Abstract
Extracellular matrix is a complex network of macromolecules that constitute a microenvironment of normal tissues and malignancies such as the primary brain tumor glioblastoma (GBM). The unique composition of the GBM ECM, compared with the brain, contributes to angiogenesis, invasion, and therapeutic resistance of GBM. On the other hand, components of tumor ECM and related aberrant signaling pathways offer opportunities for various therapeutic strategies that are under active investigations. Here we provide a comprehensive overview of emerging therapeutic approaches for GBM that target or utilize its unique ECM via antibodies or ligands, RNA interference, pharmacological agents and modification of ECM molecules. Furthermore, drug-loaded nanoparticles displaying ECM-directed antibodies or peptides enable tumor selective delivery of the payload. As an in vitro research platform, 3D tumor cell culture incorporating ECM can advance our understanding of tumor-ECM interactions.
Keywords: Extracellular matrix, glioblastoma, tumor microenvironment, antibodies, peptides, oncolytic viruses, nanoparticles, chemotherapy, 3D culture
Introduction
Glioblastoma (GBM) is a primary brain tumor that is notorious as one of the most difficult cancers to treat. Standard therapies for GBM include surgery, radiation, and chemotherapy with alkylating agent temozolomide (TMZ). Still, with a 5-year survival rate of only 5%, it remains to be a devastating tumor [1]. The proliferative and highly invasive nature of the tumor cells and resistance to therapies make GBM management difficult and challenging. The extracellular matrix (ECM) is an intricate network made up of many multi-domain macromolecules organized in a tissue specific manner and is present in both normal and tumor tissue environments. The brain tumor ECM differs from the ECM of normal brain tissue, and supports the aggressive biology of brain neoplasms, thereby providing opportunities for GBM therapy. Whether the therapy involves antibodies against specific components in the ECM, RNA interference, physical modification of ECM components, enzymatic breakdown of ECM components, or tumor microenvironment modulation, each utilizes different properties of brain tumor ECM, and thus has varying mechanisms of action and levels of efficacy. The goal of this review is: (1) to overview the characteristics of brain tumor ECM and its components, and (2) to provide comprehensive summary of anti-GBM therapeutics approaches that utilize or target the GBM ECM.
Brain ECM
The brain ECM has several important features. Specifically, the brain ECM has the unique composition of a complex mixture of matrix molecules that include: glycoproteins-fibronectin, laminins, and tenascins, glycosaminoglycans represented by hyaluronic acid (HA, hyaluronan) and proteoglycans such as chondroitin sulfate proteoglycan (CSPG) [2] (Figure 1). Contribution of collagen to the brain ECM is limited, compared with other parts of the body, since the presence of collagen in the brain is restricted to blood vessels (the basement membrane) and the glia limitans in the brain. The main assembly of the adult brain ECM is believed to be a hyaluronan-lectican-tenascin-R-complex [3]. Physically, mature brain ECM is more flexible than tumor ECM. Brain ECM, however, plays more than a structural role in the tissue. Proteins of the ECM interact with each other and with neighboring cells through specialized matrix receptors which allows the ECM to influence different signaling pathways in the cells. ECM is involved in determining cell fate, cell migration, cell maturation and differentiation, cell survival, and tissue homeostasis in the brain. For example, tenascins have been shown to help regulate the differentiation and migration of neurons.
Figure 1.
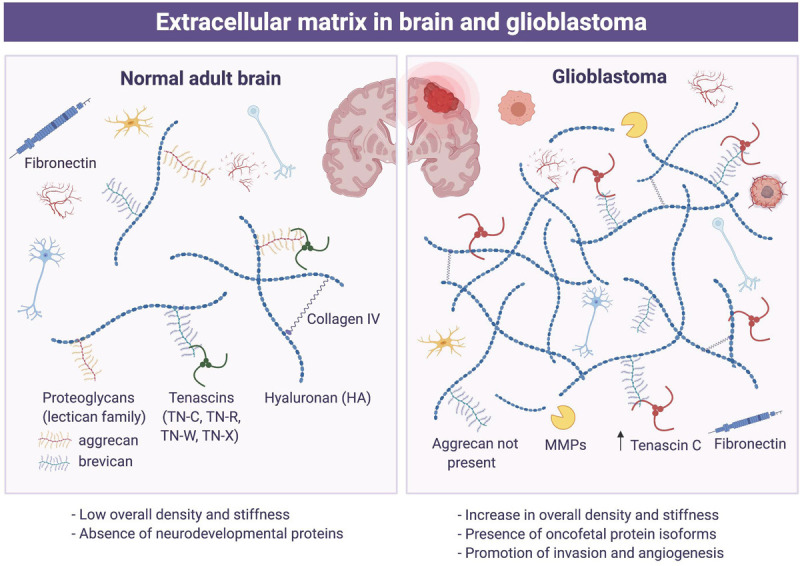
Extracellular matrix in brain and glioblastoma. Schematic presentation of relative abundance of a variety of extracellular matrix components in the brain (left) and glioblastoma (right).
Brain tumor ECM
Brain tumor ECM shares many of the same components as mature adult brain ECM. Essentially all the common components of ECM including proteoglycans and proteins (laminins, fibronectin, or tenascins) influence the behavior of glioma cells (Figure 1). Collagens I, II, and IV are detected in basal lamina of tumor blood vessels. Glioma cells overexpress ECM components like HA, brevikan, tenascin-C, fibronectin and thrombospondin as well as specific integrins and other receptors interacting with ECM components, which lead to adhesion and migration of glioma cells. Indeed, glioma cells can actively migrate using blood vessels or axons as guide paths through interaction with ECM. Witnessed in the tumor ECM is an alteration of HA levels, which increase as the malignancy of tumors increases. Fibronectin and HA promote the mobility and invasiveness of glioma cells. Brain tumors also show ECM-dependent metabolic activity [4]. Research using various assays has identified signals driven by ECM that shape phenotypes of the tumor. Furthermore, tumors trigger altered synthesis of proteins promoting degradation of the ECM at the invasive front of the tumor as well as an elevated synthesis of ECM components by normal tissues in the vicinity of the tumor. Glioma cells express specific integrins and other receptors interacting with ECM components including receptors not present in corresponding normal cells, which facilitates tumor cell migration and invasion. Thus, ECM in brain tumors plays a significant role in cell survival, proliferation, and differentiation processes in tumor growth, offering excellent opportunities for therapeutic approaches to treat GBM. Moreover, overexpression of ECM components makes tumor ECM more condensed and less flexible in a way that might diminish the diffusion of neuroactive molecules or therapeutic agents. With such a low survival rate and a poor response to common therapeutic approaches, targeting GBM ECM might present new strategies that help combat this malignant disease [4].
