Abstract
Hormone-positive breast cancer (BC) is a unique heterogeneous disease with a favorable prognosis compared to other types of breast cancer. As tumor biology influences the prognosis and clinical treatment, a deep understanding of how the molecular mechanisms regulate hormone sensitivity or resistance is critical in improving the efficacy and overcoming the endocrine resistance. This article comprehensively reviews the endocrine resistance in hormone-positive BC from a molecular and genetic perspective, encompassing the updated treatment and developing direction. This review includes the mechanisms of hormone resistance, which vary from epigenetic changes, crosstalk between signaling networks, cell cycle aberrance, and even change in the tumor microenvironment (TME) or stem cell. These mechanisms may contribute to treatment resistance. Current targeted therapy for hormone-resistant tumors includes PI3K/AKT/mTOR and cdk4/6 inhibitors. Several relevant pathways, biomarkers, and predictor genes have also been identified. Immunotherapy so far has a relatively less crucial role in hormone-positive than in triple-negative BC. Furthermore, the methodology to identify the PDL1 is not standardized. In a molecule and gene study, next-generation sequencing with circulating tumor DNA (ctDNA) has recently appeared as a sensitive and minimally invasive tool worth investigating.
Keywords: Breast cancer, endocrine resistance, hormone-positive, estrogen, signaling pathway, ESR1, tumor microenvironment, next-generation sequencing
Introduction
Breast cancer (BC) is one of the most relevant causes of death among women worldwide [1]. It is a heterogeneous disease with multiple intrinsic tumors, categorized into various subgroups presenting diverse biological and clinical behaviors [2].
There are four subtypes of BC based on the presentation of estrogen or progesterone receptors (PR) and human epidermal growth factor 2 (HER2). Luminal A is hormone-responsive BC (estrogen-receptor or progesterone-receptor positive, HER2 negative, and has low protein Ki-67), with a favorable prognosis that can be treated with endocrine therapies. Luminal B (estrogen-receptor or progesterone-receptor positive, and either HER2 positive or HER2 negative with high levels of Ki-67) generally grow slightly faster than luminal A cancers, and their prognosis is worse when compared with luminal A. Luminal B subtype can be treated with hormone regimen or antiHER2 targeted therapy if it is HER2 positive. The other two types are HER2 enrich type, which response to anti-HER2 regimen such as Trastuzumab or Pertuzumab [3], and triple-negative BC, which can be treated with an immune checkpoint inhibitor. As tumor biology influences the prognosis and clinical treatment, and 70% of BCs are estrogen receptor (ER) positive, a deep understanding of how the molecular mechanisms regulate hormone sensitivity or resistance is critical in improving the efficacy and overcoming the resistance to endocrine therapy [4]. Furthermore, many gene expressions determine the prognosis and affect the treatment strategy; that is why multi-gene assays, such as Oncotype Dx, Mammaprint, and EndoPredict, are currently available [5]. Whether other estrogen-response genes could be identified will be an issue in predicting prognosis and guiding treatment [6].
This article reviews the endocrine resistance in hormone-response BC from the perspective of signaling pathways, anti-endocrine resistance, potential or predictive biomarkers for the treatment to contribute a novel insight for the BC microenvironment (Figure 1).
Figure 1.
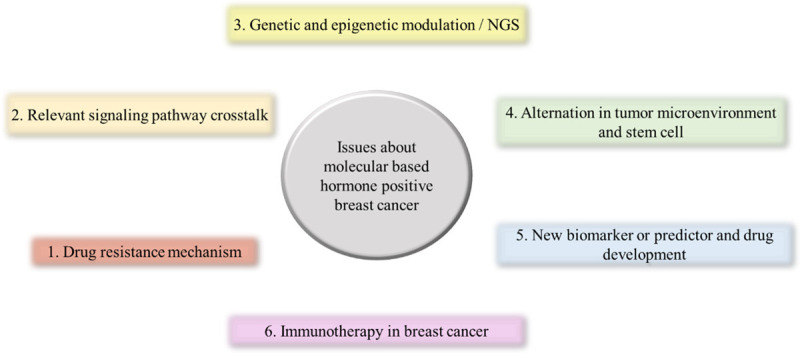
Issues about molecular based Hormone-positive breast cancer.
The characteristics of hormone-positive BC and current therapy
The presentation of ER and PR are two crucial molecules that determine the heterogeneity of BC and prognosis, as ER/PR double positive has better survival than ER or PR single positive BC [7]. Studies have shown that the characteristics of single or double hormone receptor-positive subtypes are very different and warranted varied treatments for further investigation [8]. The two subtypes of ER are alpha (ERα) and beta (ERβ). ERα is the primary form of ER in BC and presents both protein and mRNA levels. ERα activation is considered responsible for the enhanced proliferation of breast epithelial cells, whereas ERβ exerts an antiproliferative effect [9].
Estrogen Receptor α (ERα) targets the treatment with antiestrogens, steroidal or non-steroidal molecules [10]. Nevertheless, scholars do not completely understand the mechanisms of the expression of ERα. Gene amplification was thought to be one of the most important factors regulating protein expression. However, around 40% of ERα-positive tumors showed an absence of response to endocrine therapy, and the majority of those with response eventually become resistant [11,12].
The current therapies for Hormone-positive BC contain drugs that suppress estrogen presentation (aromatase inhibitors, GnRH agonists) and direct inhibitors of the ER (selective estrogen receptor modulators (SERM)) or selective estrogen receptor degraders (SERD), the uses of which vary among premenopausal and menopausal women [13] (Table 1). It is well that levels of the two ER subtypes change during BC progression, so the independent measurement of both ERs might help predict the disease prognosis and response to endocrine therapies. Selective activation of ERα or ERβ depends not only on binding affinity to the selective receptor but also on various activation of each receptor subtype [14].
Table 1.
Current hormone and target therapy for HR positive Breast cancer (BC)
| Drug character | Drug | mechanism | Indication | Others |
|---|---|---|---|---|
| Hormone therapy | Tamoxifen | Block ERs on BC cells | 1. HR+ invasive BC treated with surgery, or breast-conserving surgery in ductal carcinoma in situ (DCIS) | associated with increased endometrial cancer risk |
| selective estrogen receptor modulator (SERM) | 2. mBC with HR+ | |||
| selective estrogen receptor degrader (SERD) | Fulvestrant (Faslodex) | Block and damages ERs | Alone to treat advanced BC not or underwent other hormone drugs | CONFIRM trial |
| combined with a CDK4/6 inhibitor or PI3K inhibitor to treat metastatic BC | FALCON trial | |||
| FACT trial | ||||
| Aromatase Inhibitor | steroidal (exemestane) | Block the aromatase activity, thus inhibiting estrogen biosynthesis | Post-menopausal women, either alone or after tamoxifen | Better than taking just tamoxifen for 5 years |
| non-steroidal AIs (letrozole/anastrozole) | ||||
| LHRH analogs | goserelin (Zoladex) and leuprolide | Lower estrogen levels | Used alone or combined with other hormone drugs such as tamoxifen, aromatase inhibitors, or fulvestrant as hormone therapy in pre-menopausal women | Mechanism: |
| inhibition of pituitary and gonadal function | ||||
| PI3K Inhibitors | Pictillisib | Inhibiting PI3K signaling | Resistance to endocrine | BELLE-2/SOLAR-1/ |
| Buparilisib | therapy in HR-positive BC | SANDPIPER Trial | ||
| MTOR inhibitors | Everolimus | Inhibit the intracellular | Resistance to endocrine therapy in HR-positive BC | BOLERO-2/HORIZON/ |
| temsirolimus | protein FKB12, interacting with mTORC1 and inhibiting | BALLET Trial | ||
| mTOR signaling | ||||
| Cyclin-dependent Kinase (CDK) inhibitor | Ribociclib | Prevents the cell cycle from proceeding G1 to S phase, then inhibiting cell proliferation | Alone or combine endocrine therapy for post-menopausal women | PALOMA/MONALEESA trial |
| Palbociclib | ||||
| Abemaciclib |
Fulvestrant, which lowers both ER and PR levels, referring to a selective ER down regulators, is not inferior to tamoxifen and similar or superior to aromatase inhibitors in metastatic disease in the FALCON trial [15,16] and sub-group analysis [16,17]. Other ovarian suppression medication includes leuprorelin or goserelin, which can be used for premenopausal patients [18].
Furthermore, the updated therapy for hormone response BC in metastatic setting includes hormone therapy, chemotherapy, or target therapy such as CDK4/6 inhibitors [19], PI3K/AKT/mTOR inhibitors (Everolimus), and PI3K inhibitor (Alpelisib) [20]. The novel topic in developing a new drug or enhancing efficacy will also focus on the cross-talk between various signal transduction and endocrine receptor pathways, which will be discussed later.
The mechanism of the endocrine resistance in hormone-positive BC
Drug resistance refers to both hormone resistance and multidrug resistance in Hormone-positive BC. The progression to drug resistance is considered a gradual, step-wise process. Several mechanisms have been proposed, but the resistance phenomenon still cannot be entirely explained by only one [21]. Regarding endocrine resistance, tumors with the biological character of hormone resistance formed de novo before therapy is referred to as primary resistance. At the same time, tumors that lost sensitivity to the effects of antiestrogens during endocrine therapy are referred to as secondary resistance [22]. Investigators have proposed several mechanisms such as downregulation of ER; ER gene mutation or epigenetic changes; overexpression of positive cell cycle regulators by activating CDKs; cross-talk between ER and other signaling networks; and change in the tumor microenvironment (TME) [23]. A brief review of these resistance mechanisms will be presented as follows (Figure 2).
Figure 2.
