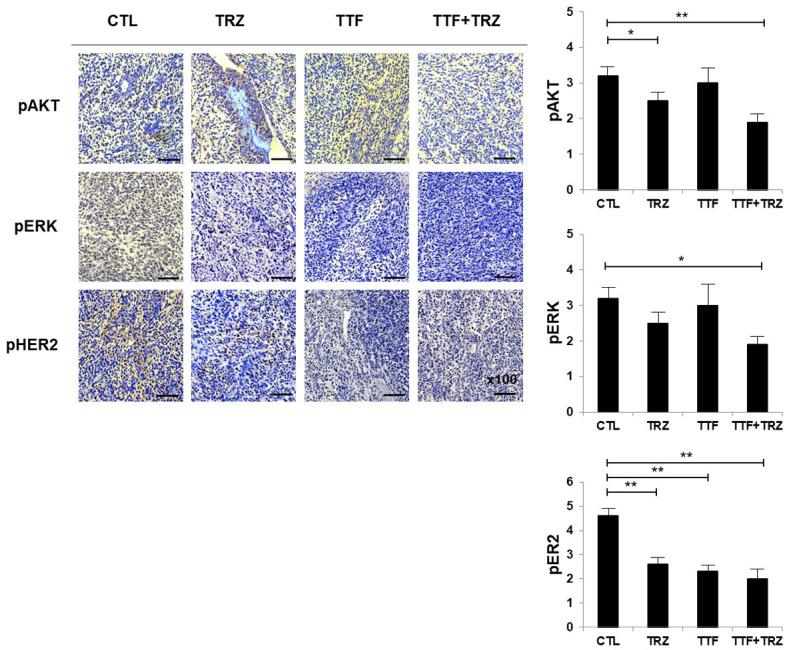
Effects of TTF on the growth factor receptor 2 of human epidermal and downstream signaling pathways. pAKT, pERK, and pHER2 expressions in xenografts were examined using immunohistochemistry. *P < 0.05, **P < 0.01.
Treatment with TTF increases Alexa 488-trastuzumab penetration from the tumor vessel in vivo
Antibody accumulation was determined by analyzing fluorescence intensity in tumor sections. The result shows that Alexa 488-TRZ (green) accumulation was improved with TTF. The TRZ penetration from the tumor vessel in the entire tumor section was ~33% greater in TTF combined treated tumors (P = 0.0211) (Figure 5). TTF significantly improved the extravasation of TRZ within 60 μm from the tumor vessel.
Figure 5.
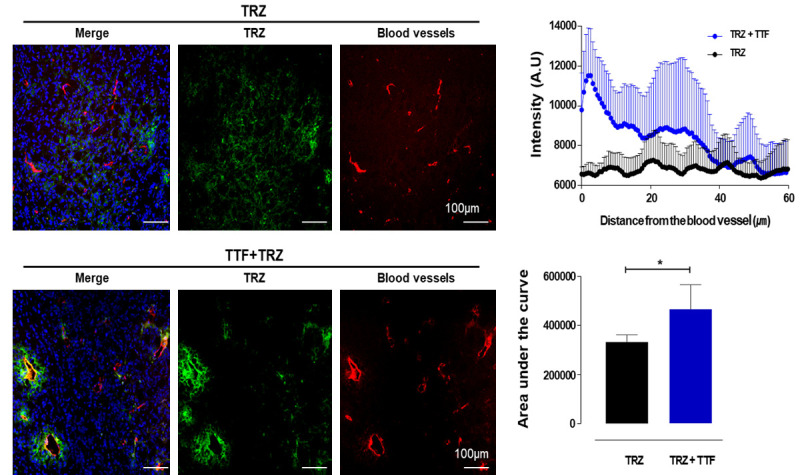
TTF increase the penetration of trastuzumab. Fluorescent images were obtained with 10 × objective lenses, using in cell analyzer. The channels are as follow: DAPI for nuclei (shown in blue), Alexa 488-TRZ (shown in green) and Rhodamine lectin to detect blood vessel (shown in red). The penetration of TRZ from the tumor vessel was plotted with line profiling by intensity measurement from the vessel in various ROI from peripheral and central regions in each tumor. Additionally, the area under the curve was calculated from three tumors with 10 sections in each tumor in two groups. *P < 0.05.
