Abstract
The tumor microenvironment (TME) plays a central role in tumor initiation, development, immune escape, and clinical treatment. Hypoxia, an important characteristic of the TME, mediates vascular endothelial factor (VEGF) signaling through direct or indirect mechanisms. Directly, hypoxia promotes the expression of VEGF through hypoxia-inducible factor (HIF) induction. Indirectly, VEGF inhibits dendritic cell (DC) maturation and function by binding to VEGF receptors (VEGFRs) and co-receptors expressed on cell membranes. Additionally, HIF can bypass VEGF/VEGFR and activate downstream signaling factors to promote tumor development. Currently, DC vaccine, anti-HIF and anti-VEGF therapies are widely used in clinical treatment, but their long-term effects remain limited. Therefore, a further understanding of the effects of hypoxia and VEGF signaling on DCs will help in the development of innovative combination therapies and the identification of new targets.
Keywords: Hypoxia, HIF, VEGF, dendritic cell, combination therapy
Introduction
Cancer incidence is rapidly increasing worldwide and is likely to be the leading cause of death in the 21st century [1]. Tumor metastasis and drug resistance limit the efficacy of cancer treatment. Multiple therapies targeting specific signaling pathways have been developed; however, their long-term effects remain limited [2]. A growing understanding of tumor initiation and progression has revealed the importance of the tumor microenvironment (TME) and its potential applications in clinical treatments. The TME is the internal environment in which tumor cells develop and exist. This is also where symbiotic nutrient sharing, nutrient competition, and signaling molecule interference occur [3], and it has been demonstrated to affect angiogenesis, tumor development, and metastasis [4]. Increasing studies have revealed that perturbation of the TME strongly influences the functions of immune cells [3,5]. Tumors can drive dendritic cell (DC) differentiation and maturation, resulting in a poor immune response [6,7] and making DCs a crucial target for tumors to escape immunosurveillance mechanisms [8]. Combining anti-angiogenic therapy with chemotherapy and immunotherapy is believed to be an important strategy to overcome the immunosuppressive TME [7,9]. Understanding the complex networks in the TME can help in the development of novel drugs and treatments.
Growing evidence shows that the level of hypoxia, vascular endothelial factor (VEGF) expression, and immune cell infiltration are important in the TME. Furthermore, the relationships among these factors in the TME are also important. One of the common characteristics of the TME is hypoxia, which is defined as an oxygen pressure around cells lower than 5-10 mmHg [10]. The level of hypoxia is positively correlated with cancer mortality [11]. Usually, hypoxia in normal organs or tissues (e.g., the liver) and pathological tissues is induced by inflammation, hypoglycemia, and tumor development [12]. Extensive clinical data have demonstrated that hypoxia regulates repetitive-injury regeneration [5], angiogenesis, tumor survival [13], immune responses, and drug resistance [2].
Hypoxia-inducible factor (HIF) is one of the most important signaling molecules in the hypoxia signaling pathway. HIF is a heterodimeric transcription factor consisting of an α subunit and a β subunit. The composition of subunits is regulated by the surrounding concentration of oxygen [14]. A low oxygen level stabilizes the expression and activation of HIF. Thus, HIF can be applied as an indicator of the surrounding hypoxia level. Moreover, HIFs are responsible for transducing signals to downstream factors. HIF-1 and HIF-2 regulate gene transcription using similar mechanisms, whereas HIF-3 functions as a regulator of HIF-1/2 by modulating glucose uptake, metabolism, and cell proliferation [2,10]. Therefore, HIF is considered the main transducer of hypoxia signaling pathways in tumor development [13].
Hypoxia, which can be induced by chemoembolization, plays a significant role in the TME by upregulating VEGF through HIF. VEGF rebound can thus lead to treatment failure and poor survival rates in tumor patients [15]. VEGF is synthesized by most types of tumor cells and DCs, and it acts as a central regulatory factor and an important indicator of tumor migration, invasion, and regulation [7,16-19]. In addition to preventing tumor angiogenesis, the inhibition of VEGF signaling can also improve the function of DCs in the TME [7].
VEGF has several isoforms, including VEGFA-E and placental growth factor [20]. These isoforms are generated through alternative splicing, and they can perform distinct functions by binding to various receptors [21]. The three tyrosine kinases receptors VEGFR1, VEGFR2, and VEGFR3 are the most common transmembrane receptors activated by the ligand binding of VEGF. All three receptors are dimers with similar protein organization but different functions [22-24]. Both VEGFR1 and VEGFR2 are essential factors in suppressing DC functions [17,25]. The former mainly inhibits DC maturation, and the latter primarily inhibits DC migration. VEGFR3 can prevent TLR4 (Toll-like receptor)/NF-κB (Nuclear Factor of κ-light-chain-enhancer of activated B cells) activation [18] and suppress the trafficking of DCs to draining lymph nodes to mediate delayed-type hypersensitivity (DTH) in corneal transplantations [26]. However, the downstream effectors of the VEGFR3 signaling pathway remain unclear, and further study is needed. Besides VEGFR1/2/3, other membrane proteins have similar functions, including Neuropilins (NRP1 and NRP2). As co-receptors, they play a crucial role in VEGF signal transduction [20]. We will discuss their functions in the co-receptor section below.
Although HIF/VEGF signaling pathway has been proved to be involved in tumor immunity, the effects on DC are not thoroughly explained. In this review, we first describe the crosstalk between VEGF and DCs at the molecular level upon HIF activation, especially the signal transduction through the three VEGF receptors and co-receptors. Then, we introduce the mechanisms that activate VEGF downstream signaling pathways independent of VEGF/VEGFR interactions. Finally, we discuss the efficacy of molecules targeting HIF and VEGF signaling pathways in clinical cancer treatment.
Dual impacts of hypoxia on DCs
DCs, the most powerful antigen-presenting cells, originate from bone marrow-derived pluripotent hematopoietic stem cells and can be divided into different subtypes according to their source: conventional dendritic cells (cDC) and plasmacytoid dendritic cells (pDC) [27-29]. In addition, these can be further divided into several subpopulations according to the distribution of surface antigens [30].
DCs can induce antitumor immunity by activating both innate and adaptive immune systems [19]. cDCs are crucial in antigen presentation [31]. When stimulated, cDCs become activated, carry antigens from tumors to lymph nodes, activate T cells, and initiate tumor cell killing [31,32]. Unlike cDCs, pDCs were thought to have a low antigen uptake capacity [31]. However, the level of pDCs is positively related to the prognosis of patients, indicating that pDCs may play an important role in antitumor immunity [31]. pDCs produce type I interferon (IFN-1), which subsequently stimulates natural killer (NK) cells, macrophages, and other innate immune cells by activating Toll-like receptor 7 or 9 [33,34]. IFN-1 can also promote Th1/Th2 polarity shift, B cell differentiation, apoptotic cell responses, and other adaptive immune cells [30,33]. Recently, pDCs were also demonstrated to function as antigen-presenting cells (APCs) by presenting antigens and activating autoimmunity [30]. Because DCs are essential in antitumor immunity, modulating DCs may be a promising cancer strategy and potentially overcome tumor-induced immunosuppression [30].
However, cancer cells can influence the immunogenic or tolerogenic functions of pDCs to inhibit immune responses in the TME, and hypoxia is one of the key pathways [30]. The effects of hypoxia on DCs are complex. First, the response of cells in the hypoxic state is highly associated with the degree of hypoxia. Different durations and severities of hypoxia can cause varying degrees of cell responses. For example, cells survive under moderate hypoxia, whereas prolonged hypoxia can cause cell death [35]. Second, hypoxia can affect DCs directly or indirectly by regulating both the quality and intensity of the immune response [36]. In mice, hypoxia itself does not activate DCs directly, but it can enhance the expression of costimulatory molecules, pro-inflammatory cytokine synthesis, allogeneic lymphocyte proliferation, and glucose use in the presence of lipopolysaccharide (LPS) [27]. However, hypoxia also suppresses immune function through other induced factors. Third, hypoxia can affect both pro-survival and pro-apoptotic pathways [37]. Additionally, hypoxia can have completely distinct effects on tumor development in different tissues and patients, and it may even have different effects on the same drugs [38]. In clinical settings, long-term hypoxia is generally considered to be unfavorable, and its suppression of immunity usually promotes the proliferation and metastasis of tumor cells.
Hypoxia can affect DCs by regulating the levels of several molecules, such as reactive oxygen species (ROS). As the Warburg effect explains, in low oxygen conditions, cells will produce ATP through a higher rate of anaerobic glycolysis, resulting in an increased level of reactive oxygen species (ROS) in both tumor cells and immune cells [35]. A lack of energy can inhibit cell development, and a high level of ROS can prevent DC maturation via p38-MAPK and ERK1/2 pathways and influence antigen presentation. Moreover, ROS can modulate pro-inflammatory signaling pathways in DCs [27,39] and thus affect the physiological state of DCs.