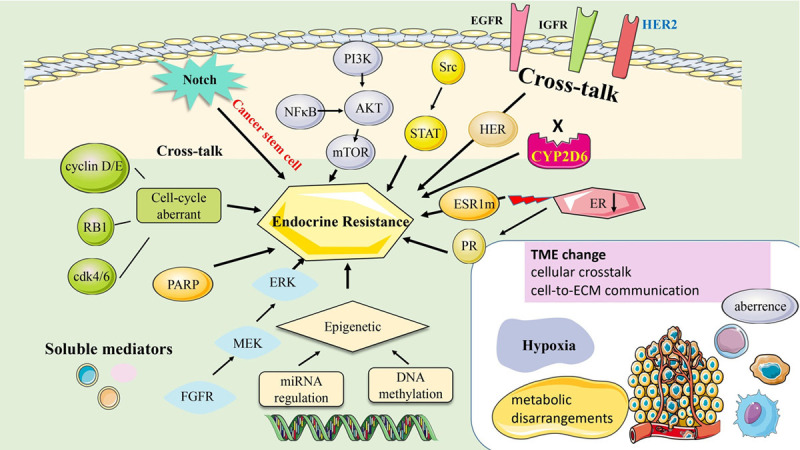
The mechanism of endocrine resistance in Hormone-positive breast cancer. A. ER down regulation (such as CYP2D6 variants; lack of ERα expression). B. The profiles of DNA methylation or miRNA regulation with differential adaptation of breast cancer cells to the hormone therapy, and hence could be cause of hormone resistance. C. Tumors harboring mutations of ESR1. D. Cross-talk between ER and other signaling networks, such as HER2/IFGR/EGFR/MAPK/mTOR). Acquired resistance to endocrine therapy involves alterations in translation signals through increasing the expression and activity of tyrosine kinase receptor family proteins (HER2, EGFR, and IGFR). These events result in aberrant activation of cAMP/PKA, MAPK/ERK, and PI3K/AKT signaling pathways. E. Aberrance of cell cycle regulators (c-myc; cyclin D1/E; CDK4/6). F. Resistance from Cancer stem cell: breast cancer stem cells to generate resistant BCs containing part of BCSCs; the other is via the dedifferentiation of BCs will gnerate tumors resistant to endocrine therapy and leading to the acquisition of BCSCs. G. Resistance from tumor microenvironment (cellular crosstalk and cell-to-ECM communication, inducing the release of soluble factors in charge of immune evasion and ECM remodeling, which further promote therapy resistance. Abbreviations: ER, Estrogen receptor; ECM, Extracellular Matrix; ESR1, Estrogen Receptor 1; EGFR, epidermal growth factor receptor; FGF2, fibroblast growth factor 2; FGFR, fibroblast growth factor receptor; GFR, growth factor receptor; HER2, human epidermal growth factor receptor 2; IGF, insulin-like growth factor; IGFR, insulin-like growth factor 1 receptor; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3 kinase; PR, Progesterone receptor; TME, tumor microenvironment.
ER down-regulation and epigenetic change
Lack of ERα expression is recognized as a primary mechanism for de novo endocrine resistance. Phosphorylation of specific serines on ERα is also related to tamoxifen resistance in BC [24]. The presence of active CYP2D6, the enzyme responsible for tamoxifen activation, decreases the response to tamoxifen therapy [25]. Endocrine resistance is also indicated due to DNA methylation and non-coding RNAs [26,27]. DNA methylation in the promoter region of genes is predictive biomarkers to different modalities of endocrine therapies [28]. In the strategy of miRNA, post-transcriptional regulation of miRNAs, including overexpression of miR-221/222; miRNA-519a, which co-regulates a network of TSGs, have been related to hotmone resistance [29,30].
Furthermore, downregulation of microRNA-27b-3p strengthens tamoxifen resistance through increasing NR5A2 and CREB1 expression [31]. Therefore, manipulating these miRNA expressions or using inhibitors of such miRNAs may serve as a novel therapeutic approach to combat hormone resistance in BC [32]. Furthermore, the quantitative measurement of circulating cell-free DNA (ccf DNA) from liquid biopsies is also a promising tool in detecting aberrant DNA methylation [33,34]. Recently, epigenetic modifications, such as DNA methylation, acetylation of histone proteins, and miRNA expression alteration all influence protein synthesis patterns [35].
Other epigenetic change includes silencing Spalt-like transcription factor 2 (SALL2) induced downregulation of ERα and PTEN and activated Akt/mTOR signaling, rendering estrogen-independent growth and tamoxifen resistance ERα-positive BC [36]. Prostaglandin E2 receptor 4 (PTGER4) is essential for estrogen-independent growth, and it contributes resistance to endocrine therapy for BC on activation [37]. The expression of MTA1, relating to cancer progression, is also correlated to poor prognosis. The activation of AMPK and subsequent autophagy contributes to tamoxifen resistance in BC [38]. Some alterations in other pathways and related genes, such as MAPK, EGFR, KRAS, etc. or ER transcriptional regulators (MYC, CTCF, FOXA1, and TBX3) were also lead to endocrine resistance in BC.
Altogether, these data suggest that diverse gene expression, DNA methylation, or miRNA regulation profiles in BC cells correlate with differential adaptation to hormone therapy, and they could be the targets of hormone resistance [26]. We summarize the genomic alternation in Hormone-positive BC in Table 2.
Table 2.
The over-review of genomic alternation in Hormone-positive breast cancer (BC)
| Gene | Alteration | Implication and comments | incidence |
|---|---|---|---|
| PIK3CA [95] | Hot spot mutation in exons 9 and 20 | PTEN loss and PIK3CA mutation were frequently concordant, suggesting different contributions to pathophysiology | 30-40% |
| ESR1 [130] | Mutation in ligand-binding domain | Hot-spot activating missense mutations (E380Q, Y537S/C/N, D538G). Treatment with AIs may exert a selective pressure favoring the expansion of ESR1-mutated clones | 18-39% |
| FGFR1 [131] | amplification | 1. 11q12-14 chromosome | Around 15-23% |
| 2. resistance to hormone therapies can be motivated by FGFR1 amplifications | |||
| 3. related to a lower PFS correlation between FGFR1 amplification and resistance to CDK4/6 inhibitors ribociclib or palbociclib | |||
| CCND1 [82,132] | amplification | 1. 11q13 chromosome BC | 9-30% |
| 2. CCND1 amplification could serve as a biomarker for the prediction of response to cyclin-dependent kinase 4/6 inhibitors | |||
| ERBB2 [133,134] | amplification | 1.Hot-spot activating missense mutations (e.g. S310F/Y, L755S, V777L); Inframe insertion exon 20 (Ex: Y772_A775dup) | In 10% ER+ BC |
| 2. HR and ERBB2 cross-talk as a mechanism for resistance to endocrine therapy | |||
| PTEN [135] | loss or mutation | Homozygous deletions; Loss-of-function mutations: truncated mutations and known inactivating missense mutations (Ex: R130Q/G) | Mutations: 5-10% |
| acquired PTEN mutation is a resistance mechanism to PI3Kα inhibitors in ER+ PIK3CA mutant BC | Loss: 30% | ||
| MDM2 [136] | amplification | Most frequently co-occurred with MDM2 amplification were CDK4 amplification, CDKN2A/B loss, and MYC amplification | 5.7% |
| AKT1 [137,138] | E17K mutation | 1. E17K mutation activates AKT1 by recruiting it to the membrane through a PI3K-independent mechanism | In 3% of ER+ BC |
| 2. E17K-AKT1, PIK3CA, and PTEN mutations are usually mutually exclusive | |||
| BRCA1/BRCA2 [139] | mutation | Truncated mutations: insertions or deletions, splice-site, nonsense (except known truncating polymorphic variant) | 3% |
| RB1 [140] | Loss of function mutations/deletions | RB-pathway dysregulation is strongly correlated with poor response to tamoxifen therapy | 20% |
| EGFR [141] | Amplifications | Aberrant EGFR expression was associated with poor prognosis in ER+ BCs, especially the Luminal B subtype | 10% of ER+ BCs |
| ERBB2 [142] | Mutations or amplification | 1. Hot-spot activating missense mutations (e.g. S310F/Y, L755S, V777L) | 20% |
| 2. A potential signaling cross-talk between EGFR or IGF1R and ERBB2, which could influence response of ERBB2-positive BCs to inhibitors |
Tumors harboring mutations of estrogen receptor 1 (ESR1)
Recently, a mechanism for acquired endocrine resistance noted as mutations in the gene encoding ERα, ESR1, have drawn attention in metastatic BC more than in primary BC [39]. In the BC population, nearly 20% of metastatic tumors evolve ESR1 mutations during hormone therapy [40]; furthermore, ESR1 and TP53 were the most mutually exclusive gene pair in Hormone-positive mBCs [41]. Patients with an ESR1 mutation had decreased progression-free survival (PFS) and worse overall survival (OS) than patients with WT ESR1, and a clear association between ESR1 mutation and AI resistance was noted [42]. The missense mutations have been recognized in more than 50 other residues; most occur within the ER ligand-binding domain (LBD). The most common ESR1 mutations in endocrine resistance were the missense mutations Y537 and D538. Other models demonstrated cell lines transfected with a Y537C, D538G, L536Q, Y537N, S463P, Y537S, or E380Q ESR1 mutation activity in the lack of estrogen [43]. In brief, ESR1 mutation exerts activity in the absence of estrogen, and ESR1 amplification is associated with a poor outcome [39,44]. However, whether ESR1 gene mutation could be a biomarker is still uncertain since the detection methods are varied. Many centers apply circulating tumor DNA (ctDNA) to detect ESR1 LBD mutations in a non-invasive manner. Some studies indicated that ctDNA might better reflect the total ESR1 mutation burden across multiple metastatic sites since the ESR1 mutant content is usually higher in ctDNA than in match tumor tissue in the same patients with both samples [45]. So far, by utilizing the NGS with genome or exome sequencing, we can detect ESR1 mutations [46,47]. Recently, ddPCR detection of ESR1 mutation is increasingly being adopted in solid tumors and liquid biopsies for cost-effective and more powerful sensitivity, compared with NGS technology [45]. A clinical, updated study noted that BC with ESR1 mutations was more sensitive and better response to fulvestrant-containing regimen [48].