Discussion
The present study validates the postulation that supporting a generalized TRZ drug, which is used to exercise control over life-threatening HER2 positive breast cancerous genes, with Tumor Electrofields Therapy may amplify the inhibition of such TRZ resistant cells. The research also aims to identify and unravel the workings of a TTField system on involved TRZ-resistant, HER2 positive breast cancer cell lines. By doing so, it brings up the value of TTF’s for use in future studies. In September 1998, the FDA approved the use of TRZ and its incorporation in devising the treatment plan of GBM or the Glioblastoma Multiforme, a rare malignant tumor. This need for TRZ application for therapy is issued because of the exaggerated activity of the HER2 tyrosine kinase receptor gene. In response to the proliferation, the rate at which breast cancer progresses into its secondary stages increases alarmingly. TRZ was, thus, predicted to work as a humanized anti-HER2 monoclonal IgG1 antibody [18]. However, this study as well as other instances in the past, evidence clearly that a HER2 gene that is past its primary stages remains indifferent to the influence of TRZ. Hence, treatment therapy for breast cancer is at a large disadvantage due to the TRZ resistance it faces. Mechanisms that contribute to TRZ’s failure to operate upon HER2 are intensively studied, and include the following general observations; i) obstacles are faced by TRZ in binding to HER2; ii) upregulation of signaling pathways causes HER2 downstream; iii) TRZ’s tendency of signaling through alternate pathways and iv) failure to trigger immune-mediated mechanisms to destroy tumor cells [6].
This study shows that the TRZ resistant nature of cancerous genes can be compensated by TTF. This study is first of its’ own, that destroys cancerous cells by using the Trastzumab antibody, and sensitizing it within a well-constructed electromagnetic TTF. This finding was confirmed that the penetration and uptake of Alexa 488-TRZ TRZ was enhanced after TTF treatment. The authors showed that TTF increased the penetration of TRZ. Possible underlying mechanism could be explained by decreased interstitial pressure after TTF treatment. Figure 4 shows that TTTield increased apoptotic region measured by TUNEL assay. Solid tumor has a phenomenon in which the interstitial tumor pressure is high and the penetration of anti-cancer drugs, including antibody drugs, is limited [20,21]. To overcome limited penetration of antibody, co-administration of anti-cancer drugs were tried [20,21]. Previously, co-administration of paclitaxel during antibody therapy showed that reduce of the interstitial tumor pressure and increase of the penetration and total uptake of the antibody. A similar study can be observed that when HIFU was used, interstitial pressure inside the tumor was reduced, increasing the penetration of the antibody [22]. Previously, we demonstrated that atorvastatin could increase the total uptake of rituximab due to inducing apoptosis after atorvastatin treatment [23]. In addition, penetration and uptake of TRZ or anti-PD1 antibody were also enhanced after inducing apoptosis after a combination gene therapy [24]. Therefore, in our study, enhanced penetration and uptake of Alexa 488-TRZ could be explained due to decreased interstitial pressure after TTF treatment.
The consequence of these applications is a prohibitive one that suppresses HER2 receptor cells. This study stands in opposition to the present milieu in which oncology regards the combination of chemotherapy with TRZ as the most accurate way of dealing with tumor cells. Despite this promulgation, many patients with HER2-overexpressing breast cancer develop de novo or acquired resistance to such methods. However, the results stated in this study that a TTF integrated solution with TRZ can work wonders as a HER2 therapy.
In this research, we illustrated the in vitro and in vivo effects of this novel suggestion. This aim is achieved by analyzing the context-dependent roles that TTF plays in TRZ resistant breast cancer cells- the general consensus is a positive outcome.
This speculation is supported through previous studies, in which TTF is responsible for GBM arrest, deregulating kinase expression that is dependent on cyclin, inducing caspase-3 activation, as well as spindle formation along with cell death in vitro [25,26]. TTF is also responsible for inhibiting the proliferation and invasion of GBM [3,21]. Further, in vivo and in vitro experiments [16] have agreed at the ideality of TTFields, as it targets cancerous cells specifically, and presents as an attractive alternative to conventional cancer treatment. Numerous in vivo experiments have pointed towards the promising results of TTF’s in executing anti-tumor activities. Further, TTF’s are also capable of impeding the human lung tumor as demonstrated in the xenograft models and, GBM or GBM patient-derived stem cell tumor-bearing models [27,28].