Hypoxia induces HIF expression in DCs. Several HIF-mediated pathways are altered under hypoxic conditions [40]. In rats, the use of HIF inhibitors can strongly increase the anti-tumor activity of DC-based vaccines [13].
Although short-term hypoxia can slightly increase the migratory ability of immature DCs from the periphery into draining lymph nodes [41,42], hypoxia mainly inhibits cell maturation and induces cell death. Data suggest that as a transcription factor, HIF upregulates the expression of the pro-apoptotic protein BAX, induces the hypoxia-inducible gene BNIP3, and promotes cell death. HIF can also regulate the PI3K/Akt pathway. The downregulation of Akt is associated with cell survival and can affect DC activation. The life span of DCs may vary from different oxygen levels depending on different molecules. Additionally, DC migration can be inhibited. New evidence also shows that HIF inhibits the migratory ability of DCs via metabolic reprogramming toward glycolysis and preventing the upregulation of CCR7 [43-45]. By inducing apoptosis and regulating cell migration, HIF can greatly affect the number and immune function of DCs. Furthermore, the secretory function of DCs is also affected by HIF. Hammami et al. recently found that HIF could decrease IL-12 production from DCs, leading to immature Th1 development [44]. In addition, increased IL-22 secretion was observed in the hypoxia microenvironment, whereas the levels of IL-10, IL-6, TNF-α, IL-1β, and IL-23 were decreased [42].
However, the functions of hypoxia are complex because it can have different effects on mature and immature DCs [7]. For instance, under 2% O2, which is close to the oxygen level in lymph nodes, immature DCs die, but mature DCs survive and can still perform their functions [36]. Although hypoxia reduces the cell growth of bone marrow-derived DCs (BMDCs), it can enhance their phenotypic maturation [42]. These effects may depend on the duration and extent of hypoxia and DC subtypes. Furthermore, HIF-1 and HIF-2 may have opposing effects [42], and elucidating their distinct functions requires additional studies (Figure 1).
Figure 1.
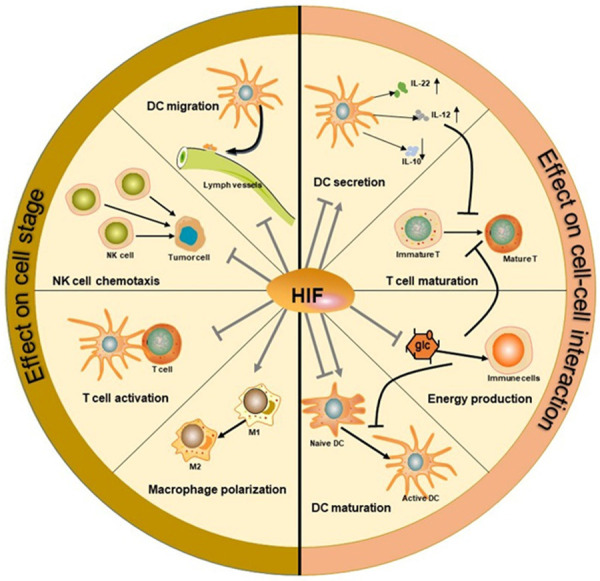
Hypoxia effects on immune cells. As a transcription factor, HIF can directly affect the differentiation and function of different immune cells, such as T cells, dendritic cells, NK cells, and macrophages (the left side). Additionally, it can mediate the interactions between cells and thus influence the maturation and activation of immune cells (the right side).
Hypoxia induces VEGF activation
In addition to directly affecting the expression of intracellular signaling molecules, hypoxia can indirectly regulate the maturation and function of DCs by inducing the secretion of particular cytokines. The VEGF signaling pathway is one of the most important downstream pathways and plays a crucial role in immunity. Clinical studies have confirmed the positive correlation between VEGF signaling pathway activation and the overall survival of cancer patients. Reagents targeting VEGF or VEGF receptors (e.g., bevacizumab, sunitinib, and sorafenib) have been applied in clinical cancer trials [16]. These drugs not only impair angiogenesis but also suppress immune responses.
As an important hypoxia-associated factor, VEGF is not just a downstream molecule of HIF. The crosstalk between HIF and VEGF is complex. HIF and VEGF are both negatively correlated with the prognosis of cancer patients, suggesting that there may be a relationship between these two factors. In fact, HIF-1 can induce the production of VEGF, which subsequently promotes angiogenesis to increase the oxygen content. In addition, HIF overexpression has been found to promote angiogenesis [46,47]. For example, by activating NF-κB and AP-1 signaling pathways, ROS promote angiogenesis with increased VEGF secretion [35]. Subsequently, increased HIF expression or hypoxia levels can promote VEGF expression to some extent. Accumulating findings support HIF as an upstream regulatory factor of VEGF. Specifically, HIF can upregulate VEGF gene expression by modulating DNA transcription.
In fact, HIF can affect the function of DCs through several signaling pathways. The HIF/VEGF pathway is a significant mechanism, and VEGF can influence the development and function of DCs through different receptors and signaling molecules. The HIF/VEGF signaling pathway can influence both antigen presentation and immune factor secretion, thereby increasing the probability of immune escape.
VEGF and DCs
VEGFR1 signaling pathway
The VEGFR1 signaling pathway impairs DC maturation directly in the early phase by regulating NF-κB activity and influences the expression of NF-κB subunits, such as rel-B. Therefore, it can affect the antigen-presenting and secretory functions of DCs.
VEGFR1 binds to VEGFA, VEGFB, and placental growth factor. It mainly inhibits DC maturation during the early phase [19]. At physiological concentrations, VEGF signaling via VEGFR1 has a minimal effect on DC maturation; it only mediates maturation at high ligand concentrations [48]. Several findings have shown that the VEGFR1 signaling pathway can block NF-κB activation and impair hemopoietic progenitor cell (HPC) differentiation into DCs [49]. It is worth noting that this pathway only functions in the early phase of differentiation, both in vivo and in vitro. In other words, only HPCs and not mature DCs can respond to VEGF through the VEGFR1 pathway [49].
NF-κB is a transcription factor present in almost all animal cells. At first, it was discovered to be able to bind to the enhancer element of the immunoglobulin κ light-chain of activated B cells [50]. NF-κB can form both homo- and heterodimeric complexes consisting of five subunits: RelA (p65), RelB, c-Rel, NF-κB1 (p105), and NF-κB2 (p100). Different combinations determine the specificity of the transcriptional response [50]. The most common NF-κB dimer is the heterodimer of RelA and p50. Notably, its subunits may also play a role. For example, recent data suggest that RelB is crucial for DC development, and knocking out RelB in mice prevents DC maturation [51]. Accumulating evidence indicates that NF-κB can regulate gene transcription and thus mediate inflammation, angiogenesis, proliferation, differentiation, and apoptosis. However, the role of NF-κB is quite controversial. It can act both as a tumor promoter and suppressor under different circumstances [50].
Tsunehiro et al. found that increased VEGF levels rapidly decreased the specific DNA binding ability of NF-κB and drastically reduced the expression of proteins downstream of NF-κB signaling. However, the mRNA level of NF-κB subunits was only slightly reduced during the very early phase (less than 7 days) [49]. This suggested that the aberrant function of NF-κB may be due to its reduced activity [52].
The regulatory effects of VEGF on NF-κB may be related to the dissociation of inhibitor-κB (I-κB) and NF-κB in the cytoplasm. The activation of NF-κB requires co-activators, co-repressors, and other transcription factors. It can also be inhibited by various regulators, such as I-κB. Research has shown that even after blocking NF-κB activity during early differentiation stages, a dominant I-κB inhibitor can still restore the effects of VEGF [49].
In the cytoplasm, I-κB can bind to NF-κB and inhibit its translocation into the nucleus and subsequent binding to DNA. Phosphorylation or acetylation can disassociate them and active NF-κB. Data revealed that the VEGFR1 signaling pathway inhibits the disassociation between NF-κB and I-κB. Thus, NF-κB-regulated transcription pathways are inhibited, and protein synthesis is also reduced. For example, the production of MHC class II molecules, costimulatory molecules (CD40, CD86), and pro-inflammatory cytokines is substantially inhibited [20]. After VEGF treatment, the level of RelB is also decreased.
VEGFR2 signaling pathway
VEGFR2 can bind to VEGFA, VEGFC, VEGFD, and VEGFE (except mouse VEGFD). It is the most well-known receptor and is indispensable for angiogenesis. In the absence of VEGFR2 tyrosine kinase signaling, early hematopoiesis is abnormal. As for DCs, VEGFR2 signaling has a weak effect on maturation but a significant influence on early hemopoietic differentiation and cell migration [48].