Cross talk between ER and other signaling networks
BC is as intelligent as developing new pathways to obtain resistance to new agents. Concerning Hormone-positive BC, we learned that a complicated biological network formed the ER signaling pathway. Different intracellular signaling pathway activation in preclinical models results in endocrine resistance, and targeting specific pathways will be a potential solution to prevent resistance [49]. In detail, the hormone resistance encompasses several regulators and “cross-talk” between ER and membrane receptor tyrosine kinases such as epidermal growth factor receptor (EGFR), epidermal growth factor receptor 2 (human HER2), and insulin-like growth factor 1 receptor (IGFR) or fibroblast growth factor receptor (FGFR), mitogen-activated protein kinase (MAPK) and famous mTOR (mechanistic target of rapamycin) pathway. Activation of these alternative signaling pathways that promote cell proliferation will induce tamoxifen resistance. Recent studies revealed that breast malignancies harboring FGFR1 amplification display resistance to endocrine treatment, and TORC1 inhibitors play a therapeutic role in treating ER+/FGFR1+ mBC [50].
According to a previous study, estrogen could activate the growth stimulatory properties of the IGF pathway via ER’s genomic and non-genomic functions [51]. The role of the IGF system in endocrine-resistant BC has also been investigated. So far, the only study to report results that involved monoclonal antibodies against IGFR-1 is ganitumab, which regulates the PI3K/Akt and MAPK Pathways [52]. Cross-talk exists between IGF1R and the ERBB family, activating downstream signaling pathways such as PI3K/AKT [53]. Endocrine-resistant metastatic BC could acquire mutations in ER and MAPK pathways, including activating ERBB2 (which encodes HER2) [41,54].
A recent study showed RBP2 (retinoblastoma-binding protein 2) could be a critical target molecule for tamoxifen-resistant ER+ BC since it enforces the cross-talk between IGF1R and ErbB receptors through promoting the stability of EGFR and HER2 protein, and activating the PI3K/AKT pathway and thereby leads to tamoxifen resistance [55].
Regarding HER pathways, the bidirectional cross-talk between the ER and HER pathways results in resistance to the endocrine therapy and anti-HER2 treatments [56]. It has been revealed that HER signaling members can reduce ER expression both at the mRNA and protein levels. The bidirectional ER/HER cross-talk can influence these pathways at their expression or activity [57].
The PI3K/AKT/mTOR/signaling pathway is a critical driver in many tumors. Aberrations in this intracellular pathway also related to hormone therapy resistance [58]. Based on the previous study, patients with HR+/HER2- BC and PIK3CA mutation have worse overall survival and were less sensitive to chemotherapy due to the HR+/HER2- BC with enrichment of PIK3CA mutations usually lost HR expression in the metastatic setting [59]. However, longer PFS was founded in patients with the PIK3CA/H1047R mutation than patients with wild-type or other mutant forms of PIK3CA. The PIK3CA/H1047R mutation might be a potential predictor of sensitivity to everolimus [60,61]. ER levels increase by blocking the PI3K pathway in vitro, and PI3K inhibitor is promising in overcoming the hormone resistance.
It is well-known that treatment strategies targeting only sure of the pathways usually lead to the up-regulation of others and ultimately contribute to treatment resistance. Hence, combining different targeted agents with a broader blockade of multiple direction pathways may be more effective in reversing resistance development.
Aberrance of cell cycle regulators
The cell cycle’s deregulation is a character of cancer that leads to limitless cell division. In Hormone-positive BC, by binding to the ER, estrogen drives cell cycle progression, generating its dimerization and translocation to the nucleus and induces the transcriptional activity at estrogen response elements (EREs) [62]. C-myc gene regulation explains more than half of estrogen-regulated genes and comprised more than 80% of the cell growth signature.
Overexpression of MYC has been revealed to contribute resistance to tamoxifen in vitro; more deeply, the siRNA-mediated MYC downregulation impairs estrogen-induced growth [63]. The expression of MYC is up-regulated through the cross-talk between ER and HER2 in AI-resistant BC cells. The mechanism is probably due to the glutamine transporter solute carrier (SLC) family 1A5 was significantly up-regulated in AI-resistant BC cells [64].
On the other hand, the aberrant expression of cell-cycle regulators (cyclin A, cyclin D1, cyclin E, RB1, p21, and p27) will lead to chromosomal instability; thus, overexpression of cyclin D1 was significantly correlated with increased chromosomal instability [65], and dysregulation of the cyclin D1/CDK4/6 pathway is confirmed as a crucial hallmark for breast tumorigenesis [66].
Many studies noted that CCNE1/RB1 ratio could be served as a potential biomarker of cdk4/6 inhibitor such as palbociclib resistance, which has been discussed in the above section [67]. The cell division cycle associated 8 genes (CDCA8) in humans are presented as a putative oncogene reported to be up-regulated in many cancers. Research also noted that overexpression of CDCA8 would promote the growth of tamoxifen-sensitive BC cells and induce tamoxifen resistance [68].
Resistance from Cancer stem cell and tumor microenvironment (TME)
Cancer stem cells (CSCs) play an essential role in cancer evolution and treatment resistance. In BC, hormone therapy contributes to maintain cells harboring elevations in ER signaling, growth factor receptor (GFR)-PI3K-AKT-mTOR, NOTCH, or other pathways GFR-PI3K-AKT-mTOR pathway inhibits the ER and NOTCH signaling. Further, these pathways render the tumors progression with endocrine therapy resistance [69].
The NOTCH signaling pathway is critical in hormone resistance by promoting breast cancer stem cells (BCSCs) [70]. Upregulation of NOTCH4 confers resistance to tamoxifen and contributes to the stemness of tamoxifen-resistant MCF7 cells [71]. Two models have been proposed by Dr. David et al., and one is endocrine therapy inducing the existed BCSCs to produce resistant BCs containing a proportion of BCSCs; the other is via the dedifferentiation of BCs will produce tumors resistant to endocrine therapy and leading to the acquisition of BCSCs [69].
In addition, many researchers have shown that the TME is critical in determining tumor sensitivity or resistance to target therapies [72]. Antiestrogens can induce immunosuppression through a TGFβ-dependent mechanism in the TME, contributing to the development of antiestrogen resistance in BC [73]. A study screened a group of micro-environmental proteins that revealed fibroblast growth factor 2 (FGF2) is a potent mediator of resistance to anti-estrogens [74]. Moreover, exogenous and endogenous FGF2 promotes FGFR-MEK-ERK signaling to drive cell cycle progression and survival in ER+ BC, rendering FGF2 a potential therapeutic target in ER+ BC- microenvironment [74]. In the BC microenvironment, infiltrating immune cells produce growth factors, cytokines, and chemokines that contribute to angiogenesis and remodel the extracellular matrix (ECM); cytokines such as TNF, IL1β, IL6 have been correlated to suppression of ERα in BCs; and increased IL6 serum levels also lead to hormone therapy resistance [75]. On the other hand, an ECM gene cluster has been connected to endocrine resistance [76,77]. Fibronectin, lysyl oxidase, SPARC, and TIMP3 expression levels were related to the prognosis in BC, while levels of tenascin C are associated with tamoxifen resistance [78].
To sum up, the noncancerous components of the TME, both noncellular components such as ECM, cytokines, and oxygenation, and cellular components such as fibroblasts, endothelial cells, immune cells, and adipocytes play an essential role in modulating treatment response. Further research should focus on TME and CSC in improving drug resistance or new drug development.
Recent development in hormone inhibitors (cdk4/6) and relevant signaling pathways to overcome endocrine resistance
The cyclinD1/cyclin-dependent kinase 4/6 cell cycle pathways, and PI3K/AKT/mTOR cell signaling pathway, are two major pathways that have recently been extensively investigated for therapeutic intervention in endocrine-resistant breast tumors [79].
CDK4/6 cell cycle pathway
In metastatic BC, the mechanism behind the observed efficacy of CDK inhibition is related to a dependence of HR+ BC on CDK4/6 activity to override Rb mediated repression of cell cycle progression [80]. The D-type cyclins form complexes with cyclin-dependent kinases (CDK4/6), which then hypophosphorylated the retinoblastoma protein (Rb) in phase G1. Once hypophosphorylated, Rb is primed for hyperphosphorylation by cyclin E-CDK2 complexes, leading to the E2F transcription factors releasing which is vital for entering the S phase. Other CDK-cyclin pairs operate during late phases of the cell cycle [81]. From a molecular aspect, RB transcriptional control is a crucial node in controlling the therapeutic response to ER antagonists; therefore, compromised RB-mediated transcriptional control, presumably as a result of cyclin D1 deregulation, might be an essential feature of ER-positive BC biology [80].
Recent trials showed that cdk4/6 inhibitors, plus fulvestrant, significantly improved PFS compared with fulvestrant in the context of advanced HR+/HER2- BC [82-84]. Despite this combination’s benefit, nearly 80% of patients develop resistance after using cdk4/6 inhibitor for 12 to 36 months [84]. Some research has explored the development of acquired resistance after exposure of HR+ BC cell lines to CDK4/6 inhibition in vitro. CCNE1 overexpression and loss of RB1 expression occurred in HR+ BC cells cultured to resistance in the prolonged presence of palbociclib as well as in ribociclib [85]. Another study indicated that the CCNE1/RB1 ratio could be a potential biomarker in HR+ BC and to be able to differentiate palbociclib-sensitive versus resistant populations [67]. Some revealed that PI3K inhibitors might prevent resistance to CDK4/6 inhibitors, yet they failed to resensitize cells once resistance had been acquired. Nevertheless, cells resistant to CDK4/6 inhibitors due to CCNE1 amplification can probably be resensitized by targeting CDK2 [85]. Furthermore, loss of RB1 and FAT1 tumor suppressor could serve as another resistance mechanism to CDK4/6i through Hippo signaling in ER+ BC [86].