Tumor extracellular matrix as barriers to therapeutic efficacy in solid cancer
Although not necessarily proven in the context of brain tumors, tumor ECM has been shown to diminish efficiency of cancer therapies via multiple mechanisms (Figure 2). In solid cancers, the ECM components, like HA and collagens, are overexpressed, creating barriers surrounding and protecting the tumor [5]. This limits the ability of chemotherapeutic drugs to diffuse and penetrate the tumor, reducing their effectiveness. Hyaluronidase treatment of HA has been shown to enhance the actions of various chemotherapeutic agents in patients [6]. Elevated levels of ECM components on the other hand can diminish supply of nutrients and oxygen to the tumor and cause tumor hypoxia and metabolic stress, leading to resistance to radiation therapy and chemotherapy, respectively. Furthermore, ECM components, particularly collagens, mediate immune trapping through inhibiting migration of T cells [7], contributing to exclusion of effector immune cels and dysfunction of immune surveillance [8]. In human and mouse GBM, lower T cell infiltration was found in tumor areas that contain abundant HA, compared with HA-low tumor areas [9].
Figure 2.
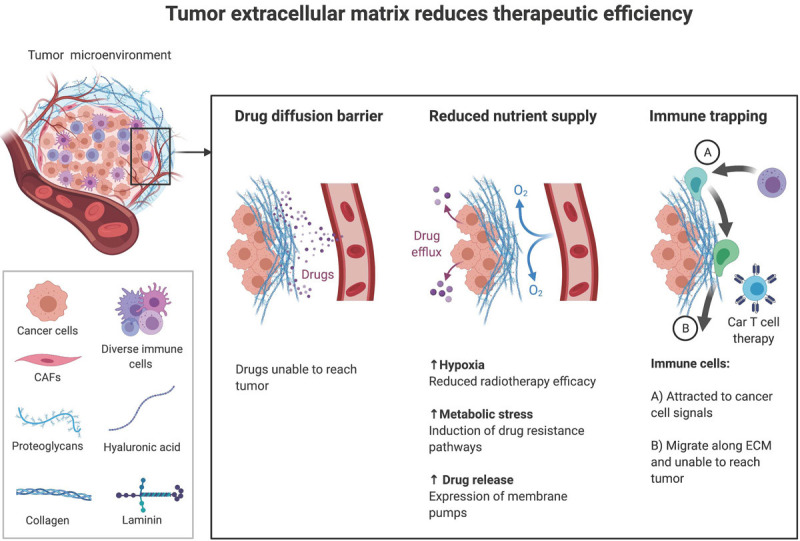
Tumor extracellular matrix reduces efficacy of anti-cancer therapies. Illustration shows the roles of extracellular matrix in serving as barriers to drug diffusion, nutritional supply, and immune cell migration.
Major components of the glioblastoma extracellular matrix
Tenascins
Tanascins glycoprotein family includes different types of tenascins: TN-C, TN-R, TN-W, and TN-X, but TN-C is the most prominent and active member in brain ECM [2]. TN-C is mainly involved in embryonic development, and less in later adulthood. TN-C can affect cell behavior in a multitude of ways. TN-C can either bind to integrins as its main receptor type to affect the cell directly, or it can act through binding to ECM molecules and affect the cell indirectly. TN-C can promote cell migration, angiogenesis, and proliferation, and mediate changes in constituents of brain ECM. TN-C is expressed at the highest concentrations in glioma cells compared to other types of cancer. TN-C is highly associated with blood vessels, which is beneficial to glioma cells because of their consistent need for nutrients available from the circulation. Amount of TN-C in tumor blood vessels is directly correlated with the malignancy of the tumors. Moreover, the alternatively spliced domains of TN-C are thought to have an important role in tumor cell migration [10]. Thus, TN-C contributes to the capability of glioma cells to invade into healthy parenchyma and make new tumor foci, the most fatal attribute of glioma, creating a significant challenge for therapeutic approaches.
Fibronectin
Fibronectin is a glycoprotein that is a part of the ECM of brain tissue and brain tumors. Fibronectin increased adherence by glioma stem-like cells with increased matrix metallopeptidases in a concentration dependent manner. Cell differentiation, cell adhesion, proliferation, and chemoresistance of glioma cells are all promoted by fibronectin. Fibronectin also suppresses p53-mediated apoptosis and upregulates P-glycoprotein expression, making glioma stem-like cells more chemoresistant [11].
Fibulin-3
Fibulin-3, a glycoprotein that forms the ECM scaffolding of GBM, promotes tumor progression by driving Notch and NF-kB signaling. Fibulin-3 is critical for the signaling functions of ECM in GBM. Fibulin-3 is sparsely detected in the body, and is essentially absent in the adult brain, but highly expressed in GBMs where it gains novel functions as an autocrine/paracrine activator of Notch and NF-kB signaling. Fibulin-3 enhances GBM invasion, vascularization, and survival of the tumor initiating cell population, correlating with poor patient survival and acting as a marker for regions of active tumor progression in GBM and other invasive cancers [12,13].
Hyaluronic acid (HA)
Hyaluronic acid (Hyaluronan, HA) is a high molecular weight glycosaminoglycan, and is a common component of brain tumor ECM. HA plays a main structural role in the brain ECM and a significant role in the biology of tumors. Glioma cell migration is definitely affected by the presence and molecular weight of HA. High production of HA is associated with tumor progression, yet abnormally high levels of HA can potentially lead to opposite behavior. HA turnover is required for GBM tumor malignancy [14]. GBMs have more HA than lower grade astrocytomas, and HA plays a prominent role in the aggressive invasion of GBM cells.