VEGF induces VEGFR2 complex assembly [24], and VEGF/VEGFR2 binding can impair the motility, antigen-presenting, and secretory function of mature DCs through the RhoA-COF1 pathway [53]. Researchers found that increased VEGF can upregulate the expression of RhoA and that the effect of VEGF on DCs is reversed with a RhoA inhibitor. Previous results suggest that RhoA is a downstream molecule of VEGF/VEGFR2 [53].
When VEGF binds to VEGFR2, it activates the RhoA/ROCK signaling pathway. RhoA is a small G protein belonging to the Rho GTP subfamily of GTPases. It is activated by several cytokines and inflammatory mediators. Active RhoA can bind to GTP and then activate its downstream serine/threonine protein kinase Rho-associated kinase (ROCK) [54], which subsequently affects the production and secretion of cellular molecules. Active ROCK can inactivate downstream myosin phosphatase, which dephosphorylates the myosin light chain (MLC), thereby preventing MLC dephosphorylation and resulting in increased actin-myosin interactions. As a result, cell contraction and migration are influenced [55].
The RhoA/ROCK signaling pathway is an important mediator of transcription, cytoskeleton remodeling, cell proliferation, migration, invasion, and differentiation in several immune cells. One of the downstream molecules of the RhoA/ROCK pathway is cofilin1 (COF-1). COF-1 is an actin-binding protein in eukaryotic cells. It can promote the cycle of actin fibers by ensuring the rapid polymerization and depolymerization of actin fibers. Therefore, it can affect the adhesion of cells and their extracellular matrix, thereby promoting cell movement, migration, division, and apoptosis.
In the presence of high VEGF levels, the phosphorylation of COF-1 is increased. Normally, COF-1 is dephosphorylated when DCs undergo maturation [56]. Thus, abnormally elevated levels of COF-1 phosphorylation inhibit DC maturation. Meanwhile, the abnormal structure of COF-1 leads to its dysfunction, which impairs DC cytoskeleton remodeling and directional motility. The antigen-presenting activity of DCs also depends on motility because they need to migrate to the lymph nodes to perform their function. A further study found that the mRNA level of COF-1 was increased, and COF-1 localization was altered following VEGF stimulation. The function of COF-1 also depends on its location, and this change further compromises the immune function of DCs [53]. Therefore, the VEGFR2/RhoA/COF-1 pathway inhibits the immune function of DCs.
The VEGFR2/RhoA/COF-1 axis is just one key signaling pathway. VEGFR2 can also influence other signaling molecules in DCs. For example, the combination of VEGF and VEGFR2 has been found to affect the phosphorylation of extracellular regulated protein kinases (ERK1/2), which are also important serine/threonine protein kinases in signal transduction. ERK1/2 signals through MAPK cascades and the PI3K/AKT/PKB pathway [57]. When the expression of VEGF increases, VEGF can assist in the recruitment of DC precursors to the tumor via the VEGFR2 pathway [46].
Following VEGF stimulation, some immature DCs can also differentiate into endothelial-like cells (ELC), thereby reducing the number of mature DCs. VEGF can promote angiogenesis and also induce the differentiation of cells into ELCs to promote neovascularization. DCs can promote angiogenesis by secreting VEGF and interleukin and interacting with endothelial cells [43,58]. In addition, the secretory ability of DCs is modulated to promote vascular formation. For instance, IL-2 secretion by DCs suppresses angiogenesis. The detailed signaling pathway has not yet been revealed, but the shift from DCs to ELCs is likely related to ERK signaling [59]. Some VEGFR2-mediated signaling pathways have been clearly elucidated in T cells, macrophages, or other immune cells, and researchers recently showed that these might also function in DCs.
Co-receptors: the NRP signaling pathway
As co-receptors of VEGF, NRPs can bind to VEGF165, VEGF145, and VEGFC on DCs, regulatory T cells, and macrophages. NRPs can form complexes with VEGFR2 and VEGF to enhance signal transduction. Therefore, they are known as co-receptors [60]. Recent studies indicate that co-receptors also play significant roles in mediating the effects of VEGF. VEGF co-receptors include NRP1 and NRP2. NRP1 was first identified in 1995 and thought to be a co-receptor of the neural guidance factor semaphorin 3A [46]. Although NRPs have no intrinsic signaling capability, they can form complexes with VEGF RTKs (VEGFR1 and VEGFR2) and thus increase the affinity of VEGF by more than 4-fold [21].
NRPs are known to be involved in angiogenesis, immune responses, tumor development, and epithelial-mesenchymal transition (EMT) [61]. In cancer patients, aberrantly enhanced NRP1 and NRP2 expression [20,62] promotes tumor tissue angiogenesis, tumor cell growth, and metastasis. NRPs also participate in regulating immune responses. They impair T cell activation, regulate myeloid cell migration, and strongly inhibit BMDC maturation [20]. In the absence of NRP1, BMDC physiology is not impaired, but these cells exhibit a reduced ability to respond to VEGF, resulting in deficient growth, maturation, and T cell stimulation [20,63]. In addition, NRPs can inhibit LPS stimulation initiated by TLR signaling. TLR4 activates downstream MyD88/IRAK4 signaling to modulate the phosphorylation of ERK1/2 and NF-κB. After receiving the signal from VEGF, NRPs are upregulated and disrupt the downstream TLR4 signaling pathway to reverse the phosphorylation of ERK1/2 and NF-κB [20]. ERK1/2 and NF-κB pathways are crucial for DC maturation and differentiation, MHC II expression, and costimulatory molecule (CD40, CD86) secretion. Further research revealed that this reverse was caused by direct interactions between NRPs and TLR4. There is also a negative feedback mechanism in this TLR4 signaling pathway whereby the suppression of ERK and NF-κB signaling and reduced secretion of inflammatory cytokines inhibit ligand-bound NRP1 [20]. Regarding other immune cells, NRP can interact with VEGFR2. However, in BMDCs, there is no direct evidence of their interaction, and its role in other DC subtypes is not well understood (Figure 2).
Figure 2.
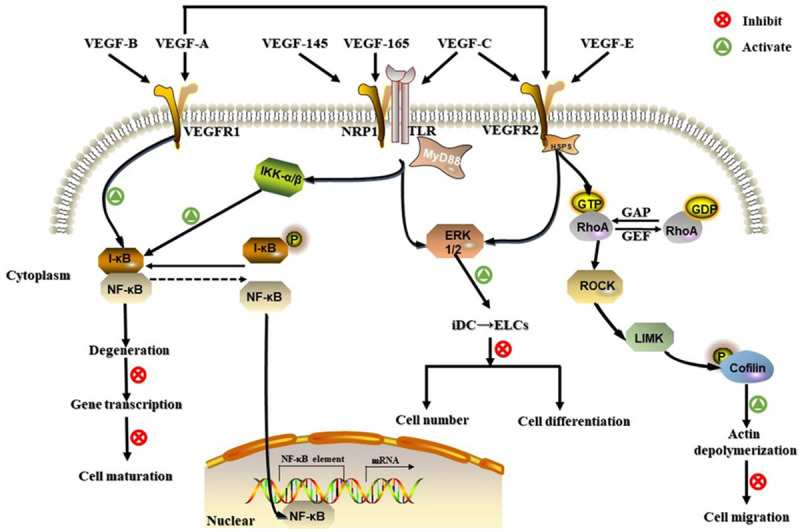
Three VEGF/VEGFR signaling pathways involved in DC regulation. VEGFR1, VEGFR2, and NRPs can bind to different VEGF subtypes. The VEGFR1 pathway inhibits cell maturation by promoting the binding between I-κB and NF-κB in the early phase. VEGFR2 can impair cell migration by activating RhoA/ROCK and increasing phosphorylated (P)-cofilin levels. VEGFR2 may also participate in the ERK1/2 signaling pathway, resulting in abnormal cell differentiation and decreased DC numbers. NRP1 influences both NF-κB and ERK1/2 signaling pathways associated with Toll-like receptors.
Other responses
After stimulation with VEGF, the mechanisms underlying many of the changes that occur in DCs are unknown.
Compared with wild-type mice, the number of DCs was increased in VEGF transgenic mice, and enhanced VEGFR2 but decreased VEGFR1 expression were observed [21,32]. Following VEGF stimulation, DCs strongly express u-PA [52], and changes in the expression of more than 33 proteins have been reported [53]. In addition, Takuya et al. found that the proportion of DC subtypes (DC 1/DC2) was decreased [52]. Although the detailed signaling pathways have not yet been explained, these variants are associated with VEGF stimulation.