PI3K/AKT/mTOR cell signaling pathway
Alterations in the PI3K signaling pathway are critical in tumor initiation and survival, angiogenesis, and the development of resistance to cancer therapies [87]. AKT is a crucial downstream target of the PI3K signaling pathway, regulating the cell survival, proliferation, growth, apoptosis, and glycogen metabolism, while mTOR is a downstream effector AKT [88]. Endocrine therapy has significantly worse efficacy in the AKT-positive than in the AKT-negative patients. AKT activation induces endocrine resistance irrespective of the endocrine agents that were given for metastatic BC [58]. The irregular activation of the PI3K/AKT/mTOR pathway, such as the amplification or mutations of PI3K subunits and PI3K modulators, was noted in more than half of HR-positive metastatic BC [89]. Prominent alterations in the PI3K pathway included mutations [e.g. PIK3CA (E542K), AKT1 (E17K)], amplifications (MDM2), frameshifts [TSC2 (R1753fs*58+)] and loss [PTEN]. The highest number of alterations was observed in the CCND1 gene, followed by alterations in the RB1 gene [90]. The mTOR1 inhibitors, including sirolimus and its analogs (temsirolimus, everolimus, and deforolimus), are irreversible allosteric inhibitors of mTOR1 kinase [91]. Everolimus, which targets mTOR1, is approved for the treatment of HR+/HER2-BC [92]; and even after or on previous AI treatment, the everolimus-exemestane combination remains effective and well-tolerated regardless of PIK3CA mutational status [93].
Furthermore, in patients who have resistance to mTOR inhibitors after treating with endocrine therapy and mTOR inhibitors, PI3K inhibitors such as Buparlisib plus endocrine therapy (Fulvestrant) might still be efficient in BELLE 3 trial [94]. Another PI3K inhibitor, alpelisib, combined with fulvestrant, also benefits in prolonging progression-free survival in PIK3CA-mutated, HR+ HER2-advanced BC who had received hormone therapy previously [95].
Moreover, regarding the aromatase inhibitors acquired resistance, the study also noted that inhibiting autophagy or PI3K pathway could probably revert exemestane resistance through disruption of the cell cycle, apoptosis promotion, and inhibiting cell survival pathways [96].
However, some cancer cells eventually evolve resistance to PI3K inhibitors, and preclinical studies demonstrated that PI3Ki insensitiveness is evident by persistent RB phosphorylation and can be effectively suppressed by combining a CDK inhibitor with a PI3Ki due to the synergetic effect in depressing the growth and proliferation of BC cells in vitro [90,97].
Find the predictive biomarker and response gene in hormone-positive BC
Recently, the most popular genomic test is the Oncotype DX. It can survey the expression of 21 genes by RT-PCR. The current consensus is that it is a marker of good prognosis in Hormone-positive tumors (ER and PR) and a worse prognosis marker in triple-negative BC. However, the study also revealed that the absence of PR expression in the lymph node metastases of ER-positive, HER2-negative BC is correlated with relapse on tamoxifen [98].
In the strategy of Hormone-positive cancer, the ER signaling pathway, from PI3K/AKT/MTOR pathway, CDK4/6-RB pathway to p53/MDM2 pathways are all complicated with much network crosstalk.
Each of these pathways may lead biomarkers to confer sensitivity or resistance to endocrine therapy [13].
For example, the PALOMA-3 clinical trial investigates the intrinsic resistance of the combining use of palbociclib and fulvestrant and noted that CCNE1 mRNA levels associated with the degree of benefit; patients with low levels of CCNE1 had marked improvement with the addition of palbociclib, referring that CCNE1 could be a potential biomarker [99]. Another study in 2018 noted that PIK3CA mutation, with high levels of ERα phospho-Ser167 and low levels of AKT phospho-Ser473, are positive prognostic indicators in ER+ BC [100].
As has been mentioned, the ESR1 mutations can be prognostic and predictive biomarkers for ESR1 amplification, which is associated with a poor outcome [101]. The development of targeted therapy directed to ESR1-mutated clones is an appealing idea.
On the other hand, MYC, a transcription factor, plays an important signaling hub in multiple cellular processes that sustains growth [102]. A previous study indicated that an activated MYC and high MYC protein levels predict a shorter time to recurrence following adjuvant tamoxifen, rendering MYC a therapeutic target in endocrine-resistant BCs. [63]. Further, HSPC111 as estrogen and c-Myc target gene, over-expressed in BC, refers to poor outcome [103]. So far, only cdk4/6 inhibitors and mTOR inhibitors have been found with corresponding drug development; most of the studies remain in a preclinical state. Furthermore, since some miRNAs can be detectable in blood and their dysregulations are associated with the development or progression of the disease; such as, the suppression of miR-221 and miR-222 could increase the sensitivity of estrogen receptor-positive MCF-7 BC cells to tamoxifen [104]; thus, miRNA can be utilized as a non-invasive biomarker [105].
Whether there are other biomarkers or response genes over the related pathway is worth investigating.
Immunotherapy in HR+ BC
Nowadays, we are in the era of personalized medicine where the crosstalk between genomics and immunology contributes to successful cancer treatment. Some solid tumors that harbor TILs and express PD-L1 are tended to respond to programmed cell death-1 (PD-1)/PD-L1 blockade [106], which could also cause BCs.
Consequently, the evaluation of tumor-infiltrating lymphocytes becomes increasingly relevant with the emerging role of immunotherapy in BC, and it has been noted that the TILs are correlated with higher PD-1 and PD-L1 expression in BC [107]. Nevertheless, it may be intrinsically less immunogenic in luminal ER-positive/HER2 negative BC as they arise from a less antigenic cell of origin.
Lower levels of stromal tumor-infiltrating lymphocytes (sTIL) and PD-L1 refer to inadequate responses to checkpoint inhibitor therapy [108], which contrast to the triple-negative and HER2+ BC with high immunogenicity and high proliferation index [109]. Moreover, determination and standardization of PD-L1 expression has not been reported, leading to no standardization of assays, percentage cutoff for establishing positivity, or scoring methodology. Therefore, a unified PD-L1 expression measurement might need to be established in future studies [110]. An early clinical trial indicated that in previously treated, PD-L1-positive, ER+/HER2-advanced BC, pembrolizumab has been noted with well-tolerated and results in durable overall responses in certain patients [111].
Another checkpoint inhibitor targeting CTLA4 (cytotoxic T-lymphocyte antigen 4), which is noted on Treg cells, could promote suppressive Treg activity. CTLA-4 downstream signaling may inhibit T cell activation directly; some studies of CTLA-4 blockade have been conducted with ipilimumab or tremelimumab in diverse cancer types, including BC [110].
The new aspects of the progress on endocrine-resistant HR-positive BC
Regarding the progress on endocrine-resistant HR+ BC, on the one hand, in molecular respect, TME has been a hot spot for treatment target as the cellular crosstalk and cell-to-ECM communication are active in TME. These interactions induce soluble factors and contribute to immune evasion and ECM remodeling, which lead to therapy resistance [112]. The updated study indicates that the characteristics of the TME and its effects on treatment resistance, including the sum of interaction between carcinoma-associated fibroblasts (CAFs), adipocytes, immune cells, endothelial cells, pericytes, the ECM, and soluble factors, all contribute to tumor evolution [113]. Hypoxia or metabolic disarrangements such as lactate, lipid, or reactive oxygen species (ROS) metabolic aberrance are linked to drug or endocrine resistance [114]. The ECM might have a central role in establishing mechanical cues and directly regulate the impact of hormone action in BC cells. In ECM, fibronectin (FN) is associated with disease progression in BC. Culturing ERα human BC cells on FN leads to endocrine resistance through binding to b1 integrin [79,115]. TME is indeed a promising area in evaluating how cancer evolves and how to overcome the treatment resistance.
On the other hand, in clinical respect, in hormone-resistant HR+ BC, apart from fulvestrant (selective ER down regulator) combined with CDK4/6 inhibitor or mTOR inhibitor, patients with PIK3CA mutation could consider alpelisib, which can prolong progression-free survival [93,95]. Furthermore, in a population with germline wildtype BRCA genes and an actionable somatic BRCA mutation, olaparib can be considered as later-line therapy for ER+, HER2-mBC, for which PARPi could provide substantial clinical benefits [116]. At the same time, talazoparib is suggested for BRCA2 nutation in EMBRACA trial [117]. Lastly, nearly 30% of endocrine-resistant BC is still dependent on ERα signaling; the need for the next generation of ERα antagonists to overcome aberrant ERα activity is critical [118]. A recent study indicates that H3B-5942, a novel ERα antagonist, exhibits enhanced potency due to its ability to target a unique cysteine in ER [119] covalently. Besides, next-generation oral selective estrogen receptor modulators (SERMs) or selective estrogen receptor down regulators (SERDs) that target both wild-type and mutant ER are clinically developed [120]. For instance, elacestrant and SAR439859, oral SERD, have been proved with preliminary clinical activity in ESR1-mutant MBCs with tolerable toxicity profiles [121,122], yet most of these new drugs are pending further data of phase II or III trials [123].
Discussion: the prospect and implication for overcome endocrine resistance in HR-positive BC
This article reviewed endocrine-resistant BC from a molecular to clinical perspective, examining the mechanism, recent new drug development, and crosstalk between relevant pathways. The prospect for overcome endocrine resistance should focus not only on advanced technology to identify some mutation and biomarker but also more attention in changes in the TME after multiple lines of treatment.