3D bioengineered in vitro tumor models incorporating ECM
ECM engagement with integrin or CD44 receptors on cells can activate Src protein-tyrosine kinase. Bioengineered hydrogel biomaterials composed of HA and integrin binding sites based on the “RGD” adhesive tripeptide were developed to surround 3D cultured, patient-derived GBM cells [15]. In hydrogel cultures, HA-CD44 and RGD-integrin interactions acted together to drive chemotherapy resistance. Src activation was identified as a key signaling event mediating both chemotherapy resistance and invasive morphology. GBM cells in HA high hydrogels grew significantly better and were less responsive to alkylating chemotherapies than those cultured in low HA content hydrogels. When treated with TMZ or carmustine incorporation of RGD, additional protection against apoptosis was observed. Therapies targeting the downstream molecules of matrix mediated resistance, such as Src, may be beneficial to overcome resistance. Dasatinib can inhibit Src activation and promote apoptosis when given with TMZ chemotherapy, representing a potential therapeutic agent against GBM [15]. Therefore, hydrogel with HA offers a better tool for pre-clinical screening evaluation of therapeutic strategies than 2D culture methods.
Simiarly, patient-derived tumor cells and tunable ECM microenvironmental scaffolds were combined to generate a 3D bioengineered brain tumor culture system [4]. This platform allowed live imaging of tumor cell behavior as well as studies of ECM-dependent phenotypes through transcriptomic and cytokine/MMPs release profiling. Nanofibers can also mimic the topographical features of brain ECM and help in vitro studies to investigate the interactions of GBM cells and ECM [16].
Therapeutic approaches that target brain tumor ECM
As our knowledge accumulates that ECM overall promotes tumor malignant phenotypes, increasing efforts have been directed at developing novel therapeutic modalities that specifically target the ECM in GBM. Such approaches may have an inherent advantage since many ECM components are ubiquitously located throughout the tumor parenchyma. So far, most antitumor approaches with a focus on ECM have concentrated on ECM associated proteins rather than the actual structural components of the ECM. Strategies that have been translated to the clinical setting include inhibition of matrix-degrading metalloproteinases and integrin. Targeting of various structural ECM molecules can also facilitate other modalities of treatment such as immunotherapy. Here, we discuss therapeutic approaches that target brain tumor ECM by classifying them into: the deployment of antibodies, RNA interference, and drug therapies targeting ECM molecules and pathways; and modification of ECM components.
Antibodies, peptide ligands and their anti-cancer drugs or radio-isotopes conjugates
Fibulin-3
Because of its high enrichment in GBM and low expression in normal tissues, fibulin-3 is an appealing target to test anti-ECM strategies. The antibody mAb428.2 blocks the unique functional motif in fibulin-3 resulting in complete inhibition of its signaling functions in GBM cells [13]. This antibody has antitumor efficacy against GBM, making it the first example of a rationally developed, function-blocking antibody against a structural ECM protein and capable of inhibiting cancer signaling. mAb428.2 is one of the first antibodies that focuses on identifying and blocking signaling mechanisms that are triggered or regulated by ECM in GBM. By inhibiting pro-tumoral signaling, mAb428.2 increased tumor cell apoptosis, decreased vascularization, and showed efficacy against intracranial GBM after intravenous administration. The antibody also reduced GBM invasion in organotypic cultures of brain tissue, suggesting that it probably has a similar effect in vivo. Since fibulin-3 increases tumor invasion, apoptotic resistance, and vascularization of intracranial GBM via Notch and ADAM17/NF-kB activation, the effects of mAb428.2 match what would be expected from a widespread inhibition of fibulin-3 signaling in the tumor.
The tumoricidal effects of mAb428.2 may have some limitations. First, mAB428.2 may be restricted by its rapid clearance. Repeated injections may be necessary to maintain the level of mAb428.2 in the brain sufficient to elicit antitumor effects. Another limitation is the limited solubility of mAb428.2 in phosphate, which may hinder intratumoral accumulation. Despite these limitations, the mAb428.2 antibody effectively inhibited fibulin-3 signaling pathways and ultimately increased the rate of apoptosis in GBM cells and prevented tumor progression.
Fibronectin
Extra domain B (EDB) of fibronectin represents one of the most promising neovascular markers because it is present in the perivascular space of many aggressive solid tumors. Antibody L19-SIP was generated to specifically target the EDB of fibronectin in angiogenesis. Angiogenesis is the formation of new blood vessels from preexisting ones and is a typical feature of many malignant tumors like GBM. Intravascularly administered L19-SIP ends up targeting tumor vessels in a time and blood flow dependent manner. L19-SIP has selectivity for neo-angiogenic tumor vessels and extravasates into tumor interstitium in a secondary process, which leads to an optimal signal-to-noise ratio occurring in glioma models 4 hours after antibody application [17].
Tumors with a high angiogenic activity and a strong vascular turnover appear preferred L19-SIP targets, and it will bind to well-perfused tumor areas. Successful delivery of L19-SIP has been demonstrated as a radioimmunotherapy or a microvascular biodistribution study (Figure 3). Radioimmunotherapy with iodine-131 labeled L19-SIP to target the EDB domain of fibronectin showed efficacy, as the median survival rate of treated rats was significantly improved compared with that of the control group, indicating the activity of this antibody. Yet, to truly test the efficacy of L19-SIP and radioimmunotherapy, studies performed on tumors of a substantial size would be advised. When used for microvascular biodistribution, L19-SIP represented a useful tool to specifically target tumor microvessels, making it a reasonable choice in targeting glioma [18]. In the case of therapy resistant tumor vessels, antiangiogenic therapy might be used to change tumor hemodynamics with the potential to improve the microvascular binding process and efficacy of L19-SIP-mediated therapies.
Figure 3.