Moreover, VEGF can indirectly affect the development and function of DCs by regulating other cells. VEGF inhibits the maturation of myeloid cells (MCs), and the coexistence of iMCs can inhibit the maturation of DCs [17]. Of course, VEGF also affects the entire immune system by modulating the maturation and function of other cells via the regulation of DCs. On the one hand, DCs regulate surrounding immune cells by secreting cytokines through direct interactions. For instance, inhibition of the transcription factor kruppel-like factor 2 (KLF2) in DCs can inhibit Th2 cell development [64]. On the other hand, the abnormal secretion of molecules from DCs may disrupt the entire immune system [65]. In addition, abnormal levels of TNF-α, which is secreted by DCs, may promote tumor development.
Hypoxia interference
Hypoxia can both inhibit and activate DCs depending on the degree and duration of hypoxia and patient characteristics [36]. This may due to the complex interaction between the hypoxia pathway and immune cell activation pathways [35]. It was found that the effect of hypoxia on downstream signaling molecules in both VEGFR1 and VEGFR2 pathways was not completely consistent with the effect of VEGF, suggesting that hypoxia may interfere with the VEGF pathway through other signaling pathways.
NF-κB pathway: opposite effect
In contrast to VEGF, HIF can promote the action of NF-κB. Walmsley. et al. found that the activation and expression of NF-κB were regulated by HIF-1, and its expression was significantly increased in the hypoxic microenvironment [66]. In fact, NF-κB was induced primarily by inflammation stimulation and hypoxia functions through a secondary mechanism under inflammatory conditions [67]. By increasing the production of costimulatory molecules and pro-inflammatory cytokines, hypoxia can enhance NF-κB production and activation. Currently, NF-κB is thought to be an indispensable intermediate molecule between hypoxia and inflammation. In turn, NF-κB can also induce HIF mRNA production [68].
There is also extensive crosstalk between the HIF and NF-κB pathways. For example, some cytokines can both activate the NF-κB pathway and promote the expression of HIF, or some cytokines can enhance their function through NF-κB pathways or HIF pathways [67]. The interaction between NF-κB and HIF is complex and can occur at different levels from activators to the target genes [50]. For VEGF, DC maturation was inhibited only in the early phase. HIF function is not only affected during the early phase. It can function during DC maturation and even in mature DCs. Therefore, the role of hypoxia in regulating NF-κB in DCs needs to be discussed in terms of specific stages of development.
During different developmental periods or in different cell types, NF-κB exhibits various functions. It can promote both development and apoptosis depending on the different stages of cell development and cell types. For example, NF-κB was found to induce the apoptosis of T cells [69]. Although these results have not been confirmed in DCs, this study suggested that we could not determine the effects of HIF simply by assessing NF-κB expression or activation because the developmental stages of DCs are also important. The detailed effects of NF-κB in DCs have not been completely revealed, and further studies are still needed.
RhoA/ROCK pathway: similar effect
For molecules in the VEGFR2 signaling pathway, hypoxia has a similar effect as VEGF. Unlike the VEGFR1 signaling pathway, RhoA and ROCK are thought to function upstream of HIF [70]. In hypoxic microenvironments, the amount of GTP-bound RhoA increases, and thus the activity of RhoA is enhanced. Additionally, the level of ROCK2 but not ROCK1 was increased. However, the expression and stabilization of HIF did not affect RhoA/ROCK expression, whereas the mRNA and protein expression levels of HIF were altered in response to RhoA/ROCK signaling under low oxygen conditions [70]. These results show that RhoA/ROCK can be an upstream factor of HIF but not hypoxia. In addition, VEGF can inhibit EMT, similar to HIF. In this case, hypoxia cannot be represented directly by HIF, but these changes are consistent with the effect of VEGF.
Normally, hypoxia impairs the activity and expression of COF-1 because RhoA/ROCK is activated. In addition, some studies found that hypoxia could promote F-actin depolymerization by inhibiting COF-1 activation in intestinal epithelial cells [71]. However, this study was not conducted in DCs. In fact, there is currently no definite conclusion on the relationship between hypoxia and COF-1 in DCs.
From these studies, HIF inhibits DCs by inducing VEGF, but the regulatory effects are not the ultimate effect of HIF. In previous experiments, the effects of these two proteins were usually studied independently, but their interactions may cause DCs to respond differently.
Furthermore, there are still some limitations to the related studies. First, it is difficult to determine the specific effect of hypoxia. In fact, studies have generally focused on either hypoxia or VEGF rather than directly studying their interaction. Additionally, the studies on the crosstalk between hypoxia and VEGF have focused on HIF and VEGF themselves rather than the downstream signaling molecules. Second, most signaling pathway studies have been conducted in mouse models, and some studies have shown that there may be differences in DCs and VEGF between humans and mice [32,51,53]. Third, most studies on DC mechanisms were based on previous results from other cells, such as T cells. Because some are well-known signaling pathways, complete validation using in vitro and in vivo experiments is sometimes not performed. Finally, studies on different VEGFRs have focused on a single receptor pathway, overlooking their connection with other receptors [71].
Applications and prospects
Because HIF/VEGF signaling promotes tumor development and may cause drug resistance, controlling the expression and activation of this signaling pathway is important in clinical treatments [72]. Many drugs targeting the HIF/VEGF signaling pathway have been developed, and some drugs that have used effectively in the past are also found to be related to the downregulation of HIF/VEGF [73]. Asparagus polysaccharide in asparagus, a type of traditional Chinese herb, can downregulate the HIF/VEGF pathway [18]. Anti-VEGF and anti-VEGFR targeted therapies are widely used in clinical treatment [16,74]. VEGF can not only promote tumor development by enhancing angiogenesis, but it also affects the function of immune cells through the VEGFR pathway [52,75]. Therefore, anti-VEGF/VEGFR therapy can either target tumors by preventing angiogenesis or promote an immune-supportive tumor microenvironment. However, anti-VEGF/VEGFR therapy can lead to drug resistance by inducing a hypoxic microenvironment [9,76]. Therefore, it only achieves a short-term effect in patients [11,16].
In recent years, the use of combination therapies, also termed “acceleration on” strategies, has been promoted in clinical treatment. Using anti-VEGF therapy in combination with DC immunotherapy, tumor vaccine therapy, or chimeric antigen receptor T-cell therapy can increase treatment efficacy and reduce the possibility of drug resistance [6,52,77]. DC-based vaccines, one type of tumor vaccination, is a common combination therapy [78]. However, the synthesis of DC-based vaccines is complicated, and their efficacy remains limited. Thus, highly effective, long-term, and stable treatments are still needed.
In addition, HIF inhibitors (e.g., halofuginone and topotecan) were applied in clinical trials and shown to be effective in various settings [79]. A number of HIF inhibitors are currently being investigated in clinical trials, which have shown promising results for future cancer treatments.
The HIF/VEGF pathway and DCs have been demonstrated to play a role in many tumors and affect the outcomes of clinical treatment. Angiogenesis plays an important role in the tumorigenesis and development of multiple myeloma (MM). The proteasome inhibitor bortezomib, which is used to treat MM, was also found to inhibit VEGF production and associated angiogenesis [80]. HIF helps maintain MM homeostasis. Approximately 35% of MM patients constitutively produce HIF-1α [81]. Therefore, the suppression of HIF-1α may be an effective therapy. Recent studies found that the suppression of HIF-1α enhanced the anti-tumor effect of lenalidomide [82]. Although DC function is impaired in many MM patients, the effects of DC vaccines are unsatisfactory. Shinde et al. reported that the combination of a stem cell-derived DC vaccine and an anti-CTLA-4 antibody was a promising treatment for MM [83].
The HIF/VEGF signaling pathway also participates in the regulation of colorectal cancer (CRC) cell migration and invasion [84]. Anti-angiogenic therapy is used to treat CRC, but acquired resistance develops within several months. Thus, the efficacy of anti-VEGF therapy is insufficient. However, a recent study found that a combination treatment consisting of anti-VEGF therapy could control tumor growth and inhibit drug resistance [74,85]. Therefore, a combination of anti-VEGF drugs and HIF inhibitors may benefit cancer patients in clinical trials [86]. Moreover, anti-VEGF therapy inhibits the liver metastasis of CRC [87]. DC vaccines are an attractive therapeutic strategy for CRC treatment. DC vaccines have not been approved for CRC treatment, but clinical trials have demonstrated that DC vaccines could enhance immune responses and improve survival in CRC patients without severe adverse effects [88,89].