Firstly, a methodology such as next-generation sequencing (NGS) is also a critical issue. In past years, the methodologies have evolved from tissue to plasma, followed by digital droplet PCR (ddPCR), and eventually entered an NGS era [124]. Advanced NGS platforms and modified ddPCR will facilitate treatment decisions efficiently and benefit patients [125]. Other new methods include Nanopore technology and BEAMing. The Nanopore technology is independent of amplification steps involving with fluorescence labeling and DNA polymerase even with high sequencing error rate [126]; the BEAMing is a modified digital PCR using beads, emulsion, amplification, and magnetics which has been applied in a large-scale clinical trial such as PALOMA-3 [127,128].
So far, what we concerned about is how to accurately translate NGS into the clinical setting since the interpretation of cell sequencing data is complicated by various amplification biases introduced in the cell’s DNA or RNA sample; therefore, combined artificial intelligence to make NGS a more powerful tool could be a direction [129]. Second, the change in TME in therapy-resistant HR+ BC has sparked researcher’s attention; as TME is also in a dynamic change which contributes to drug resistance, a further understanding of the interaction between various components of the TME may stimulate the development of new therapies. Therefore, future study needs to focus more on net-talk or cytokine change in TME.
Last but not least, though immunotherapy role applied in hormone-positive BC is not as critical as in triple-negative and HER2 positive cancer, there are heterogeneous genes and phenotypes in hormone-positive BC. Accordingly, further investigation to merge immunotherapy and conventional therapy (chemotherapy, radiotherapy, hormone therapy) according to more detailed gene expression or correspondent TME change is worthwhile.
In summary, BC is a molecularly complicated disease. The more we understand the diversity of molecule and epigenetic character about BC, the more precise decision we can make in treating the disease.
Acknowledgements
The study is supported by Ministry of Science and Technology-MOST, Taiwan grant 107-2635-B-532-003- and grant 108-2320-B-532-001-MY3.
Disclosure of conflict of interest
None.
References
- 1.Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. doi: 10.1007/978-3-030-20301-6_2. [DOI] [PubMed] [Google Scholar]
- 2.Roulot A, Hequet D, Guinebretiere JM, Vincent-Salomon A, Lerebours F, Dubot C, Rouzier R. Tumoral heterogeneity of breast cancer. Ann Biol Clin (Paris) 2016;74:653–660. doi: 10.1684/abc.2016.1192. [DOI] [PubMed] [Google Scholar]
- 3.Huszno J, Nowara E. Current therapeutic strategies of anti-HER2 treatment in advanced breast cancer patients. Contemp Oncol (Pozn) 2016;20:1–7. doi: 10.5114/wo.2016.58495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17:152–163. doi: 10.2174/1871520616666160502122724. [DOI] [PubMed] [Google Scholar]
- 5.Audeh W, Blumencranz L, Kling H, Trivedi H, Srkalovic G. Prospective validation of a genomic assay in breast cancer: the 70-gene mammaprint assay and the mindact trial. Acta Med Acad. 2019;48:18–34. doi: 10.5644/ama2006-124.239. [DOI] [PubMed] [Google Scholar]
- 6.Hikichi S, Sugimoto M, Tomita M. Correlation-centred variable selection of a gene expression signature to predict breast cancer metastasis. Sci Rep. 2020;10:7923. doi: 10.1038/s41598-020-64870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Yang D, Yin X, Zhang X, Huang J, Wu Y, Wang M, Yi Z, Li H, Li H, Ren G. Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw Open. 2020;3:e1918160. doi: 10.1001/jamanetworkopen.2019.18160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Tu Y, Wu Q, Wang Z, Li J, Zhang Y, Sun S. Clinical characteristics and outcomes of single versus double hormone receptor-positive breast cancer in 2 large databases. Clin Breast Cancer. 2020;20:e151–e163. doi: 10.1016/j.clbc.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 10.Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors α (ERα) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali S, Coombes RC. Estrogen receptor α in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–281. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- 12.Chanrion M, Negre V, Fontaine H, Salvetat N, Bibeau F, Mac Grogan G, Mauriac L, Katsaros D, Molina F, Theillet C, Darbon JM. A gene expression signature that can predict the recurrence of tamoxifen-treated primary breast cancer. Clin Cancer Res. 2008;14:1744–1752. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: insights to sequencing treatment and overcoming resistance based on clinical trials. Front Oncol. 2019;9:510. doi: 10.3389/fonc.2019.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitman DC, Paruthiyil S, Vivar OI, Saunier EF, Herber CB, Cohen I, Tagliaferri M, Speed TP. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr Opin Pharmacol. 2010;10:629–636. doi: 10.1016/j.coph.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, Shparyk Y, Cardona-Huerta S, Cheung KL, Philco-Salas MJ, Ruiz-Borrego M, Shao Z, Noguchi S, Rowbottom J, Stuart M, Grinsted LM, Fazal M, Ellis MJ. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi S, Ellis MJ, Robertson JFR, Thirlwell J, Fazal M, Shao Z. Progression-free survival results in postmenopausal Asian women: subgroup analysis from a phase III randomized trial of fulvestrant 500 mg vs anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON) Breast Cancer. 2018;25:356–364. doi: 10.1007/s12282-018-0838-8. [DOI] [PubMed] [Google Scholar]
- 17.Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, Watanabe T, Morris C, Webster A, Dimery I, Osborne CK. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J. Clin. Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 18.Segura C, Avenin D, Gligorov J, Selle F, Esteso A, Beerblock K, Emile G, Do Huyen N, Lotz JP. The use of GnRH analogues in early and advanced breast carcinomas. Gynecol Obstet Fertil. 2005;33:914–919. doi: 10.1016/j.gyobfe.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland RL, Musgrove EA. CDK inhibitors as potential breast cancer therapeutics: new evidence for enhanced efficacy in ER+ disease. Breast Cancer Res. 2009;11:112. doi: 10.1186/bcr2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12:342–354. doi: 10.7497/j.issn.2095-3941.2015.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rani A, Stebbing J, Giamas G, Murphy J. Endocrine resistance in hormone receptor positive breast cancer-from mechanism to therapy. Front Endocrinol (Lausanne) 2019;10:245. doi: 10.3389/fendo.2019.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherbakov AM, Krasil’nikov MA, Kushlinskii NE. Molecular mechanisms of hormone resistance of breast cancer. Bull Exp Biol Med. 2013;155:384–395. doi: 10.1007/s10517-013-2160-y. [DOI] [PubMed] [Google Scholar]
- 23.Higgins MJ, Stearns V. Understanding resistance to tamoxifen in hormone receptor-positive breast cancer. Clin Chem. 2009;55:1453–1455. doi: 10.1373/clinchem.2009.125377. [DOI] [PubMed] [Google Scholar]
- 24.Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13(Suppl 1):S15–S24. doi: 10.1677/erc.1.01273. [DOI] [PubMed] [Google Scholar]
- 25.Rae JM, Sikora MJ, Henry NL, Li L, Kim S, Oesterreich S, Skaar TC, Nguyen AT, Desta Z, Storniolo AM, Flockhart DA, Hayes DF, Stearns V COBRA investigators. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009;9:258–264. doi: 10.1038/tpj.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, Salisbury JD, Cheng AS, Li L, Abbosh PH, Huang TH, Nephew KP. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 27.Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates estrogen receptor α and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2016;291:22859. doi: 10.1074/jbc.A116.806041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 29.Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, Burow ME, Ivan M, Croce CM, Nephew KP. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30:1082–1097. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward A, Shukla K, Balwierz A, Soons Z, Konig R, Sahin O, Wiemann S. MicroRNA-519a is a novel oncomir conferring tamoxifen resistance by targeting a network of tumour-suppressor genes in ER+ breast cancer. J Pathol. 2014;233:368–379. doi: 10.1002/path.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Zou Z, Nie P, Kou X, Wu B, Wang S, Song Z, He J. Downregulation of microRNA-27b-3p enhances tamoxifen resistance in breast cancer by increasing NR5A2 and CREB1 expression. Cell Death Dis. 2016;7:e2454. doi: 10.1038/cddis.2016.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutanzi KR, Yurchenko OV, Beland FA, Checkhun VF, Pogribny IP. MicroRNA-mediated drug resistance in breast cancer. Clin Epigenetics. 2011;2:171–185. doi: 10.1007/s13148-011-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Galan J, Torres B, Del Moral R, Munoz-Gamez JA, Martin-Oliva D, Villalobos M, Nunez MI, Luna Jde D, Oliver FJ, Ruiz de Almodovar JM. Quantitative detection of methylated ESR1 and 14-3-3-sigma gene promoters in serum as candidate biomarkers for diagnosis of breast cancer and evaluation of treatment efficacy. Cancer Biol Ther. 2008;7:958–965. doi: 10.4161/cbt.7.6.5966. [DOI] [PubMed] [Google Scholar]