The above-stated facts and figures show a distinct positive outline displayed by the TTF that must be studied to and examined to discuss the therapeutic potential of TTF in combination with TRZ in HER2-positive breast cancer. In this research, BT474 cell lines are employed into in vivo and in vitro experiments, and their resultant changes are noted and analyzed. From the observations, it has been concluded that the use of a TTField leads to the sensitization of the primary TRZ-resistant cell line. Consequently, this facilitates apoptosis by increasing the expression of caspase 3/7 assay. Additionally, TTFs can inhibit phosphorylation of the expression levels of HER2, p-AKT, and p-MAPK in HER2-positive BT474 cells. In turn, this suppresses migration and invasion of cells, and successfully inhibits tumor cell growth in vivo model. The experiments also work towards studying molecular signaling pathways in vivo models, and signify the validity of the results. By evaluating the pathways, the study also identifies TTF therapy as an alternate solution to patients who are resistant to chemotherapy from antibody like TRZ. Thus, it is essential to study the methods to get through primary drug resistance.
The experiments exalted in this research, lay a biological basis for the use of TTF-sensitizer to enhance the therapeutic efficacy against breast cancer cells. Future research must involve evaluating the toxicity to normal cells, as it can help determine the clinical advantages of combination treatment in contrast to mono-therapy.
In conclusion, TTF can overcome the TRZ resistance in vitro and in vivo. These results also necessitate that further studies and exploration of the therapeutic properties of TTF in treating TRZ-resistant breast cancer patients will prove to be invaluable in the future of cancerous research.
Acknowledgements
The authors thank to Novocure for reporting the study. This work was supported partly by Program Ministry of Science and ICT (NRF-2021M2E8A1039980) and by the Rare Isotope Science Project of Institute for Basic Science funded by Ministry of Science and ICT and NRF of Korea (2013M7A1A1075764).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lv F, Yu Y, Zhang B, Liang D, Li ZM, You W. Inhibitory effects of mild hyperthermia plus docetaxel therapy on ER (+/-) breast cancer cells and action mechanisms. J Huazhong Univ Sci Technol Med Sci. 2013;33:870–876. doi: 10.1007/s11596-013-1214-8. [DOI] [PubMed] [Google Scholar]
- 2.Bedard PL, Cardoso F, Piccart-Gebhart MJ. Stemming resistance to HER-2 targeted therapy. J Mammary Gland Biol Neoplasia. 2009;14:55–66. doi: 10.1007/s10911-009-9116-x. [DOI] [PubMed] [Google Scholar]
- 3.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 4.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26:60–70. [PubMed] [Google Scholar]
- 5.Dubska L, Andera L, Sheard MA. HER2 signaling downregulation by trastuzumab and suppression of the PI3K/Akt pathway: an unexpected effect on TRAIL-induced apoptosis. FEBS Lett. 2005;579:4149–4158. doi: 10.1016/j.febslet.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 6.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 8.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbalý V, Ram Z, Villano JL, Rainov N, Weinberg U, Schiff D, Kunschner L, Raizer J, Honnorat J, Sloan A, Malkin M, Landolfi JC, Payer F, Mehdorn M, Weil RJ, Pannullo SC, Westphal M, Smrcka M, Chin L, Kostron H, Hofer S, Bruce J, Cosgrove R, Paleologous N, Palti Y, Gutin PH. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Mun EJ, Babiker HM, Weinberg U, Kirson ED, Von Hoff DD. Tumor-treating fields: a fourth modality in cancer treatment. Clin Cancer Res. 2018;24:266–275. doi: 10.1158/1078-0432.CCR-17-1117. [DOI] [PubMed] [Google Scholar]