In patients with prostate cancer (PCa), anti-VEGF therapy increased their survival time [90]. HIF-1α can induce immune escape, suggesting its potential as a therapeutic target. However, the application of HIF inhibitors in PCa requires more studies [91]. Moreover, DC vaccines successfully induced an immune response in all PCa patients [92]. Similar to PCa, VEGF is also essential in lung cancer, and anti-VEGF therapy has been approved for the clinical treatment of lung cancer [93]. HIF-1α promotes immune escape by increasing PD-L1 in non-small cell lung cancer (NSCLC) cells [94]. Therefore, the downregulation of HIF-1α can inhibit proliferation and angiogenesis in NSCLC patients [95]. DC vaccines are also considered effective for the treatment of lung cancer. DC vaccines inhibit lung cancer cell proliferation in mice [96]. The combination of a DC vaccine and cytokine-induced killer cell therapy can also benefit patients without adverse effects [97].
In addition, anti-HIF/VEGF therapy and DC therapy are also effective in renal, esophagogastric, breast, oral, and endometrial cancers (Figure 3) [98-106]. However, individual treatments failed to achieve the promising expectations in some cancers (Table 1). This treatment failure may be caused by different patient conditions, drug resistance, or the complex crosslink between HIF, VEGF, and DCs [107]. Hence, “acceleration on” strategies may increase the efficacy of cancer immunotherapy in the future. VEGF is one of significant TME barrier and can suppress effects on DC vaccine [108]. Theoretically anti-VEGF therapy could increase antitumor responses of DC-based immunotherapy. Since hypoxia can function through bypass pathways and have dual impacts on DC, the combination of HIF inhibitor and DC vaccine may have more complex effects and hypoxia may mask the efficacy of VEGF therapy. The interference of hypoxia should also be controlled in the production and application of DC vaccines. Therefore, the degree and duration of hypoxia should be considered in the specific treatment strategies.
Figure 3.
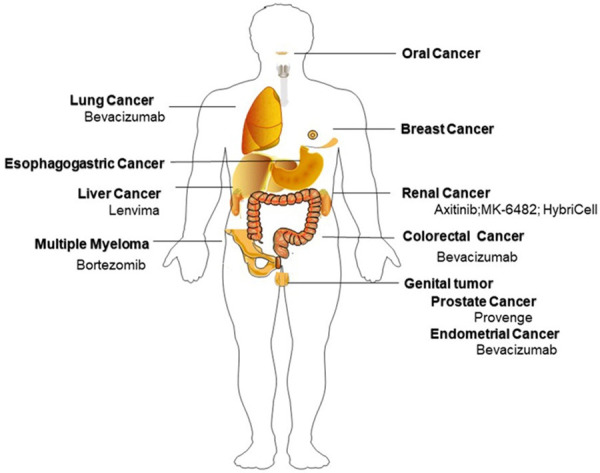
Potential targets of HIF/VEGF/DC therapy and FDA approved drugs. Abbreviations: VEGF: vascular endothelial growth factor; DC: dendritic cell; HIF: hypoxia-inducible factor; ROS: reactive oxygen species; TME: tumor microenvironment; NRP1: Neuropilin-1; NF-κB: Nuclear Factor of κ-light-chain-enhancer of activated B cells; MLC: myosin light chain; COF-1: Cofilin1; ERK: extracellular regulated protein kinases; ELCs: endothelial-like cells; TLR: toll-like receptor; EMT: epithelial-mesenchymal transition; LPS: lipopolysaccharide.
Table 1.
HIF/VEGF/DC therapy in several cancers
| Cancer | Therapy | Common Drug | Impact | Clinical Course | Ref |
|---|---|---|---|---|---|
| Multiple Myeloma | Anti-VEGF | Bortezomib | Improve overall survival | FDA approved | [80,110] |
| HIF inhibitor | EZN-2968 | Reduces cancer cell viability | Pre-clinical trail | [81] | |
| DC vaccine | SC-DC vaccine | Induce antitumor response | Clinical trail | [83,111] | |
| Colorectal Cancer | Anti-VEGF | Bevacizumab | Acquired resistance develop | FDA approved | [84,112] |
| HIF inhibitor | DNMT inhibitors: zebularine | Overcome oxaliplatin resistance | Pre-clinical | [85] | |
| DC vaccine | / | Enhance immune | Clinical trail | [88,89] | |
| response; | |||||
| Improve survival | |||||
| Prostate Cancer | Anti-VEGF | Bevacizumab, Sutent | Improve survival | Clinical trail | [90,113] |
| HIF inhibitor | Acriflavine | Potent effects | Pre-clinical trail | [91,114] | |
| DC vaccine | Provenge | Induce immune response | FDA approved | [115] | |
| Lung Cancer | Anti-VEGF | Bevacizumab | Improve progression free survival | FDA approved | [93,94,116] |
| Improve survival | |||||
| HIF inhibitor | SOCS3 | Inhibit proliferation and angiogenesis | Pre-clinical trail | [95] | |
| DC vaccine | / | Induce immune response; | Clinical trail | [97] | |
| Improve survival | |||||
| Renal Cancer | Anti-VEGF | Axitinib, Sutent | Improve survival | FDA approved | [103,117] |
| HIF inhibitor | MK-6482 | Greater activity than sutent | FDA approved | [105,106] | |
| DC vaccine | HybriCell | Induce immune response with well torlerance | FDA approved | [104] |
Understanding the HIF/VEGF signaling pathway also contributes to the development of strategies for clinical diagnosis and precision medicine. Ye et al. analyzed the data from existing databases (The Cancer Genome Atlas) and found that drug efficacy was indeed affected by the level of hypoxia, and different drugs may have various sensitivities or tolerances at different hypoxia levels [38]. Therefore, the assessment of hypoxia levels in cancer patients is critical to improve the efficacy of drugs. The criteria for evaluating hypoxia and immune levels in cancer patients still need to be improved and modified. Understanding the relationship between HIF/VEGF and immune cells can help in the adjustment of these hypoxia-immune correlation scales. Importantly, these correlation scales can promote the development of precision medicine and improve the prognosis of cancer patients.
More research is necessary and required. Although most studies showed that VEGF plays a negative role on DC through VEGFR1, VEGFR2 and NRP pathways, the role of VEGFR3 has not been clearly studied. Also, the impact and mechanism of hypoxia on DC require further exploration. HIF could be a crucial regulator in the balance of the immune status of dendritic cells. Factors influenced by hypoxia should be valued, because these factors may be involved in the bypass pathway of HIF signal pathways and modulate efficacy of HIF inhibitor. Understanding the crosstalk between hypoxia, VEGF, and DC is essential for designing clinical therapies. Building upon current knowledge, further investigations should focus on the dosage and duration of anti-VEGF agents combined with DC vaccine. It is worth looking forward to further findings which may realize the promise of immunology and immunotherapeutics.
Conclusion
Studies have shown that HIF is involved in tumor development. In the VEGF/VEGFR signaling pathway, hypoxia is not just an upstream signaling molecule, but it participates in the downstream pathways of VEGF in DCs. Although different VEGF/VEGFR pathways can all affect the function of DCs, their specific impacts are distinct.
Since its discovery, HIF has been studied in different types of cancer. The complex HIF pathways, which were elucidated in previous studies, increase the difficulty of analyzing and understanding its specific mechanisms. The complexity also affects the clinical application of anti-HIF therapies [109]. Additionally, most reviews of HIF/VEGF pathways focus on the mechanism in tumor cells and T cells, and the reviews on the mechanisms in DCs are limited. DC vaccines provide a new direction for cancer treatment and also highlight the necessity for mechanistic research on the TME. This review provides a comprehensive summary of the existing molecular pathways and may help promote the development of novel clinical treatments. In cancer therapy, more attention has been paid to the control of hypoxia, VEGF levels, and DC function. The hypoxic pathway in the TME is expected to become an important target of cancer treatment in the future.