- 34.Stewart CM, Tsui DWY. Circulating cell-free DNA for non-invasive cancer management. Cancer Genet. 2018;228-229:169–179. doi: 10.1016/j.cancergen.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama R, Choudhury S, Kowalczyk A, Bessarabova M, Beresford-Smith B, Conway T, Kaspi A, Wu Z, Nikolskaya T, Merino VF, Lo PK, Liu XS, Nikolsky Y, Sukumar S, Haviv I, Polyak K. Epigenetic regulation of cell type-specific expression patterns in the human mammary epithelium. PLoS Genet. 2011;7:e1001369. doi: 10.1371/journal.pgen.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Lin C, Wang X, Li Q, Li Y, Wang M, Zhao Z, Wu X, Shi D, Xiao Y, Ren L, Jian Y, Yang M, Ou R, Deng G, Ouyang Y, Chen X, Li J, Song L. Epigenetic silencing of SALL2 confers tamoxifen resistance in breast cancer. EMBO Mol Med. 2019;11:e10638. doi: 10.15252/emmm.201910638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiken JF, McDonald JI, Decker KF, Sanchez C, Hoog J, VanderKraats ND, Jung KL, Akinhanmi M, Rois LE, Ellis MJ, Edwards JR. Epigenetic activation of the prostaglandin receptor EP4 promotes resistance to endocrine therapy for breast cancer. Oncogene. 2017;36:2319–2327. doi: 10.1038/onc.2016.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MH, Koh D, Na H, Ka NL, Kim S, Kim HJ, Hong S, Shin YK, Seong JK, Lee MO. MTA1 is a novel regulator of autophagy that induces tamoxifen resistance in breast cancer cells. Autophagy. 2018;14:812–824. doi: 10.1080/15548627.2017.1388476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angus L, Beije N, Jager A, Martens JW, Sleijfer S. ESR1 mutations: moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat Rev. 2017;52:33–40. doi: 10.1016/j.ctrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L, Gursky A, Siddiqui J, Tomlins SA, Roychowdhury S, Pienta KJ, Kim SY, Roberts JS, Rae JM, Van Poznak CH, Hayes DF, Chugh R, Kunju LP, Talpaz M, Schott AF, Chinnaiyan AM. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, Soria JC, Dien AT, Adnani Y, Kamal M, Garnier S, Meurice G, Jimenez M, Dogan S, Verret B, Chaffanet M, Bachelot T, Campone M, Lefeuvre C, Bonnefoi H, Dalenc F, Jacquet A, De Filippo MR, Babbar N, Birnbaum D, Filleron T, Le Tourneau C, Andre F. Genomic characterization of metastatic breast cancers. Nature. 2019;569:560–564. doi: 10.1038/s41586-019-1056-z. [DOI] [PubMed] [Google Scholar]
- 42.Clatot F, Perdrix A, Augusto L, Beaussire L, Delacour J, Calbrix C, Sefrioui D, Viailly PJ, Bubenheim M, Moldovan C, Alexandru C, Tennevet I, Rigal O, Guillemet C, Leheurteur M, Gouerant S, Petrau C, Thery JC, Picquenot JM, Veyret C, Frebourg T, Jardin F, Sarafan-Vasseur N, Di Fiore F. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget. 2016;7:74448–74459. doi: 10.18632/oncotarget.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, Li Z, Gala K, Fanning S, King TA, Hudis C, Chen D, Taran T, Hortobagyi G, Greene G, Berger M, Baselga J, Chandarlapaty S. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita S, Zhang Z, Nakano M, Ibusuki M, Kawazoe T, Yamamoto Y, Iwase H. Estrogen receptor α gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci. 2009;100:1012–1017. doi: 10.1111/j.1349-7006.2009.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dustin D, Gu G, Fuqua SAW. ESR1 mutations in breast cancer. Cancer. 2019;125:3714–3728. doi: 10.1002/cncr.32345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paolillo C, Mu Z, Rossi G, Schiewer MJ, Nguyen T, Austin L, Capoluongo E, Knudsen K, Cristofanilli M, Fortina P. Detection of activating estrogen receptor gene (ESR1) mutations in single circulating tumor cells. Clin Cancer Res. 2017;23:6086–6093. doi: 10.1158/1078-0432.CCR-17-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, Hastings RK, Luo J, Pringle JH, Stebbing J, Coombes RC, Ali S, Shaw JA. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin Chem. 2015;61:974–982. doi: 10.1373/clinchem.2015.238717. [DOI] [PubMed] [Google Scholar]
- 48.Turner NC, Swift C, Kilburn LS, Fribbens C, Beaney M, Garcia-Murillas I, Buzdar AU, Roberston JF, Gradishar W, Piccart M, Schiavon G, Bliss JM, Dowsett M, Johnston SRD, Chia SK. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res. 2020;26:5172–5177. doi: 10.1158/1078-0432.CCR-20-0224. [DOI] [PubMed] [Google Scholar]
- 49.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 50.Drago JZ, Formisano L, Juric D, Niemierko A, Servetto A, Wander SA, Spring LM, Vidula N, Younger J, Peppercorn J, Yuen M, Malvarosa G, Sgroi D, Isakoff SJ, Moy B, Ellisen LW, Iafrate AJ, Arteaga CL, Bardia A. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR+) breast cancer. Clin Cancer Res. 2019;25:6443–6451. doi: 10.1158/1078-0432.CCR-19-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:423–429. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 52.Johnston SR. Enhancing endocrine therapy for hormone receptor-positive advanced breast cancer: cotargeting signaling pathways. J Natl Cancer Inst. 2015;107:djv212. doi: 10.1093/jnci/djv212. [DOI] [PubMed] [Google Scholar]
- 53.Gallardo A, Lerma E, Escuin D, Tibau A, Munoz J, Ojeda B, Barnadas A, Adrover E, Sanchez-Tejada L, Giner D, Ortiz-Martinez F, Peiro G. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106:1367–1373. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson P, Penson A, Shen R, Pareja F, Kundra R, Middha S, Cheng ML, Zehir A, Kandoth C, Patel R, Huberman K, Smyth LM, Jhaveri K, Modi S, Traina TA, Dang C, Zhang W, Weigelt B, Li BT, Ladanyi M, Hyman DM, Schultz N, Robson ME, Hudis C, Brogi E, Viale A, Norton L, Dickler MN, Berger MF, Iacobuzio-Donahue CA, Chandarlapaty S, Scaltriti M, Reis-Filho JS, Solit DB, Taylor BS, Baselga J. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–438. e426. doi: 10.1016/j.ccell.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi HJ, Joo HS, Won HY, Min KW, Kim HY, Son T, Oh YH, Lee JY, Kong G. Role of RBP2-induced ER and IGF1R-ErbB signaling in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2018;110 doi: 10.1093/jnci/djx207. [DOI] [PubMed] [Google Scholar]
- 56.Giuliano M, Trivedi MV, Schiff R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care (Basel) 2013;8:256–262. doi: 10.1159/000354253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 58.Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, Morita M, Kakeji Y, Baba H, Maehara Y. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13:137–144. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- 59.Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, Le Tourneau C, Sablin MP, Lacroix L, Enrico D, Miran I, Jovelet C, Bieche I, Soria JC, Bertucci F, Bonnefoi H, Campone M, Dalenc F, Bachelot T, Jacquet A, Jimenez M, Andre F. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020;31:377–386. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Yi Z, Ma F, Liu B, Guan X, Li L, Li C, Qian H, Xu B. Everolimus in hormone receptor-positive metastatic breast cancer: PIK3CA mutation H1047R was a potential efficacy biomarker in a retrospective study. BMC Cancer. 2019;19:442. doi: 10.1186/s12885-019-5668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zardavas D, Te Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, Razis E, Papaxoinis G, Joensuu H, Moynahan ME, Hennessy BT, Bieche I, Saal LH, Stal O, Iacopetta B, Jensen JD, O’Toole S, Lopez-Knowles E, Barbaraeschi M, Noguchi S, Azim HA Jr, Lerma E, Bachelot T, Wang Q, Perez-Tenorio G, Can de Velde CJH, Rea DW, Sabine V, Bartlett JMS, Sotiriou C, Michiels S, Loi S. Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J. Clin. Oncol. 2018;36:981–990. doi: 10.1200/JCO.2017.74.8301. [DOI] [PubMed] [Google Scholar]
- 62.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Miller TW, Balko JM, Ghazoui Z, Dunbier A, Anderson H, Dowsett M, Gonzalez-Angulo AM, Mills GB, Miller WR, Wu H, Shyr Y, Arteaga CL. A gene expression signature from human breast cancer cells with acquired hormone independence identifies MYC as a mediator of antiestrogen resistance. Clin Cancer Res. 2011;17:2024–2034. doi: 10.1158/1078-0432.CCR-10-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z, Wang Y, Warden C, Chen S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J Steroid Biochem Mol Biol. 2015;149:118–127. doi: 10.1016/j.jsbmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lung JC, Chu JS, Yu JC, Yue CT, Lo YL, Shen CY, Wu CW. Aberrant expression of cell-cycle regulator cyclin D1 in breast cancer is related to chromosomal genomic instability. Genes Chromosomes Cancer. 2002;34:276–284. doi: 10.1002/gcc.10072. [DOI] [PubMed] [Google Scholar]
- 66.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- 67.Guarducci C, Bonechi M, Benelli M, Biagioni C, Boccalini G, Romagnoli D, Verardo R, Schiff R, Osborne CK, De Angelis C, Di Leo A, Malorni L, Migliaccio I. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4:38. doi: 10.1038/s41523-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu D, Shi L, Bu Y, Li W. Cell division cycle associated 8 is a key regulator of tamoxifen resistance in breast cancer. J Breast Cancer. 2019;22:237–247. doi: 10.4048/jbc.2019.22.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez D, Ramkairsingh M, Lin X, Kapoor A, Major P, Tang D. The central contributions of breast cancer stem cells in developing resistance to endocrine therapy in estrogen receptor (ER)-positive breast cancer. Cancers (Basel) 2019;11:1028. doi: 10.3390/cancers11071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Acar A, Simoes BM, Clarke RB, Brennan K. A role for notch signalling in breast cancer and endocrine resistance. Stem Cells Int. 2016;2016:2498764. doi: 10.1155/2016/2498764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lombardo Y, Faronato M, Filipovic A, Vircillo V, Magnani L, Coombes RC. Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells. Breast Cancer Res. 2014;16:R62. doi: 10.1186/bcr3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sidaway P. Breast cancer: microenvironment mediates differential resistance. Nat Rev Clin Oncol. 2017;14:458. doi: 10.1038/nrclinonc.2017.89. [DOI] [PubMed] [Google Scholar]