- 11.Kirson ED, Dbaly V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S, Gurvich Z, Schneiderman R, Wasserman Y, Salzberg M, Ryffel B, Goldsher D, Dekel E, Palti Y. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 13.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 14.Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci. 2013;1291:86–95. doi: 10.1111/nyas.12112. [DOI] [PubMed] [Google Scholar]
- 15.Dizon DS, Krilov L, Cohen E, Gangadhar T, Ganz PA, Hensing TA, Hunger S, Krishnamurthi SS, Lassman AB, Markham MJ, Mayer E, Neuss M, Pal SK, Richardson LC, Schilsky R, Schwartz GK, Spriggs DR, Villalona-Calero MA, Villani G, Masters G. Clinical cancer advances 2016: annual report on progress against cancer from the American Society of Clinical Oncology. J. Clin. Oncol. 2016;34:987–1011. doi: 10.1200/JCO.2015.65.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo Y, Hwang SG, Jin YB, Sung J, Jeong YK, Baek JH, Cho JM, Kim EH, Yoon M. Selective toxicity of tumor treating fields to melanoma: an in vitro and in vivo study. Cell Death Discov. 2018;4:46. doi: 10.1038/s41420-018-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong H, Sung J, Oh S, Jeong S, Koh EK, Hong S, Yoon M. Inhibition of brain tumor cell proliferation by alternating electric fields. Appl Phys Lett. 2014;105:203703. [Google Scholar]
- 18.Goetz M, Berry D, Klein T International Tamoxifen Pharmacogenomics Consortium. Adjuvant tamoxifen treatment outcome according to cytochrome P450 2D6 (CYP2D6) phenotype in early stage breast cancer: findings from the International Tamoxifen Pharmacogenomics Consortium. Cancer Res. 2009;69:33. [Google Scholar]
- 19.Singer CF, Kostler WJ, Hudelist G. Predicting the efficacy of trastuzumab-based therapy in breast cancer: current standards and future strategies. Biochim Biophys Acta. 2008;1786:105–113. doi: 10.1016/j.bbcan.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Zaheer J, Kim H, Lee YJ, Kim JS, Lim SM. Combination radioimmunotherapy strategies for solid tumors. Int J Mol Sci. 2019;20:5579. doi: 10.3390/ijms20225579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HG, Yu AR, Lee JJ, Lee YJ, Lim SM, Kim JS. Measurement of tumor pressure and strategies of imaging tumor pressure for radioimmunotherapy. Nucl Med Mol Imaging. 2019;53:235–241. doi: 10.1007/s13139-019-00598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Shin IS, Hancock H, Jang BS, Kim HS, Lee SM, Zderic V, Frenkel V, Pastan I, Paik CH, Dreher MR. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J Control Release. 2012;162:218–24. doi: 10.1016/j.jconrel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim EH, Ko HY, Yu AR, Kim H, Zaheer J, Kang HJ, Lim YC, Cho KD, Joo HY, Kang MK, Lee JJ, Lee SS, Kang HJ, Lim SM, Kim JS. Inhibition of HIF-1α by atorvastatin during 131I-RTX therapy in Burkitt’s lymphoma model. Cancers (Basel) 2020;12:1203. doi: 10.3390/cancers12051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung BK, Ko HY, Kang H, Hong J, Ahn HM, Na Y, Kim H, Kim JS, Yun CO. Relaxin-expressing oncolytic adenovirus induces remodeling of physical and immunological aspects of cold tumor to potentiate PD-1 blockade. J Immunother Cancer. 2020;8:e000763. doi: 10.1136/jitc-2020-000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EH, Song HS, Yoo SH, Yoon M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7:65125–65136. doi: 10.18632/oncotarget.11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim EH, Kim YH, Song HS, Jeong YK, Lee JY, Sung J, Yoo SH, Yoon M. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. 2016;7:62267–62279. doi: 10.18632/oncotarget.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EH, Jo Y, Sai S, Park MJ, Kim JY, Kim JS, Lee YJ, Cho JM, Kwak SY, Baek JH, Jeong YK, Song JY, Yoon M, Hwang SG. Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene. 2019;38:6630–6646. doi: 10.1038/s41388-019-0882-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim EH, Kim MS, Lee KH, Koh JS, Jung WG, Kong CB. Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma. Oncotarget. 2016;7:70869–70880. doi: 10.18632/oncotarget.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