Acknowledgements
This work was supported by the WBE Liver Fibrosis Foundation (CFHPC 2020021), Beijing Dongcheng District outstanding talent funding project, CAMS Innovation Fund for Medical Sciences (CIFMS), and Natural Science Foundation of Gansu Province: Research on the construction of hepatobiliary model and the key technologies of 3D printing in preoperative planning system (18JR3RA029).Melissa Crawford, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38:614–622. doi: 10.1038/aps.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27:863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Xiong XX, Qiu XY, Hu DX, Chen XQ. Advances in hypoxia-mediated mechanisms in hepatocellular carcinoma. Mol Pharmacol. 2017;92:246–255. doi: 10.1124/mol.116.107706. [DOI] [PubMed] [Google Scholar]
- 6.Wijesekera DPH, Yuba E, De Silva NH, Watanabe SI, Tsukamoto M, Ichida C, Izawa T, Itoh K, Kanegi R, Hatoya S, Yamate J, Inaba T, Sugiura K. Manipulation of the tumor microenvironment by cytokine gene transfection enhances dendritic cell-based immunotherapy. FASEB Bioadv. 2020;2:5–17. doi: 10.1096/fba.2019-00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 8.Boissel N, Rousselot P, Raffoux E, Cayuela JM, Maarek O, Charron D, Degos L, Dombret H, Toubert A, Rea D. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia. 2004;18:1656–1661. doi: 10.1038/sj.leu.2403474. [DOI] [PubMed] [Google Scholar]
- 9.Tamura R, Tanaka T, Akasaki Y, Murayama Y, Yoshida K, Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: perspectives for therapeutic implications. Med Oncol. 2019;37:2. doi: 10.1007/s12032-019-1329-2. [DOI] [PubMed] [Google Scholar]
- 10.Noman MZ, Messai Y, Muret J, Hasmim M, Chouaib S. Crosstalk between CTC, immune system and hypoxic tumor microenvironment. Cancer Microenviron. 2014;7:153–160. doi: 10.1007/s12307-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao JH, Barbi J, Pan F. Hypoxia-inducible factors in T lymphocyte differentiation and function. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C580–589. doi: 10.1152/ajpcell.00204.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheshtchin N, Arab S, Ajami M, Mirzaei R, Ashourpour M, Mousavi N, Khosravianfar N, Jadidi-Niaragh F, Namdar A, Noorbakhsh F, Hadjati J. Inhibition of HIF-1alpha enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother. 2016;65:1159–1167. doi: 10.1007/s00262-016-1879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degrossoli A, Bosetto MC, Lima CB, Giorgio S. Expression of hypoxia-inducible factor 1alpha in mononuclear phagocytes infected with Leishmania amazonensis. Immunol Lett. 2007;114:119–125. doi: 10.1016/j.imlet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Dong G, Lin XH, Liu HH, Gao DM, Cui JF, Ren ZG, Chen RX. Intermittent hypoxia alleviates increased VEGF and pro-angiogenic potential in liver cancer cells. Oncol Lett. 2019;18:1831–1839. doi: 10.3892/ol.2019.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaszai J, Schmidt MHH. Trends and challenges in tumor anti-angiogenic therapies. Cells. 2019;8:1102. doi: 10.3390/cells8091102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 18.Cheng W, Cheng Z, Xing D, Zhang M. Asparagus polysaccharide suppresses the migration, invasion, and angiogenesis of hepatocellular carcinoma cells partly by targeting the HIF-1alpha/VEGF signalling pathway in vitro. Evid Based Complement Alternat Med. 2019;2019:3769879. doi: 10.1155/2019/3769879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 20.Oussa NA, Dahmani A, Gomis M, Richaud M, Andreev E, Navab-Daneshmand AR, Taillefer J, Carli C, Boulet S, Sabbagh L, Labrecque N, Sapieha P, Delisle JS. VEGF requires the receptor NRP-1 to inhibit lipopolysaccharide-dependent dendritic cell maturation. J Immunol. 2016;197:3927–3935. doi: 10.4049/jimmunol.1601116. [DOI] [PubMed] [Google Scholar]
- 21.Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. 2016;13:206–214. doi: 10.20892/j.issn.2095-3941.2015.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 24.Claesson-Welsh L. VEGF receptor signal transduction - A brief update. Vascul Pharmacol. 2016;86:14–17. doi: 10.1016/j.vph.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother. 2007;56:761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW, Dana MR. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 27.Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, Volke M, Glasner J, Warnecke C, Wiesener MS, Eckardt KU, Steinkasserer A, Hensel M, Willam C. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 28.Hong CH, Lee CH, Chen GS, Chang KL, Yu HS. STAT3-dependent VEGF production from keratinocytes abrogates dendritic cell activation and migration by arsenic: a plausible regional mechanism of immunosuppression in arsenical cancers. Chem Biol Interact. 2015;227:96–103. doi: 10.1016/j.cbi.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Song ZY, Hu HY, Deng L, Wu BY, Guo KY, Zhang MX. Effect of small interfering RNA targeting multidrug resistance-related protein and bcl-2 on drug resistance and apoptosis of K562 and K562/ADM cells. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1306–1308. [PubMed] [Google Scholar]
- 30.Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. 2018;322:63–73. doi: 10.1016/j.jneuroim.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Oshi M, Newman S, Tokumaru Y, Yan L, Matsuyama R, Kalinski P, Endo I, Takabe K. Plasmacytoid dendritic cell (pDC) infiltration correlate with tumor infiltrating lymphocytes, cancer immunity, and better survival in triple negative breast cancer (TNBC) more strongly than conventional dendritic cell (cDC) Cancers (Basel) 2020;12:3342. doi: 10.3390/cancers12113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block MS, Nevala WK, Leontovich AA, Markovic SN. Differential response of human and mouse dendritic cells to VEGF determines interspecies discrepancies in tumor-mediated TH1/TH2 polarity shift. Clin Cancer Res. 2011;17:1776–1783. doi: 10.1158/1078-0432.CCR-10-2836. [DOI] [PubMed] [Google Scholar]
- 33.Greene TT, Jo YR, Zuniga EI. Infection and cancer suppress pDC derived IFN-I. Curr Opin Immunol. 2020;66:114–122. doi: 10.1016/j.coi.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller LME, Holmes M, Michael JL, Scott GB, West EJ, Scott KJ, Parrish C, Hall K, Stable S, Jennings VA, Cullen M, McConnell S, Langton C, Tidswell EL, Shafren D, Samson A, Harrington KJ, Pandha H, Ralph C, Kelly RJ, Cook G, Melcher AA, Errington-Mais F. Plasmacytoid dendritic cells orchestrate innate and adaptive anti-tumor immunity induced by oncolytic coxsackievirus A21. J Immunother Cancer. 2019;7:164. doi: 10.1186/s40425-019-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paardekooper LM, Vos W, van den Bogaart G. Oxygen in the tumor microenvironment: effects on dendritic cell function. Oncotarget. 2019;10:883–896. doi: 10.18632/oncotarget.26608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naldini A, Morena E, Pucci A, Miglietta D, Riboldi E, Sozzani S, Carraro F. Hypoxia affects dendritic cell survival: role of the hypoxia-inducible factor-1alpha and lipopolysaccharide. J Cell Physiol. 2012;227:587–595. doi: 10.1002/jcp.22761. [DOI] [PubMed] [Google Scholar]
- 37.Kong KH, Oh HJ, Lim BJ, Kim M, Han KH, Choi YH, Kwon K, Nam BY, Park KS, Park JT, Han SH, Yoo TH, Lee S, Kim SJ, Kang DH, Choi KB, Eremina V, Quaggin SE, Ryu DR, Kang SW. Selective tubular activation of hypoxia-inducible factor-2alpha has dual effects on renal fibrosis. Sci Rep. 2017;7:11351. doi: 10.1038/s41598-017-11829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Y, Hu Q, Chen H, Liang K, Yuan Y, Xiang Y, Ruan H, Zhang Z, Song A, Zhang H, Liu L, Diao L, Lou Y, Zhou B, Wang L, Zhou S, Gao J, Jonasch E, Lin SH, Xia Y, Lin C, Yang L, Mills GB, Liang H, Han L. Characterization of hypoxia-associated molecular features to aid hypoxia-targeted therapy. Nat Metab. 2019;1:431–444. doi: 10.1038/s42255-019-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agod Z, Fekete T, Budai MM, Varga A, Szabo A, Moon H, Boldogh I, Biro T, Lanyi A, Bacsi A, Pazmandi K. Regulation of type I interferon responses by mitochondria-derived reactive oxygen species in plasmacytoid dendritic cells. Redox Biol. 2017;13:633–645. doi: 10.1016/j.redox.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macklin PS, McAuliffe J, Pugh CW, Yamamoto A. Hypoxia and HIF pathway in cancer and the placenta. Placenta. 2017;56:8–13. doi: 10.1016/j.placenta.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Filippi I, Morena E, Aldinucci C, Carraro F, Sozzani S, Naldini A. Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1alpha and PI3K/Akt pathway. J Cell Physiol. 2014;229:2067–2076. doi: 10.1002/jcp.24666. [DOI] [PubMed] [Google Scholar]