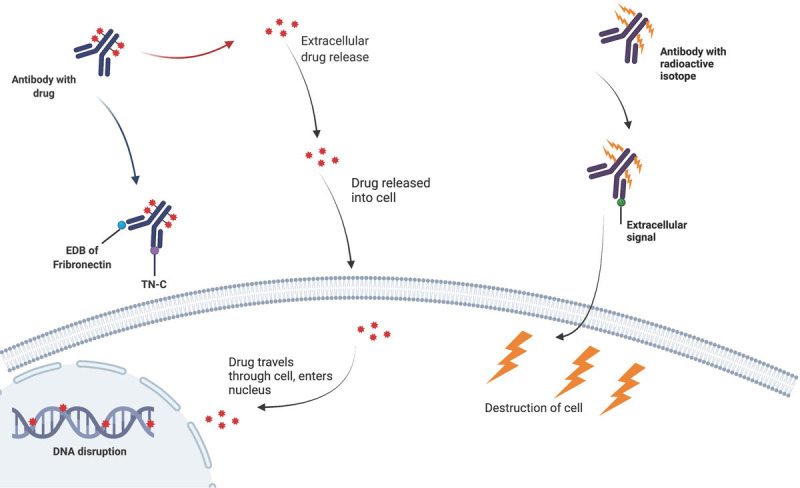
Drug or radioisotope-conjugated antibodies directed at extracellular matrix molecules. Illustration showing drug-conjugated antibody bi-specific to Extra domain B (EDB) of fibronectin and tanascin-C (TN-C) (left) and radio-labeled antibody against an extracellular matrix molecule (right), leading to damage of the tumor cell.
TN-C
Targeting the ECM component TN-C, a member of the tenascin glycoprotein family, offers an alternative approach to targeting tumors. Splice isoforms of TN-C could be considered targets for antibody-based therapeutic strategies. Radiolabeled antibodies specific to domains A1 and D of TN-C have been tested in the clinic for the treatment of glioma and lymphoma. The anti-TN-C antibody SIP (F16), directed at domain A1, has been more successful than antibodies that target other tenascin proteins. This F16 antibody accumulated specifically at the tumor site and decreased tumor growth to a level that was comparable with the anti-fibronectin L19 antibody. However, since the F16 antibody does not cross-react with the murine isoform of domain A1 it fails to detect antigen expressed in mouse organs. Yet given the success of the L19 antibodies, pursuit of the F16 targeting TN-C might prove to be successful to target tumor cells of various cancers [19].
Dual targeting of TN-C and EDB of fibronectin
Based on the activity seen with antibodies against fibronectin and TN-C separately, therapies have been suggested at dual targeting both at the same time (Figure 3). Dual targeting with affinity ligands is a way of dealing with two critical issues of affinity targeting. Firstly, the number of molecules of any tumor-associated marker is limited, and secondly there is considerable inter and intratumoral heterogeneity in the expression of such markers. Simultaneous affinity targeting of multiple molecules is more advantageous over targeting one receptor at a time. Expression of tumor ECM is heterogenous and multi-targeting may result in more uniform biodistribution of payloads. Dual targeting may alleviate issues related to the limited number of available receptors for affinity ligands, which is a major bottleneck in affinity ligands.
ECM binding affinity ligands are compatible with targeting with intracellularly acting anticancer drugs. ECM binding antibody/ligands drug conjugates are able to efficiently release their drug payloads in the extracellular environment to exert a potential therapeutic activity. Treatment with systemic PL1, a novel bi-specific peptide targeting TN-C and fibronectin, significantly reduced tumor volume and increased the lifespan of tumor-bearing mice. Both EDB and TN-C are involved in regulating endothelial cell behavior and plasticity during tumor angiogenesis. It is thus possible to envision clinical development of PL1 based approaches for precise cancer drug delivery and a method to monitor the efficacy of anticancer interventions [20].
Dual targeting of TN-C and neuropilin-1
A new Ft peptide was synthesized by coupling FHK peptide, which has a high binding affinity with TN-C, and tLyp-1 peptide, which also has a motif with a high binding affinity for neuropilin-1, a transmembrane glycoprotein. The Ft peptide synergistically targets glioma-associated TN-C and neuropilin-1 on neovasculature to enable specific penetration of nanoparticles for anti-GBM treatment. Ft peptide functionalization facilitated deep penetration into 3D glioma spheroids in vitro. Upon intravenous injection, paclitaxel-loaded Ft nanoparticles induced higher cytotoxic effects and apoptosis rate through neuropilin-1 and TN-C mediated specific penetration of nanoparticles into glioma parenchyma in the brain [21].
RNA interference delivery
ECM components such as EDB upregulated in tumors can be utilized to effectively deliver RNA interference to the tumor as a therapeutic. Nanoparticles are a powerful tool used as a specific delivery vehicle that will facilitate the intracellular uptake and cytosolic delivery of RNAi agents. Development of aptamer-like peptide (aptide), a decorated liposomal nanoplatform, facilitates systemic small interfering RNA (siRNA) delivery. This aptide is composed of classic liposome structure with an aqueous core that can encapsulate therapeutic siRNA and hydrophilic polyethylene glycol (PEG) chains on the outer shell to prolong blood circulation. The surface-encoded aptide specifically recognizes the EDB of fibronectin that is overexpressed in glioma cells. Upon administration, these siRNA delivery nanoparticles target glioma cells and efficiently inhibit GBM tumor growth by silencing the expression of cyclophilin A (CypA), which is upregulated in brain cancer, showing the potential of RNA interference as a therapeutic modality. Capitalizing on the highly expressed EDB of fibronectin, this treatment used the siRNA, siCypA, to silence the expression of the cancer associated CypA to treat GBM. Although effective in theory, this therapy needs to address challenges such as sustained blood circulation, selective accumulation at the tumor site, and efficient tumor internalization, to increase efficacy. A new nano-platform should be created that improves blood circulation, while enhancing GBM targeting ability and intracellular siRNA delivery [22]. Clinically, RNA interference was applied by direct administration of a double stranded RNA with a sequence homologous to TN-C mRNA into gliomas in a cohort of 10 patients [23].
Modification or modulation of ECM components
Modification of the physical components of ECM is a relatively new area of research and could have value as therapeutic modalities for GBM. Modifying different components of ECM, like HA, tenascins, and fibronectin have each been tested preclinically with differing approaches and levels of efficacy.