- 73.Joffroy CM, Buck MB, Stope MB, Popp SL, Pfizenmaier K, Knabbe C. Antiestrogens induce transforming growth factor beta-mediated immunosuppression in breast cancer. Cancer Res. 2010;70:1314–1322. doi: 10.1158/0008-5472.CAN-09-3292. [DOI] [PubMed] [Google Scholar]
- 74.Shee K, Yang W, Hinds JW, Hampsch RA, Varn FS, Traphagen NA, Patel K, Cheng C, Jenkins NP, Kettenbach AN, Demidenko E, Owens P, Faber AC, Golub TR, Straussman R, Miller TW. Therapeutically targeting tumor microenvironment-mediated drug resistance in estrogen receptor-positive breast cancer. J Exp Med. 2018;215:895–910. doi: 10.1084/jem.20171818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 76.Kar R, Jha NK, Jha SK, Sharma A, Dholpuria S, Asthana N, Chaurasiya K, Singh VK, Burgee S, Nand P. A “NOTCH” deeper into the epithelial-to-mesenchymal transition (EMT) program in breast cancer. Genes (Basel) 2019;10:961. doi: 10.3390/genes10120961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn JG, Sleijfer S, Foekens JA, Berns EM. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res. 2008;14:5555–5564. doi: 10.1158/1078-0432.CCR-08-0555. [DOI] [PubMed] [Google Scholar]
- 78.Holton SE, Bergamaschi A, Katzenellenbogen BS, Bhargava R. Integration of molecular profiling and chemical imaging to elucidate fibroblast-microenvironment impact on cancer cell phenotype and endocrine resistance in breast cancer. PLoS One. 2014;9:e96878. doi: 10.1371/journal.pone.0096878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, Clarke R, Knudsen ES. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ingham M, Schwartz GK. Cell-cycle therapeutics come of age. J. Clin. Oncol. 2017;35:2949–2959. doi: 10.1200/JCO.2016.69.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 83.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J. Ribociclib as first-line therapy for hr-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 84.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, Campos-Gomez S, Villanueva Vazquez R, Jung KH, Babu KG, Wheatley-Price P, De Laurentiis M, Im YH, Kuemmel S, El-Saghir N, Liu MC, Carlson G, Hughes G, Diaz-Padilla I, Germa C, Hirawat S, Lu YS. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 85.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, Pearson A, Guzman M, Rodriguez O, Grueso J, Bellet M, Cortes J, Elliott R, Pancholi S, Baselga J, Dowsett M, Martin LA, Turner NC, Serra V. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, Hsieh W, Sanchez-Vega F, Brown DN, Da Cruz Paula AF, Morris L, Selenica P, Eichenberger E, Shen R, Schultz N, Rosen N, Scaltriti M, Brogi E, Baselga J, Reis-Filho JS, Chandarlapaty S. Loss of the FAT1 tumor suppressor promotes resistance to cdk4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34:893–905. e898. doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwartzberg LS, Vidal GA. Targeting PIK3CA alterations in hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: new therapeutic approaches and practical considerations. Clin Breast Cancer. 2020;20:e439–e449. doi: 10.1016/j.clbc.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 88.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 89.Han Y, Wang J, Wang Z, Xu B. Comparative efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors in women with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and network meta-analysis. Curr Probl Cancer. 2020;44:100606. doi: 10.1016/j.currproblcancer.2020.100606. [DOI] [PubMed] [Google Scholar]
- 90.Gul A, Leyland-Jones B, Dey N, De P. A combination of the PI3K pathway inhibitor plus cell cycle pathway inhibitor to combat endocrine resistance in hormone receptor-positive breast cancer: a genomic algorithm-based treatment approach. Am J Cancer Res. 2018;8:2359–2376. [PMC free article] [PubMed] [Google Scholar]
- 91.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamamoto D, Tsubota Y, Sueoka N, Yamamoto C, Kon M. Everolimus plus exemestane in postmenopausal metastatic breast cancer patients. Gan To Kagaku Ryoho. 2015;42:1319–1321. [PubMed] [Google Scholar]
- 93.Vernieri C, Corti F, Nichetti F, Ligorio F, Manglaviti S, Zattarin E, Rea CG, Capri G, Bianchi GV, de Braud F. Everolimus versus alpelisib in advanced hormone receptor-positive HER2-negative breast cancer: targeting different nodes of the PI3K/AKT/mTORC1 pathway with different clinical implications. Breast Cancer Res. 2020;22:33. doi: 10.1186/s13058-020-01271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Leo A, Johnston S, Lee KS, Ciruelos E, Lonning PE, Janni W, O’Regan R, Mouret-Reynier MA, Kalev D, Egle D, Csoszi T, Bordonaro R, Decker T, Tjan-Heijnen VCG, Blau S, Schirone A, Weber D, El-Hashimy M, Dharan B, Sellami D, Bachelot T. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 95.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Papai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 96.Amaral C, Augusto TV, Tavares-da-Silva E, Roleira FMF, Correia-da-Silva G, Teixeira N. Hormone-dependent breast cancer: targeting autophagy and PI3K overcomes exemestane-acquired resistance. J Steroid Biochem Mol Biol. 2018;183:51–61. doi: 10.1016/j.jsbmb.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, Lockerman EL, Pollack SF, Liu M, Li X, Lehar J, Wiesmann M, Wartmann M, Chen Y, Cao ZA, Pinzon-Ortiz M, Kim S, Schlegel R, Huang A, Engelman JA. CDK4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–149. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Snell CE, Gough M, Middleton K, Hsieh M, Furnas L, Seidl B, Gibbons K, Pyke C, Shannon C, Woodward N, Armes JE. Absent progesterone receptor expression in the lymph node metastases of ER-positive, HER2-negative breast cancer is associated with relapse on tamoxifen. J Clin Pathol. 2017;70:954–960. doi: 10.1136/jclinpath-2016-204304. [DOI] [PubMed] [Google Scholar]
- 99.Chandarlapaty S, Razavi P. Cyclin E MRNA: assessing cyclin-dependent kinase (CDK) activation state to elucidate breast cancer resistance to CDK4/6 inhibitors. J. Clin. Oncol. 2019;37:1148–1150. doi: 10.1200/JCO.19.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishida N, Baba M, Hatanaka Y, Hagio K, Okada H, Hatanaka KC, Togashi K, Matsuno Y, Yamashita H. PIK3CA mutation, reduced AKT serine 473 phosphorylation, and increased ERα serine 167 phosphorylation are positive prognostic indicators in postmenopausal estrogen receptor-positive early breast cancer. Oncotarget. 2018;9:17711–17724. doi: 10.18632/oncotarget.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reinert T, Goncalves R, Bines J. Implications of ESR1 mutations in hormone receptor-positive breast cancer. Curr Treat Options Oncol. 2018;19:24. doi: 10.1007/s11864-018-0542-0. [DOI] [PubMed] [Google Scholar]
- 102.Fallah Y, Brundage J, Allegakoen P, Shajahan-Haq AN. MYC-driven pathways in breast cancer subtypes. Biomolecules. 2017;7:53. doi: 10.3390/biom7030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butt AJ, Sergio CM, Inman CK, Anderson LR, McNeil CM, Russell AJ, Nousch M, Preiss T, Biankin AV, Sutherland RL, Musgrove EA. The estrogen and c-Myc target gene HSPC111 is over-expressed in breast cancer and associated with poor patient outcome. Breast Cancer Res. 2008;10:R28. doi: 10.1186/bcr1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gan R, Yang Y, Yang X, Zhao L, Lu J, Meng QH. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 2014;21:290–296. doi: 10.1038/cgt.2014.29. [DOI] [PubMed] [Google Scholar]
- 105.Bahrami A, Aledavood A, Anvari K, Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S, Avan A. The prognostic and therapeutic application of microRNAs in breast cancer: tissue and circulating microRNAs. J Cell Physiol. 2018;233:774–786. doi: 10.1002/jcp.25813. [DOI] [PubMed] [Google Scholar]
- 106.Schutz F, Stefanovic S, Mayer L, von Au A, Domschke C, Sohn C. PD-1/PD-L1 pathway in breast cancer. Oncol Res Treat. 2017;40:294–297. doi: 10.1159/000464353. [DOI] [PubMed] [Google Scholar]
- 107.Kitano A, Ono M, Yoshida M, Noguchi E, Shimomura A, Shimoi T, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Fujiwara Y, Tsuda H, Tamura K. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open. 2017;2:e000150. doi: 10.1136/esmoopen-2016-000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waks AG, Stover DG, Guerriero JL, Dillon D, Barry WT, Gjini E, Hartl C, Lo W, Savoie J, Brock J, Wesolowski R, Li Z, Damicis A, Philips AV, Wu Y, Yang F, Sullivan A, Danaher P, Brauer HA, Osmani W, Lipschitz M, Hoadley KA, Goldberg M, Perou CM, Rodig S, Winer EP, Krop IE, Mittendorf EA, Tolaney SM. The immune microenvironment in hormone receptor-positive breast cancer before and after preoperative chemotherapy. Clin Cancer Res. 2019;25:4644–4655. doi: 10.1158/1078-0432.CCR-19-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanchez K, Page D, McArthur HL. Immunotherapy in breast cancer: an overview of modern checkpoint blockade strategies and vaccines. Curr Probl Cancer. 2016;40:151–162. doi: 10.1016/j.currproblcancer.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 111.Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Le Tourneau C, van Brummelen EMJ, Varga A, Salgado R, Loi S, Saraf S, Pietrangelo D, Karantza V, Tan AR. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24:2804–2811. doi: 10.1158/1078-0432.CCR-17-3452. [DOI] [PubMed] [Google Scholar]