- 42.Kohler T, Reizis B, Johnson RS, Weighardt H, Forster I. Influence of hypoxia-inducible factor 1alpha on dendritic cell differentiation and migration. Eur J Immunol. 2012;42:1226–1236. doi: 10.1002/eji.201142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013;24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Hammami A, Abidin BM, Heinonen KM, Stager S. HIF-1alpha hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci Rep. 2018;8:3500. doi: 10.1038/s41598-018-21891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, Chen Y, Zhu H, Li Z, Cao X. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1alpha-mediated glycolysis. Immunity. 2019;50:600–615. e615. doi: 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D, Lv FL, Wang GH. Effects of HIF-1alpha on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22:5071–5076. doi: 10.26355/eurrev_201808_15699. [DOI] [PubMed] [Google Scholar]
- 48.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 49.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 50.D’Ignazio L, Batie M, Rocha S. Hypoxia and inflammation in cancer, focus on HIF and NF-kappaB. Biomedicines. 2017;5:21. doi: 10.3390/biomedicines5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 52.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, Clay T, Morse MA. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F, Zhou J, Liu L, Qiu W, Zhang S, Ouyang Y, Ye Y, Xu G, Li L, Zeng Z. Vascular endothelial growth factor (VEGF) impairs the motility and immune function of human mature dendritic cells through the VEGF receptor 2-RhoA-cofilin1 pathway. Cancer Sci. 2019;110:2357–2367. doi: 10.1111/cas.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricker E, Chowdhury L, Yi W, Pernis AB. The RhoA-ROCK pathway in the regulation of T and B cell responses. F1000Res. 2016;5:F1000 Faculty Rev-2295. doi: 10.12688/f1000research.7522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Hernandez I, Cantelli G, Bruce F, Sanz-Moreno V. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Res. 2016;5:F1000 Faculty Rev-783. doi: 10.12688/f1000research.7909.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyronneau A, He Q, Hwang JY, Porch M, Contractor A, Zukin RS. Aberrant Rac1-cofilin signaling mediates defects in dendritic spines, synaptic function, and sensory perception in fragile X syndrome. Sci Signal. 2017;10:eaan0852. doi: 10.1126/scisignal.aan0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu J, Liu K, Zhao J, Zhao J, Ma J, Yang H, Huang Y, Qin Z, Bai R, Jiang L, Lv F, Li P, Yan W, Zhao M, Dong Z. VEGF-A not Ang2 mediates endothelial-like differentiation of immature DCs by ERK1/2 signaling in the microenvironment of human colon adenocarcinoma. Int J Oncol. 2011;38:1579–1588. doi: 10.3892/ijo.2011.989. [DOI] [PubMed] [Google Scholar]
- 58.Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28:385–392. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Gulubova M, Ivanova K, Ananiev J, Gerenova J, Zdraveski A, Stoyanov H, Vlaykova T. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol Biotechnol Equip. 2014;28:508–517. doi: 10.1080/13102818.2014.909151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cebe-Suarez S, Grunewald FS, Jaussi R, Li X, Claesson-Welsh L, Spillmann D, Mercer AA, Prota AE, Ballmer-Hofer K. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J. 2008;22:3078–3086. doi: 10.1096/fj.08-107219. [DOI] [PubMed] [Google Scholar]
- 61.Luo M, Hou L, Li J, Shao S, Huang S, Meng D, Liu L, Feng L, Xia P, Qin T, Zhao X. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-kappaB and beta-catenin. Cancer Lett. 2016;373:1–11. doi: 10.1016/j.canlet.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Lin J, Zhang Y, Wu J, Li L, Chen N, Ni P, Song L, Liu X. Neuropilin 1 (NRP1) is a novel tumor marker in hepatocellular carcinoma. Clin Chim Acta. 2018;485:158–165. doi: 10.1016/j.cca.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 63.Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol. 2010;7:83–88. doi: 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong Y, Lingrel JB, Wuthrich M, Klein BS, Vasudevan NT, Jain MK, George M, Deepe GS Jr. Transcription factor KLF2 in dendritic cells downregulates Th2 programming via the HIF-1alpha/Jagged2/notch axis. mBio. 2016;7:e00436-16. doi: 10.1128/mBio.00436-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coutant F, Miossec P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol. 2016;12:703–715. doi: 10.1038/nrrheum.2016.147. [DOI] [PubMed] [Google Scholar]
- 66.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang Y, Zhu Y, Wang X, Gong J, Hu C, Guo B, Zhu B, Li Y. Temporal regulation of HIF-1 and NF-kappaB in hypoxic hepatocarcinoma cells. Oncotarget. 2015;6:9409–9419. doi: 10.18632/oncotarget.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, Albert ML. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang JG, Zhou HM, Zhang X, Mu W, Hu JN, Liu GL, Li Q. Hypoxic induction of vasculogenic mimicry in hepatocellular carcinoma: role of HIF-1 alpha, RhoA/ROCK and Rac1/PAK signaling. BMC Cancer. 2020;20:32. doi: 10.1186/s12885-019-6501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song H, Zhang J, He W, Wang P, Wang F. Activation of cofilin increases intestinal permeability via depolymerization of F-actin during hypoxia in vitro. Front Physiol. 2019;10:1455. doi: 10.3389/fphys.2019.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu J, Tu Y, Wang Y, Xu X, Sun X, Xie L, Zhao Q, Guo Y, Gu Y, Du J, Du S, Zhu M, Song E. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1alpha/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed Pharmacother. 2020;121:109606. doi: 10.1016/j.biopha.2019.109606. [DOI] [PubMed] [Google Scholar]
- 73.Hong M, Shi H, Wang N, Tan HY, Wang Q, Feng Y. Dual effects of chinese herbal medicines on angiogenesis in cancer and ischemic stroke treatments: role of HIF-1 network. Front Pharmacol. 2019;10:696. doi: 10.3389/fphar.2019.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiffmann LM, Fritsch M, Gebauer F, Günther SD, Stair NR, Seeger JM, Thangarajah F, Dieplinger G, Bludau M, Alakus H, Göbel H, Quaas A, Zander T, Hilberg F, Bruns CJ, Kashkar H, Coutelle O. Tumour-infiltrating neutrophils counteract anti-VEGF therapy in metastatic colorectal cancer. Br J Cancer. 2019;120:69–78. doi: 10.1038/s41416-018-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 76.Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain RK, Suit HD, Boucher Y. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 77.Pedersen AE, Buus S, Claesson MH. Treatment of transplanted CT26 tumour with dendritic cell vaccine in combination with blockade of vascular endothelial growth factor receptor 2 and CTLA-4. Cancer Lett. 2006;235:229–238. doi: 10.1016/j.canlet.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 78.Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kunimi H, Miwa Y, Inoue H, Tsubota K, Kurihara T. A novel HIF inhibitor halofuginone prevents neurodegeneration in a murine model of retinal ischemia-reperfusion. Int J Mol Sci. 2019;20:3171. doi: 10.3390/ijms20133171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roccaro AM, Hideshima T, Raje N, Kumar S, Ishitsuka K, Yasui H, Shiraishi N, Ribatti D, Nico B, Vacca A, Dammacco F, Richardson PG, Anderson KC. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006;66:184–191. doi: 10.1158/0008-5472.CAN-05-1195. [DOI] [PubMed] [Google Scholar]
- 81.Borsi E, Perrone G, Terragna C, Martello M, Dico AF, Solaini G, Baracca A, Sgarbi G, Pasquinelli G, Valente S, Zamagni E, Tacchetti P, Martinelli G, Cavo M. Hypoxia inducible factor-1 alpha as a therapeutic target in multiple myeloma. Oncotarget. 2014;5:1779–1792. doi: 10.18632/oncotarget.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Storti P, Toscani D, Airoldi I, Marchica V, Maiga S, Bolzoni M, Fiorini E, Campanini N, Martella E, Mancini C, Guasco D, Ferri V, Donofrio G, Aversa F, Amiot M, Giuliani N. The anti-tumoral effect of lenalidomide is increased in vivo by hypoxia-inducible factor (HIF)-1α inhibition in myeloma cells. Haematologica. 2016;101:e107–e110. doi: 10.3324/haematol.2015.133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinde P, Melinkeri S, Santra MK, Kale V, Limaye L. Autologous hematopoietic stem cells are a preferred source to generate dendritic cells for immunotherapy in multiple myeloma patients. Front Immunol. 2019;10:1079. doi: 10.3389/fimmu.2019.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhattacharya R, Fan F, Wang R, Ye X, Xia L, Boulbes D, Ellis LM. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br J Cancer. 2017;117:848–855. doi: 10.1038/bjc.2017.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei TT, Lin YT, Tang SP, Luo CK, Tsai CT, Shun CT, Chen CC. Metabolic targeting of HIF-1α potentiates the therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene. 2020;39:414–427. doi: 10.1038/s41388-019-0999-8. [DOI] [PubMed] [Google Scholar]