Enzymatic breakdown of HA
Oncolytic virus is a replication competent anti-cancer virus that is able to kill cancer cells, as well as modify the tumor microenvironment. Genetically engineered oncolytic adenovirus is one of the most promising avenues for oncolytic virus anticancer therapies. A common problem with oncolytic adenoviruses is their inability to spread throughout tumor tissues. Degrading ECM with enzymes is a method that will improve the viral spread throughout the tumor mass. Hyaluronidase, an enzyme which selectively dissociates HA, proved to enhance the intratumoral distribution of an oncolytic adenovirus and improve its therapeutic activity [24,25]. The abundant presence of HA in GBM tumors makes it a good target for enzymatic breakdown. Hyaluronidase facilitates penetration and decreases interstitial fluid pressure permitting anticancer agents to reach malignant cells. ICOVIR17 is a replication selective adenovirus armed with PH20, a soluble form of the human sperm hyaluronidase [24]. Treatment of orthotopic GBM xenografts with ICOVIR17 helped to mediate the destruction and degradation of HA in GBM ECM, which allowed for increased spread of virus within the tumor and superior antitumor efficacy [25].
In murine GBM in immunocompetent mice, treatment with ICOVIR17 increased tumor-infiltrating immune cells and increased animal survival. Tumor infiltrating macrophages were increased by ICOVIR15 virus not expressing hyaluronidase, and further increased by ICOVIR17. Low molecular weight HA-induced genes were driven by the NF-kB signaling pathway and transcripts of this pathway were increased in tumors after ICOVIR17 injections. GBM treatment with HA-degrading ICOVIR17 has a therapeutic effect in murine GBM along with increases in tumor-infiltrating CD8+ T cells, tumor-associated macrophages, and PD-L1 levels (Figure 4). These changes in the tumor microenvironment allowed ICOVIR17 to combine with anti-PD-1 antibody to help combat aggressive GBM. Thus, ICOVIR17-mediated degradation of HA within GBM enhances the immune response in the tumor microenvironment, and offers immunotherapy combined with PD-L1/PD-1 blockade [9].
Figure 4.
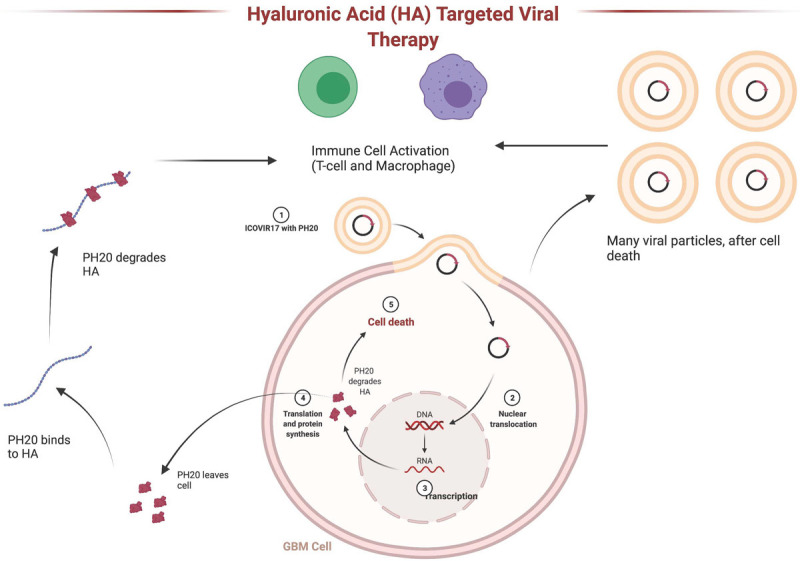
Hyaluronic acid targeted oncolytic virus therapy. Oncolytic adnovirus ICOVIR17 infects glioblastoma cells and induces expression of PH20 hyaluronidase. PH20 is secreted and degrades hyaluronic acid (HA) in the extracellular matrix. Immunogenic cell death triggered by ICOVIR17 and HA degradation result in activation of T cells and macrophages in the tumor microenvironment.
TN-C modulation
Being one of the main glycoproteins in ECM, TN-C would be a great target for ECM modification due to its elevated levels in brain tumor ECM, and contribution to tumor cell proliferation, migration, invasion, and angiogenesis. TN-C loss-of-function increased GBM neurosphere cell adhesion without affecting the cell proliferation rate. In TN-C knockdown xenografts, blood density was low and lumens of blood vessels were enlarged, suggesting altered endothelial cell behavior in response to TN-C changes in the tumor microenvironment. Decreased TN-C in the tumor microenvironment modulated the behavior of tumor stromal cells and inhibited tumor invasion, but increased tumor cell proliferation. TN-C knockdown cells were more sensitive to anti-proliferative strategies, which could ultimately lead to novel combinatory antitumor strategies that can target both tumor invasion and proliferation. Thus, TN-C modulation is a viable therapeutic method for treating GBM [26].
Fibronectin modification
Fibronectin is considered one of the most promising therapeutic targets in ECM, given its role in GBM. Functional causation is observed between the activation of fibronectin matrix assembly, FNMA, and increased tumor cell-to-cell cohesion with decreased dispersal [27]. Thus, activating FNMA can reduce dispersal and growth of primary GBM cells. Dexamethasone (Dex) treatment, which is routinely used to alleviate brain tumor-related edema, can activate FNMA and give rise to increased cell-cell cohesion and decreased capacity for dispersal. For glioma cells to disperse, they must be able to physically squeeze through pores created by astrocytes and undergo a shape change and nuclear deformation, which requires dramatic changes in cytoskeletal organization and in cellular mechanics. Dex treatment can impede this process. Administering agents that effectively increase cell-to-cell cohesion and impede cell motility after initial tumor resection may be able to effectively decrease the rate of tumor recurrence. Dex treatment that modifies fibronectin may be a potential therapy for GBM with a unique mechanism of action that physically alters a part of GBM ECM [28].
Glioma invasion via CD44-HA interaction
CD44 is a transmembrane receptor for HA that supports cell motility and promotes GBM progression and invasion. CD44 blocking antibody reduces tumor size in rats grafted with C6 glioma. Knockdown of CD44 in human GBM tumors slows tumor growth and makes tumors more susceptible to response to chemotherapeutic agents [29]. GBM cells engage HA by assembling “micro-tentacles” (McTNs) that are dynamic tubular protrusions and attach to the matrix through the CD44 receptor. CD44 is necessary for cell adhesion and the stability of McTN which allows GBM cells to invade HA-rich matrix. The IQGAP1/CLIP170 complex was found to be a cross linker of actin and microtubules in McTNs, and functionally loss of IQGAP1 disrupted CD44-mediated binding to HA and cell motility on HA, supporting a critical role of IQGAP1 in glioma cell motility. The components of McTNs may offer therapeutic targets to hinder migration of GBM cells [29].