- 112.Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol. 2021;12:656364. doi: 10.3389/fimmu.2021.656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diaz Bessone MI, Gattas MJ, Laporte T, Tanaka M, Simian M. The tumor microenvironment as a regulator of endocrine resistance in breast cancer. Front Endocrinol (Lausanne) 2019;10:547. doi: 10.3389/fendo.2019.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hultsch S, Kankainen M, Paavolainen L, Kovanen RM, Ikonen E, Kangaspeska S, Pietiainen V, Kallioniemi O. Association of tamoxifen resistance and lipid reprogramming in breast cancer. BMC Cancer. 2018;18:850. doi: 10.1186/s12885-018-4757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bae YK, Kim A, Kim MK, Choi JE, Kang SH, Lee SJ. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol. 2013;44:2028–2037. doi: 10.1016/j.humpath.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 116.Schwartzberg LS, Kiedrowski LA. Olaparib in hormone receptor-positive, HER2-negative metastatic breast cancer with a somatic BRCA2 mutation. Ther Adv Med Oncol. 2021;13:17588359211006962. doi: 10.1177/17588359211006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu Y, Elmeliegy M, Litton JK, Tudor IC, Czibere A, Zheng J, Wang DD. Talazoparib exposure-efficacy analysis in patients with advanced breast cancer and germline BRCA1/2 mutations in the EMBRACA trial. J Clin Pharmacol. 2020;60:1324–1333. doi: 10.1002/jcph.1623. [DOI] [PubMed] [Google Scholar]
- 118.Puyang X, Furman C, Zheng GZ, Wu ZJ, Banka D, Aithal K, Agoulnik S, Bolduc DM, Buonamici S, Caleb B, Das S, Eckley S, Fekkes P, Hao MH, Hart A, Houtman R, Irwin S, Joshi JJ, Karr C, Kim A, Kumar N, Kumar P, Kuznetsov G, Lai WG, Larsen N, Mackenzie C, Martin LA, Melchers D, Moriarty A, Nguyen TV, Norris J, O’Shea M, Pancholi S, Prajapati S, Rajagopalan S, Reynolds DJ, Rimkunas V, Rioux N, Ribas R, Siu A, Sivakumar S, Subramanian V, Thomas M, Vaillancourt FH, Wang J, Wardell S, Wick MJ, Yao S, Yu L, Warmuth M, Smith PG, Zhu P, Korpal M. Discovery of selective estrogen receptor covalent antagonists for the treatment of ERαWT and ERαMUT breast cancer. Cancer Discov. 2018;8:1176–1193. doi: 10.1158/2159-8290.CD-17-1229. [DOI] [PubMed] [Google Scholar]
- 119.Furman C, Hao MH, Prajapati S, Reynolds D, Rimkunas V, Zheng GZ, Zhu P, Korpal M. Estrogen receptor covalent antagonists: the best is yet to come. Cancer Res. 2019;79:1740–1745. doi: 10.1158/0008-5472.CAN-18-3634. [DOI] [PubMed] [Google Scholar]
- 120.Fanning SW, Greene GL. Next-generation ERα inhibitors for endocrine-resistant ER+ breast cancer. Endocrinology. 2019;160:759–769. doi: 10.1210/en.2018-01095. [DOI] [PubMed] [Google Scholar]
- 121.Bardia A, Kaklamani V, Wilks S, Weise A, Richards D, Harb W, Osborne C, Wesolowski R, Karuturi M, Conkling P, Bagley RG, Wang Y, Conlan MG, Kabos P. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J. Clin. Oncol. 2021;39:1360–1370. doi: 10.1200/JCO.20.02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shomali M, Cheng J, Sun F, Koundinya M, Guo Z, Hebert AT, McManus J, Levit MN, Hoffmann D, Courjaud A, Arrebola R, Cao H, Pollard J, Lee JS, Besret L, Caron A, Bangari DS, Abecassis PY, Schio L, El-Ahmad Y, Halley F, Tabart M, Certal V, Thompson F, McCort G, Filoche-Romme B, Cheng H, Garcia-Echeverria C, Debussche L, Bouaboula M. SAR439859, a novel selective estrogen receptor degrader (SERD), demonstrates effective and broad antitumor activity in wild-type and mutant ER-positive breast cancer models. Mol Cancer Ther. 2021;20:250–262. doi: 10.1158/1535-7163.MCT-20-0390. [DOI] [PubMed] [Google Scholar]
- 123.Conlan MG, de Vries EFJ, Glaudemans A, Wang Y, Troy S. Pharmacokinetic and pharmacodynamic studies of elacestrant, a novel oral selective estrogen receptor degrader, in healthy post-menopausal women. Eur J Drug Metab Pharmacokinet. 2020;45:675–689. doi: 10.1007/s13318-020-00635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McDonough SJ, Bhagwate A, Sun Z, Wang C, Zschunke M, Gorman JA, Kopp KJ, Cunningham JM. Use of FFPE-derived DNA in next generation sequencing: DNA extraction methods. PLoS One. 2019;14:e0211400. doi: 10.1371/journal.pone.0211400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee N, Park MJ, Song W, Jeon K, Jeong S. Currently applied molecular assays for identifying ESR1 mutations in patients with advanced breast cancer. Int J Mol Sci. 2020;21:8807. doi: 10.3390/ijms21228807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weirather JL, Afshar PT, Clark TA, Tseng E, Powers LS, Underwood JG, Zabner J, Korlach J, Wong WH, Au KF. Characterization of fusion genes and the significantly expressed fusion isoforms in breast cancer by hybrid sequencing. Nucleic Acids Res. 2015;43:e116. doi: 10.1093/nar/gkv562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O’Leary B, Hrebien S, Beaney M, Fribbens C, Garcia-Murillas I, Jiang J, Li Y, Huang Bartlett C, Andre F, Loibl S, Loi S, Cristofanilli M, Turner NC. Comparison of BEAMing and droplet digital PCR for circulating tumor DNA analysis. Clin Chem. 2019;65:1405–1413. doi: 10.1373/clinchem.2019.305805. [DOI] [PubMed] [Google Scholar]
- 128.Cristofanilli M, DeMichele A, Giorgetti C, Turner NC, Slamon DJ, Im SA, Masuda N, Verma S, Loi S, Colleoni M, Theall KP, Huang X, Liu Y, Bartlett CH. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur J Cancer. 2018;104:21–31. doi: 10.1016/j.ejca.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 129.Desmedt C, Voet T, Sotiriou C, Campbell PJ. Next-generation sequencing in breast cancer: first take home messages. Curr Opin Oncol. 2012;24:597–604. doi: 10.1097/CCO.0b013e328359554e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Santo I, McCartney A, Migliaccio I, Di Leo A, Malorni L. The emerging role of ESR1 mutations in luminal breast cancer as a prognostic and predictive biomarker of response to endocrine therapy. Cancers (Basel) 2019;11:1894. doi: 10.3390/cancers11121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Navid S, Fan C, O Flores-Villanueva P, Generali D, Li Y. The fibroblast growth factor receptors in breast cancer: from oncogenesis to better treatments. Int J Mol Sci. 2020;21:2011. doi: 10.3390/ijms21062011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lundberg A, Lindstrom LS, Li J, Harrell JC, Darai-Ramqvist E, Sifakis EG, Foukakis T, Perou CM, Czene K, Bergh J, Tobin NP. The long-term prognostic and predictive capacity of cyclin D1 gene amplification in 2305 breast tumours. Breast Cancer Res. 2019;21:34. doi: 10.1186/s13058-019-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Montemurro F, Di Cosimo S, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol. 2013;24:2715–2724. doi: 10.1093/annonc/mdt287. [DOI] [PubMed] [Google Scholar]
- 134.Fernandes MT, Adashek JJ, Barreto CMN, Spinosa ACB, de Souza Gutierres B, Lopes G, Del Giglio A, Aguiar PN Jr. A paradigm shift for the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer: a review of CDK inhibitors. Drugs Context. 2018;7:212555. doi: 10.7573/dic.212555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carbognin L, Miglietta F, Paris I, Dieci MV. Prognostic and predictive implications of PTEN in breast cancer: unfulfilled promises but intriguing perspectives. Cancers (Basel) 2019;11:1401. doi: 10.3390/cancers11091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-Kuraya K, Schraml P, Torhorst J, Tapia C, Zaharieva B, Novotny H, Spichtin H, Maurer R, Mirlacher M, Kochli O, Zuber M, Dieterich H, Mross F, Wilber K, Simon R, Sauter G. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64:8534–8540. doi: 10.1158/0008-5472.CAN-04-1945. [DOI] [PubMed] [Google Scholar]
- 137.Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol. 2019;30:x3–x11. doi: 10.1093/annonc/mdz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung MC, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lippi G, Mattiuzzi C, Montagnana M. BRCA population screening for predicting breast cancer: for or against? Ann Transl Med. 2017;5:275. doi: 10.21037/atm.2017.06.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ertel A, Dean JL, Rui H, Liu C, Witkiewicz AK, Knudsen KE, Knudsen ES. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–4163. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jeong Y, Bae SY, You D, Jung SP, Choi HJ, Kim I, Lee SK, Yu J, Kim SW, Lee JE, Kim S, Nam SJ. EGFR is a therapeutic target in hormone receptor-positive breast cancer. Cell Physiol Biochem. 2019;53:805–819. doi: 10.33594/000000174. [DOI] [PubMed] [Google Scholar]
- 142.Sircoulomb F, Bekhouche I, Finetti P, Adelaide J, Ben Hamida A, Bonansea J, Raynaud S, Innocenti C, Charafe-Jauffret E, Tarpin C, Ben Ayed F, Viens P, Jacquemier J, Bertucci F, Birnbaum D, Chaffanet M. Genome profiling of ERBB2-amplified breast cancers. BMC Cancer. 2010;10:539. doi: 10.1186/1471-2407-10-539. [DOI] [PMC free article] [PubMed] [Google Scholar]