- 86.Moroney J, Fu S, Moulder S, Falchook G, Helgason T, Levenback C, Hong D, Naing A, Wheler J, Kurzrock R. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biological activity. Clin Cancer Res. 2012;18:5796–5805. doi: 10.1158/1078-0432.CCR-12-1158. [DOI] [PubMed] [Google Scholar]
- 87.Feng QY, Wei Y, Chen JW, Chang WJ, Ye LC, Zhu DX, Xu JM. Anti-EGFR and anti-VEGF agents: important targeted therapies of colorectal liver metastases. World J Gastroenterol. 2014;20:4263–4275. doi: 10.3748/wjg.v20.i15.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu H, Yang X, Li J, Ren Y, Zhang T, Zhang C, Zhang J, Li J, Pang Y. Immune response, safety, and survival and quality of life outcomes for advanced colorectal cancer patients treated with dendritic cell vaccine and cytokine-induced killer cell therapy. Biomed Res Int. 2014;2014:603871. doi: 10.1155/2014/603871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu KJ, Chao TY, Chang JY, Cheng AL, Ch’ang HJ, Kao WY, Wu YC, Yu WL, Chung TR, Whang-Peng J. A phase I clinical study of immunotherapy for advanced colorectal cancers using carcinoembryonic antigen-pulsed dendritic cells mixed with tetanus toxoid and subsequent IL-2 treatment. J Biomed Sci. 2016;23:64. doi: 10.1186/s12929-016-0279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eisermann K, Fraizer G. The androgen receptor and VEGF: mechanisms of androgen-regulated angiogenesis in prostate cancer. Cancers (Basel) 2017;9:32. doi: 10.3390/cancers9040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen CH, Li SX, Xiang LX, Mu HQ, Wang SB, Yu KY. HIF-1alpha induces immune escape of prostate cancer by regulating NCR1/NKp46 signaling through miR-224. Biochem Biophys Res Commun. 2018;503:228–234. doi: 10.1016/j.bbrc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Xi HB, Wang GX, Fu B, Liu WP, Li Y. Survivin and PSMA loaded dendritic cell vaccine for the treatment of prostate cancer. Biol Pharm Bull. 2015;38:827–835. doi: 10.1248/bpb.b14-00518. [DOI] [PubMed] [Google Scholar]
- 93.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Wang XX, Wu W, Long H, Huang J, Wang Z, Li T, Tang S, Zhu B, Chen D. EZH2 regulates PD-L1 expression via HIF-1alpha in non-small cell lung cancer cells. Biochem Biophys Res Commun. 2019;517:201–209. doi: 10.1016/j.bbrc.2019.07.039. [DOI] [PubMed] [Google Scholar]
- 95.Wan J, Che Y, Kang N, Wu W. SOCS3 blocks HIF-1α expression to inhibit proliferation and angiogenesis of human small cell lung cancer by downregulating activation of Akt, but not STAT3. Mol Med Rep. 2015;12:83–92. doi: 10.3892/mmr.2015.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du YC, Lin P, Zhang J, Lu YR, Ning QZ. Studies on the enhancement of DC vaccine to mouse Lewis lung cancer by CpG oligonucleotides. Zhonghua Zhong Liu Za Zhi. 2005;27:1–5. [PubMed] [Google Scholar]
- 97.Zhang L, Yang X, Sun Z, Li J, Zhu H, Li J, Pang Y. Dendritic cell vaccine and cytokine-induced killer cell therapy for the treatment of advanced non-small cell lung cancer. Oncol Lett. 2016;11:2605–2610. doi: 10.3892/ol.2016.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Zhang C, Zhao Y, Yue X, Wu H, Huang S, Chen J, Tomsky K, Xie H, Khella CA, Gatza ML, Xia D, Gao J, White E, Haffty BG, Hu W, Feng Z. Parkin targets HIF-1α for ubiquitination and degradation to inhibit breast tumor progression. Nat Commun. 2017;8:1823. doi: 10.1038/s41467-017-01947-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang T, Meng J, Wang C, Wen T, Jia M, Ge Y, Xie L, Hao S, Liu J, Xu H. Inhibition of murine breast cancer metastases by hydrophilic As(4)S(4) nanoparticles is associated with decreased ROS and HIF-1α downregulation. Front Oncol. 2019;9:333. doi: 10.3389/fonc.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyons TG, Ku GY. Systemic therapy for esophagogastric cancer: targeted therapies. Chin Clin Oncol. 2017;6:48. doi: 10.21037/cco.2017.07.02. [DOI] [PubMed] [Google Scholar]
- 101.Lin YW, Huang ST, Wu JC, Chu TH, Huang SC, Lee CC, Tai MH. Novel HDGF/HIF-1alpha/VEGF axis in oral cancer impacts disease prognosis. BMC Cancer. 2019;19:1083. doi: 10.1186/s12885-019-6229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wright JD, Powell MA, Rader JS, Mutch DG, Gibb RK. Bevacizumab therapy in patients with recurrent uterine neoplasms. Anticancer Res. 2007;27:3525–3528. [PubMed] [Google Scholar]
- 103.Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–132. doi: 10.1016/j.phrs.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Baek S, Kim CS, Kim SB, Kim YM, Kwon SW, Kim Y, Kim H, Lee H. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: results from a phase I/II trial. J Transl Med. 2011;9:178. doi: 10.1186/1479-5876-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM, Brugarolas J. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Courtney KD, Ma Y, Diaz de Leon A, Christie A, Xie Z, Woolford L, Singla N, Joyce A, Hill H, Madhuranthakam AJ, Yuan Q, Xi Y, Zhang Y, Chang J, Fatunde O, Arriaga Y, Frankel AE, Kalva S, Zhang S, McKenzie T, Reig Torras O, Figlin RA, Rini BI, McKay RM, Kapur P, Wang T, Pedrosa I, Brugarolas J. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin Cancer Res. 2020;26:793–803. doi: 10.1158/1078-0432.CCR-19-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Incio J, Ligibel JA, McManus DT, Suboj P, Jung K, Kawaguchi K, Pinter M, Babykutty S, Chin SM, Vardam TD, Huang Y, Rahbari NN, Roberge S, Wang D, Gomes-Santos IL, Puchner SB, Schlett CL, Hoffmman U, Ancukiewicz M, Tolaney SM, Krop IE, Duda DG, Boucher Y, Fukumura D, Jain RK. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci Transl Med. 2018;10:eaag0945. doi: 10.1126/scitranslmed.aag0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiang CL, Balint K, Coukos G, Kandalaft LE. Potential approaches for more successful dendritic cell-based immunotherapy. Expert Opin Biol Ther. 2015;15:569–582. doi: 10.1517/14712598.2015.1000298. [DOI] [PubMed] [Google Scholar]
- 109.Albadari N, Deng S, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov. 2019;14:667–682. doi: 10.1080/17460441.2019.1613370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ria R, Melaccio A, Racanelli V, Vacca A. Anti-VEGF drugs in the treatment of multiple myeloma patients. J Clin Med. 2020;9:1765. doi: 10.3390/jcm9061765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen S, Haimovich J, Hollander N. Dendritic cell-based therapeutic vaccination against myeloma: vaccine formulation determines efficacy against light chain myeloma. J Immunol. 2009;182:1667–1673. doi: 10.4049/jimmunol.182.3.1667. [DOI] [PubMed] [Google Scholar]
- 112.Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int J Mol Sci. 2018;19:1232. doi: 10.3390/ijms19041232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wheler JJ, Janku F, Falchook GS, Jackson TL, Fu S, Naing A, Tsimberidou AM, Moulder SL, Hong DS, Yang H, Piha-Paul SA, Atkins JT, Garcia-Manero G, Kurzrock R. Phase I study of anti-VEGF monoclonal antibody bevacizumab and histone deacetylase inhibitor valproic acid in patients with advanced cancers. Cancer Chemother Pharmacol. 2014;73:495–501. doi: 10.1007/s00280-014-2384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Di Lorenzo G, Ferro M, Buonerba C. Sipuleucel-T (Provenge®) for castration-resistant prostate cancer. BJU Int. 2012;110:E99–104. doi: 10.1111/j.1464-410X.2011.10790.x. [DOI] [PubMed] [Google Scholar]
- 116.Meder L, Schuldt P, Thelen M, Schmitt A, Dietlein F, Klein S, Borchmann S, Wennhold K, Vlasic I, Oberbeck S, Riedel R, Florin A, Golfmann K, Schlößer HA, Odenthal M, Buettner R, Wolf J, Hallek M, Herling M, von Bergwelt-Baildon M, Reinhardt HC, Ullrich RT. Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res. 2018;78:4270–4281. doi: 10.1158/0008-5472.CAN-17-2176. [DOI] [PubMed] [Google Scholar]
- 117.D’Aniello C, Berretta M, Cavaliere C, Rossetti S, Facchini BA, Iovane G, Mollo G, Capasso M, Pepa CD, Pesce L, D’Errico D, Buonerba C, Di Lorenzo G, Pisconti S, De Vita F, Facchini G. Biomarkers of prognosis and efficacy of anti-angiogenic therapy in metastatic clear cell renal cancer. Front Oncol. 2019;9:1400. doi: 10.3389/fonc.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]