SPARCL1 modification
SPARCL1 is a member of the SPARC (secreted, acidic and rich in cyctein) protein family, and is present in the ECM as a non-structural protein. In gliomas, SPARCL1 protein expression increases concomitant with tumor grade. SPARCL1 also serves as a mediator of the complex needed for the invasion-promoting effect of subventricular zone progenitor cells during infiltration of glioma cells [30]. Overexpression of SPARCL1 in GBM cancer stem cells enhanced both neoangiogenesis and invasiveness in intracranial GBM xenografts. Interestingly, SPARCL1 also induced a recruitment of activated microglia or pro-tumorigenic M2 macrophages, which was accompanied by enhanced angiogenesis [31]. Therefore, SPARCL1 is a potential new target in the treatment of GBM.
Matrix metalloproteinases modulation
As with the case of oncolytic adenoviruses, oncolytic herpes simplex virus virotherapy of brain tumors can be limited by barriers like inadequate virus replication or poor virus distribution. A modified herpes simplex virus, armed with the ECM modifying protein matrix metalloproteinase (MMP) 9, mediated an increased viral dissemination, thereby increasing antitumor effects of the virus. Moreover, Sette and colleagues combined MMP-9-mediated ECM modulation with receptor retargeting by making the virus bind to epidermal growth factor receptor rather than the cognate HSV entry receptors, resulting in increases in the spread of the virus and the antitumor activity [32].
ECM remodeling contributes to TMZ resistance in GBM. NFE2L2 is a transcription factor upstream of MMP-2, and can be inhibited by diosgenin. Combinatorial therapy of diosgenin and TMZ helped curb resistance and enabled the dosage reduction of TMZ. Diosgenin downregulated NFE2L2, coupled with a promotion of apoptosis, offering a rationale for a new therapeutic approach [33].
IRF3 suppresses glioma invasion
Interferon regulatory factor 3 (IRF3) is a transcriptional repressor of pro-invasive ECM genes in GBM, and can serve as an endogenous inhibitor of GBM invasion. Inhibition of casein kinase 2 (CK2), a negative regulator of IRF3, by CX4945 activated IRF3, downregulated the expression of ECM collagens, and suppressed GBM invasion. IRF3 also contributed to GBM suppression in a non-cell-autonomous way by mediating the production of type I interferons and interferon-stimulated genes by GBM cells, thus endowing the local microglia with anti-tumor functions. So IRF3 activation could serve as a promising therapeutic approach to GBM [34].
HSP47 facilitates ECM modification
HSP47 (serpin H1) serves as a molecular chaperone for collagen. It is significantly overexpressed in GBM and associated with tumor grade. Overexpression of HSP47 promoted primary glioma cell tumor formation, invasion, and angiogenesis. The overexpression of HSP47 promoted the TGF-β pathway in GBM, and blocking the TGF-β pathway overcame HSP47 induced tumorigenesis and stemness. Thus, the HSP47-TGF-β signaling pathway is a potential therapeutic target in GBM [35].
Enzymatic breakdown of chondroitin sulfate proteoglycans
Chondroitin sulfate proteoglycans (CSPG) are necessary for cell-to-cell and cell-ECM interactions and are implicated in glioma growth and invasion. Chondroitinase ABC is a bacterial enzyme that cleaves chondroitin sulfate disaccharide chains from the CSPGs in the tumor ECM. Chondroitinase ABC inhibited aggregation of glioma neurospheres, by disrupting CSPG-driven glioma cell-cell interaction. An oncolytic herpes simplex virus armed with a secreted, humanized version of Chondroitinase ABC that is stable and active in mammalian cells enhanced virus spread and glioma cell killing as well as sensitized glioma neurospheres to TMZ-induced apoptotic cell death. The virus expressing secreted humanized Chondroitinase ABC combined with TMZ increased therapeutic efficacy in intracranial GBM in mice [36].
Drug therapies targeting ECM molecules and pathways
Lumefantrine
ECM is involved in epithelial to mesenchymal transition, a process that is associated with the acquisition of resistance to radiation and TMZ, and failed GBM therapy. A critical crosswalk between heat shock proteins and MMPs (MMP-2 and MMP-9) is of prime significance for ECM remodeling and promotion of epithelial to mesenchymal like characteristics [37]. Heat shock proteins present a protein signature that is associated with GBM progression, and specifically HSPB1 is up-regulated in GBM and radiation/TMZ-resistant GBM. Inhibiting the transcription of HSPB1 inhibited ECM remodeling and affected epithelial to mesenchymal transition. The transcription factor Fli-1 mediated HSPB1 expression and had a great binding affinity for lumefantrine, an antimalarial drug. Subsequently, treatment with lumefantrine downregulated ECM remodeling proteins and epithelial mesenchymal transition cell markers, while up-regulated the apoptotic protein Bax, revealing the potential of lumefantrine as a therapeutic for GBM [38].
Tetraarsenic oxide
Tetraarsenic oxide is a trivalent arsenic compound that has potential anti-cancer and anti-angiogenic effects against gliomas. Tetraarsenic oxide decreased gelatinolytic activity of MMP-2 and membrane type 1 MMP expression, leading to inhibition of glioma cell proliferation and invasion. These effects were associated with the inhibition of Akt phosphorylation, downstream of PTEN. Further studies will need to determine the effective dose of tetraarsenic oxide necessary for optimal therapeutic effects [39].
Hyaluronic acid nanoparticles
The use of hydrogel nanoparticles, particularly HA as a naturally occurring polymer of ECM, has a great potential in improving the transport of drug molecules. Crosslinked HA nanoparticles encapsulating gadolinium-diethylenetriamine pentaacetic acid, a widely used MRI contrast agent, and chemotherapeutic agent irinotecan were investigated. Active targeting of GBM cells are achieved by theranostic chemotherapeutic irinotecan decorated with angiopep-2, a targeting peptide interacting with low density lipoprotein receptor related protein-1 (LRP-1). Dual targeting is accomplished because LRP-1 is highly expressed on BBB and glioma cells. This peptide boosted selective uptake by human GBM cells and anti-tumor activity, making the nanoparticles an effective therapy [40].
Conclusions
The composition of the brain tumor ECM is complex and distinct from the ECM of healthy brain tissue. A mounting body of research has elucidated the critical roles that the brain tumor ECM play in the pathogenesis of GBM, and proposed a variety of innovative approaches that aim to target components and pathways relevant to the GBM ECM to hamper tumor growth and reverse resistance to therapy. Many of them, however, are still at preclinical developmental stage. Future research efforts to refine and translate such strategies into clinical trials are essential, and might result in clinical therapeutic paradigms that help lives of patients with this devastating malignancy.
Disclosure of conflict of interest
None.
References
- 1.Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, Lim M, Mehta MP, Mellinghoff IK, Minniti G, Nathanson D, Platten M, Preusser M, Roth P, Sanson M, Schiff D, Short SC, Taphoorn MJB, Tonn JC, Tsang J, Verhaak RGW, von Deimling A, Wick W, Zadeh G, Reardon DA, Aldape KD, van den Bent MJ. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–1113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sood D, Tang-Schomer M, Pouli D, Mizzoni C, Raia N, Tai A, Arkun K, Wu J, Black LD 3rd, Scheffler B, Georgakoudi I, Steindler DA, Kaplan DL. 3D extracellular matrix microenvironment in bioengineered tissue models of primary pediatric and adult brain tumors. Nat Commun. 2019;10:4529. doi: 10.1038/s41467-019-12420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henke E, Nandigama R, Ergun S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2019;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20:3422–3433. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 8.Gordon-Weeks A, Yuzhalin AE. Cancer extracellular matrix proteins regulate tumour immunity. Cancers (Basel) 2020;12:3331. doi: 10.3390/cancers12113331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiyokawa J, Kawamura Y, Ghouse SM, Acar S, Barcin E, Martinez-Quintanilla J, Martuza RL, Alemany R, Rabkin SD, Shah K, Wakimoto H. Modification of extracellular matrix enhances oncolytic adenovirus immunotherapy in glioblastoma. Clin Cancer Res. 2021;27:889–902. doi: 10.1158/1078-0432.CCR-20-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosicke N, Faissner A. Role of tenascins in the ECM of gliomas. Cell Adh Migr. 2015;9:131–140. doi: 10.1080/19336918.2014.1000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Q, Xue Y, Liu J, Xi Z, Li Z, Liu Y. Fibronectin promotes the malignancy of glioma stem-like cells via modulation of cell adhesion, differentiation, proliferation and chemoresistance. Front Mol Neurosci. 2018;11:130. doi: 10.3389/fnmol.2018.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B, Nandhu MS, Sim H, Agudelo-Garcia PA, Saldivar JC, Dolan CE, Mora ME, Nuovo GJ, Cole SE, Viapiano MS. Fibulin-3 promotes glioma growth and resistance through a novel paracrine regulation of Notch signaling. Cancer Res. 2012;72:3873–3885. doi: 10.1158/0008-5472.CAN-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandhu MS, Behera P, Bhaskaran V, Longo SL, Barrera-Arenas LM, Sengupta S, Rodriguez-Gil DJ, Chiocca EA, Viapiano MS. Development of a function-blocking antibody against fibulin-3 as a targeted reagent for glioblastoma. Clin Cancer Res. 2018;24:821–833. doi: 10.1158/1078-0432.CCR-17-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JE, Pedron S, Shyu P, Hu Y, Sarkaria JN, Harley BAC. Influence of hyaluronic acid transitions in tumor microenvironment on glioblastoma malignancy and invasive behavior. Front Mater. 2018;5:39. doi: 10.3389/fmats.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao W, Wang S, Zhang R, Sohrabi A, Yu Q, Liu S, Ehsanipour A, Liang J, Bierman RD, Nathanson DA, Seidlits SK. Bioengineered scaffolds for 3D culture demonstrate extracellular matrix-mediated mechanisms of chemotherapy resistance in glioblastoma. Matrix Biol. 2020;85-86:128–146. doi: 10.1016/j.matbio.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norouzi M. Recent advances in brain tumor therapy: application of electrospun nanofibers. Drug Discov Today. 2018;23:912–919. doi: 10.1016/j.drudis.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Spaeth N, Wyss MT, Pahnke J, Biollaz G, Trachsel E, Drandarov K, Treyer V, Weber B, Neri D, Buck A. Radioimmunotherapy targeting the extra domain B of fibronectin in C6 rat gliomas: a preliminary study about the therapeutic efficacy of iodine-131-labeled SIP(L19) Nucl Med Biol. 2006;33:661–666. doi: 10.1016/j.nucmedbio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Czabanka M, Parmaksiz G, Bayerl SH, Nieminen M, Trachsel E, Menssen HD, Erber R, Neri D, Vajkoczy P. Microvascular biodistribution of L19-SIP in angiogenesis targeting strategies. Eur J Cancer. 2011;47:1276–1284. doi: 10.1016/j.ejca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Brack SS, Silacci M, Birchler M, Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res. 2006;12:3200–3208. doi: 10.1158/1078-0432.CCR-05-2804. [DOI] [PubMed] [Google Scholar]
- 20.Lingasamy P, Tobi A, Haugas M, Hunt H, Paiste P, Asser T, Ratsep T, Kotamraju VR, Bjerkvig R, Teesalu T. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials. 2019;219:119373. doi: 10.1016/j.biomaterials.2019.119373. [DOI] [PubMed] [Google Scholar]
- 21.Kang T, Zhu Q, Jiang D, Feng X, Feng J, Jiang T, Yao J, Jing Y, Song Q, Jiang X, Gao X, Chen J. Synergistic targeting tenascin C and neuropilin-1 for specific penetration of nanoparticles for anti-glioblastoma treatment. Biomaterials. 2016;101:60–75. doi: 10.1016/j.biomaterials.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Saw PE, Zhang A, Nie Y, Zhang L, Xu Y, Xu X. Tumor-associated fibronectin targeted liposomal nanoplatform for cyclophilin A siRNA delivery and targeted malignant glioblastoma therapy. Front Pharmacol. 2018;9:1194. doi: 10.3389/fphar.2018.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zukiel R, Nowak S, Wyszko E, Rolle K, Gawronska I, Barciszewska MZ, Barciszewski J. Suppression of human brain tumor with interference RNA specific for tenascin-C. Cancer Biol Ther. 2006;5:1002–1007. doi: 10.4161/cbt.5.8.2886. [DOI] [PubMed] [Google Scholar]
- 24.Guedan S, Rojas JJ, Gros A, Mercade E, Cascallo M, Alemany R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol Ther. 2010;18:1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Quintanilla J, He D, Wakimoto H, Alemany R, Shah K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol Ther. 2015;23:108–118. doi: 10.1038/mt.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia S, Lal B, Tung B, Wang S, Goodwin CR, Laterra J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro Oncol. 2016;18:507–517. doi: 10.1093/neuonc/nov171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabari J, Lax D, Connors D, Brotman I, Mindrebo E, Butler C, Entersz I, Jia D, Foty RA. Fibronectin matrix assembly suppresses dispersal of glioblastoma cells. PLoS One. 2011;6:e24810. doi: 10.1371/journal.pone.0024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon S, Vaca C, Jia D, Entersz I, Schaer A, Carcione J, Weaver M, Avidar Y, Pettit R, Nair M, Khan A, Foty RA. Dexamethasone-mediated activation of fibronectin matrix assembly reduces dispersal of primary human glioblastoma cells. PLoS One. 2015;10:e0135951. doi: 10.1371/journal.pone.0135951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf KJ, Shukla P, Springer K, Lee S, Coombes JD, Choy CJ, Kenny SJ, Xu K, Kumar S. A mode of cell adhesion and migration facilitated by CD44-dependent microtentacles. Proc Natl Acad Sci U S A. 2020;117:11432–11443. doi: 10.1073/pnas.1914294117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin EY, Cooper DD, Abbott KL, Lennon J, Nagaraja S, Mackay A, Jones C, Vogel H, Jackson PK, Monje M. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170:845–859. e819. doi: 10.1016/j.cell.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagliardi F, Narayanan A, Gallotti AL, Pieri V, Mazzoleni S, Cominelli M, Rezzola S, Corsini M, Brugnara G, Altabella L, Politi LS, Bacigaluppi M, Falini A, Castellano A, Ronca R, Poliani PL, Mortini P, Galli R. Enhanced SPARCL1 expression in cancer stem cells improves preclinical modeling of glioblastoma by promoting both tumor infiltration and angiogenesis. Neurobiol Dis. 2020;134:104705. doi: 10.1016/j.nbd.2019.104705. [DOI] [PubMed] [Google Scholar]
- 32.Sette P, Amankulor N, Li A, Marzulli M, Leronni D, Zhang M, Goins WF, Kaur B, Bolyard C, Cripe TP, Yu J, Chiocca EA, Glorioso JC, Grandi P. GBM-targeted oHSV armed with matrix metalloproteinase 9 enhances anti-tumor activity and animal survival. Mol Ther Oncolytics. 2019;15:214–222. doi: 10.1016/j.omto.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajesh Y, Biswas A, Kumar U, Das S, Banerjee I, Banik P, Bharti R, Nayak S, Ghosh SK, Mandal M. Targeting NFE2L2, a transcription factor upstream of MMP-2: a potential therapeutic strategy for temozolomide resistant glioblastoma. Biochem Pharmacol. 2019;164:1–16. doi: 10.1016/j.bcp.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Pencheva N, de Gooijer MC, Vis DJ, Wessels LFA, Wurdinger T, van Tellingen O, Bernards R. Identification of a druggable pathway controlling glioblastoma invasiveness. Cell Rep. 2017;20:48–60. doi: 10.1016/j.celrep.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Zhou T, Wang Z, Qi B, Xia H. HSP47 promotes glioblastoma stemlike cell survival by modulating tumor microenvironment extracellular matrix through TGF-beta pathway. ACS Chem Neurosci. 2017;8:128–134. doi: 10.1021/acschemneuro.6b00253. [DOI] [PubMed] [Google Scholar]
- 36.Jaime-Ramirez AC, Dmitrieva N, Yoo JY, Banasavadi-Siddegowda Y, Zhang J, Relation T, Bolyard C, Wojton J, Kaur B. Humanized chondroitinase ABC sensitizes glioblastoma cells to temozolomide. J Gene Med. 2017;19:10. doi: 10.1002/jgm.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajesh Y, Banerjee A, Pal I, Biswas A, Das S, Dey KK, Kapoor N, Ghosh AK, Mitra P, Mandal M. Delineation of crosstalk between HSP27 and MMP-2/MMP-9: a synergistic therapeutic avenue for glioblastoma management. Biochim Biophys Acta Gen Subj. 2019;1863:1196–1209. doi: 10.1016/j.bbagen.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Rajesh Y, Biswas A, Kumar U, Banerjee I, Das S, Maji S, Das SK, Emdad L, Cavenee WK, Mandal M, Fisher PB. Lumefantrine, an antimalarial drug, reverses radiation and temozolomide resistance in glioblastoma. Proc Natl Acad Sci U S A. 2020;117:12324–12331. doi: 10.1073/pnas.1921531117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gwak HS, Park MJ, Park IC, Woo SH, Jin HO, Rhee CH, Jung HW. Tetraarsenic oxide-induced inhibition of malignant glioma cell invasion in vitro via a decrease in matrix metalloproteinase secretion and protein kinase B phosphorylation. J Neurosurg. 2014;121:1483–1491. doi: 10.3171/2014.8.JNS131991. [DOI] [PubMed] [Google Scholar]
- 40.Costagliola di Polidoro A, Zambito G, Haeck J, Mezzanotte L, Lamfers M, Netti PA, Torino E. Theranostic design of angiopep-2 conjugated hyaluronic acid nanoparticles (Thera-ANG-cHANPs) for dual targeting and boosted imaging of glioma cells. Cancers (Basel) 2021;13:503. doi: 10.3390/cancers13030503. [DOI] [PMC free article] [PubMed] [Google Scholar]


