Abstract
Aims
Interleukin (IL)-1β is one of the major pathogenic regulators during the pathological development of intervertebral disc degeneration (IDD). However, effective treatment options for IDD are limited. Suramin is used to treat African sleeping sickness. This study aimed to investigate the pharmacological effects of suramin on mitigating IDD and to characterize the underlying mechanism.
Methods
Porcine nucleus pulposus (NP) cells were treated with vehicle, 10 ng/ml IL-1β, 10 μM suramin, or 10 μM suramin plus IL-1β. The expression levels of catabolic and anabolic proteins, proinflammatory cytokines, mitogen-activated protein kinase (MAPK), and nuclear factor (NF)-κB-related signalling molecules were assessed by Western blotting, quantitative real-time polymerase chain reaction (qRT-PCR), and immunofluorescence analysis. Flow cytometry was applied to detect apoptotic cells. The ex vivo effects of suramin were examined using IDD organ culture and differentiation was analyzed by Safranin O-Fast green and Alcian blue staining.
Results
Suramin inhibited IL-1β-induced apoptosis, downregulated matrix metalloproteinase (MMP)-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4, and ADAMTS-5, and upregulated collagen 2A (Col2a1) and aggrecan in IL-1β-treated NP cells. IL-1β-induced inflammation, assessed by IL-1β, IL-8, and tumour necrosis factor α (TNF-α) upregulation, was alleviated by suramin treatment. Suramin suppressed IL-1β-mediated proteoglycan depletion and the induction of MMP-3, ADAMTS-4, and pro-inflammatory gene expression in ex vivo experiments.
Conclusion
Suramin administration represents a novel and effectively therapeutic approach, which could potentially alleviate IDD by reducing extracellular matrix (ECM) deposition and inhibiting apoptosis and inflammatory responses in the NP cells.
Cite this article: Bone Joint Res 2021;10(8):498–513.
Keywords: Suramin, Intervertebral disc degeneration, NF-κB, Apoptosis, Inflammation, intervertebral disc degeneration, western blotting, apoptosis, cytokines, IL-8, aggrecan, MMP-13, matrix metalloproteinases-3, ADAMTS-5, ADAMTS-4
Article focus
This study aimed to investigate the effects of suramin on interleukin (IL)-1β-induced cell degeneration in porcine nucleus pulposus (NP) cells, and its underlying molecular mechanism.
Key messages
IL-1β treatment induced the upregulation of the catabolic regulators matrix metalloproteinase (MMP)-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and ADAMTS-5, and downregulation of the anabolic regulators aggrecan and type II collagen, contributing to the degradation of disc matrix.
However, suramin treatment antagonized the IL-1β-induced extracellular matrix destruction, and downregulation of aggrecan and type II collagen, resulting in a reversal of IL-1β mediated disc degeneration.
The attenuation of disc degeneration was associated with a decrease in expression of cartilage-degrading enzymes, apoptotic markers, and nuclear factor kappa B (NF-κB) p65 transcriptional activity in disc NP cells.
Strengths and limitations
Our study may provide a new therapeutic option for the prevention and reversal of IDD.
The effects of suramin on IDD were not tested in an in vivo animal model. Although the complete pharmacological effects of suramin remain unknown, the results of this study suggested that the administration of suramin could attenuate IDD progression.
Introduction
More than 70% of adults suffer low back pain (LBP) at a certain point in their lifetime and approximately 5% of the LBP population gradually becomes disabled, 1 making LBP a serious problem in global healthcare and causing a socioeconomic burden. The intervertebral disc degeneration (IDD) is a complex process and association of degeneration with LBP is not well understood. Several studies have revealed that ageing, genetics, and intervertebral disc (IVD) disruption may be pathologically associated with IDD, causing LBP. To date, no effective IDD treatments have been developed, and drug discovery for IDD therapy is an urgent necessity. The IVD is composed of three distinct morphological regions: the inner gelatinous nucleus pulposus (NP), which is encompassed by the peripheral fibrocartilaginous (AF), and the cartilaginous endplates (CEPs). The cells in NP secrete fibrillar collagens and the proteoglycan aggrecan that constituted intricate extracellular matrix. 2 Those macromolecular assemblages form a solid hydrodynamic system that bears compressive strength, is able to withstand physical stress, and provides biomechanical support to the spine. 3,4
The striking pathological features of IDD are caused by an apoptosis-induced decrease in cell numbers, the degradation of the extracellular matrix (ECM), and altered cell phenotype. Interleukin (IL)-1β and other pro-inflammatory cytokines, such as IL-6, IL-8, and tumour necrosis factor (TNF)-α, are able to promote disc degeneration. 5-8 Of those cytokines related to pathological signalling pathways, the IL-1β-mediated potential inflammatory pathway is as a potential inflammatory mechanism contributing to IDD, and the role of IL-1β has been examined in several ex vivo and in vitro studies. The stimulation of NP cells with IL-1β activates the IL1β receptor, which activates toll-like receptors (TLRs), such as TLR-2 and TLR-4, and results in the upregulation of IL-1β, IL-6, IL-8, IL-17, TNF-α, and cyclooxygenase (COX)-2 levels. 9,10 In addition, proteases, such as matrix metalloproteinase (MMP)-3, MMP-13, and the serine protease HTRA1, are secreted at higher levels following TLR activation. With the exception of IL-1 receptor 2 (IL-1R2), all members of the IL-1 cytokine family cytokines and their receptors, and the TLR family, contain a homologous intracellular TLR/IL-1 R (TIR) signalling domain, allowing these proteins to share similar functions. 11 Recently, translational studies have indicated that TLR2 activation leads to IVD degeneration. 12
The matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) are the major proteases for ECM degradation, and increase during pathological development of IDD. Proinflammatory cytokines, including IL-1β, promote ECM remodelling through enzymatic decomposition mediated by the MMP and ADAMTS family proteins. A previous study has reported that TNF-α and IL-1β upregulate ADAMTS-4 expression in NP cells through the activation of nuclear factor-kappa B (NF-κB) and MAPK signalling pathways. 13,14 Additionally, other studies have demonstrated that IL-1β treatment facilitates ADAMTS-5 synthesis in human NP cells by NF-κB signal pathway. 15 Both p38 MAPK and c-Jun N-terminal kinase (JNK) have been found to be activated during the pathological development of IDD in humans. In contrast, the disruption of these signalling pathways enables the evasion or reduction of the effects of IL-1β to MMP-3 and MMP-13 messenger RNA (mRNA) expression. 16 The upregulation of miR-126 was found to be involved in IL-1β-induced cell viability, migration, apoptosis, and inflammatory response in human chondrocytes. 17 The exposure of NP cells to IL-1β leads to apoptosis and morphological changes 18 that might be associated with an increase in oxidative stress. 19 Glutathione, a natural peptide, is a powerful antioxidant in cells, and glutathione administration to human NP cells was able to prevent IL-1β-induced apoptosis. 20 Li et al 21 also reported that TNF-α induces an inflammatory reaction in the NP cell, resulting in apoptosis mediated by the activation of the NF-κB signalling pathway. In contrast, the A20 (also known as tumour necrosis factor alpha-induced protein 3 (TNFAIP3)) is a ubiquitin-editing enzyme that restricts NF-κB signalling and subsequently prevents the occurrence of the senescence of NP cells induced by TNF-α. 22 Therefore, the mitogen-activated protein kinase (MAPK) signalling and the NF-κB-dependent signalling are thought to be involved in the development of IDD. In addition to catabolic role in enhancing extracellular matrix breakdown, IL-1β can interfere with aggrecan transcription and translation, further tipping the scales from anabolism to catabolism. 5,23 Since 1916, suramin designed as Bayer-205 or Germanin has been used to treat the human African trypanosomiasis (sleeping sickness), which is caused by the Trypanosoma brucei. 24 Other potential therapeutic effects of suramin have since been explored including antineoplastic, 25,26 anti-inflammatiory, 27 antioxidant, 28 and anti-angiogenic activities. 29 Several studies have documented that suramin is a multifaceted inhibitor able to abrogate multiple signalling pathways, and extensive relevant effects have been reported in clinical applications. 30-32 Suramin can effectively suppress purinergic receptors signalling including the P2 × 2, P2 × 3, P2 × 5, P2 × 7, and P2Y2 receptors, and interrupt WNT signalling. 33 Pharmacologically, suramin neutralizes various growth factors, including platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), epidermal growth factor, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b-FGF), and transforming growth factor-beta (TGF-β) to their receptors. As a consequence, those cellular responses, such as endothelial cell proliferation and migration, are no longer activated. 31,34 TGF-β signalling is important for the development and growth of IVD and can play a protective role during IVD tissue regeneration by upregulating ECM synthesis and suppressing ECM catabolism, inflammatory responses, and cell apoptosis. However, the overactivation of TGF-β signalling can also be damaging to IVD, and the inhibition of aberrant TGF-β signalling has been shown to delay IDD. 34 Other studies have reported that TGF-β might activate the β-catenin-mediated connective tissue growth factor (CTGF) secretory pathway in NP cells to induce IDD. 35 IGF-1 can upregulate anabolism in IVD cells, stimulating the production of proteoglycans (PGs) in the ECM. 36 The PDGF-BB isomer has been shown to abolish apoptosis and collagen 3 (Col3) production, significantly mitigating IDD. In contrast, an animal study by Osada et al 37 have shown that VEGF expression levels are significantly upregulated in degenerative discs, correlating with the degree of degeneration and the severity of LBP. Additionally, PDGF-BB abolishes apoptosis and Col3 matrix production, and significantly mitigates disc degeneration. However, one animal study has revealed that VEGF expression levels are significantly higher in degenerative discs and correlated with the degree of degeneration and the severity of LBP. 38 However, whether suramin affects the growth factor-regulated signalling pathways thought to mediate the pathological conditions underlying IDD remains unclear. Suramin could mitigate the MMPs levels, inhibit aggrecanases activity, and inactivate metalloproteinases tissue inhibitors of matrix metalloproteinases-3 (TIMP-3) and disrupt endocytosis induced by the activation of low-density lipoprotein receptor-related protein 1 (LRP1). 39 In this study, suramin was found to increase the ECM accumulation in cartilage in vitro and limit cartilage loss in an animal model. According to the experimental findings, the pharmacological effect of suramin in ameliorating IDD is speculated. However, the functional effect and underlying mechanism of suramin for the alleviation of IDD were speculated but remain unexplained. Thus, the aim of the present study was to further investigate the potential therapeutic effect of suramin for the prevention and reversal of IDD.
Methods
Reagents and antibodies
Suramin and cycloheximide were purchased from Sigma-Aldrich (USA). IL-1* was purchased from Sino Biological Incorporation (China). The RNA extraction kit reagent was purchased from Molecular Research centre (USA); LipofectAMINE 2000 reagent was from Invitrogen (USA); Annexin V-FITC apoptosis detection kit was obtained from Biovision (USA); TLR2-specific agonist Pam3CSK4 was obtained from Abcam (Pam; ab142085); TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, was obtained from Merck (Germany); and C29, a Toll-like receptor 2 (TLR2) inhibitor, was obtained from MedChemExpress (USA). Antibodies against ADAMTS-4, ADAMTS-5, aggrecan, MMP-3, MMP-13, p38, phospho-p38, TLR2, myeloid differentiation factor 88 (MyD88), extracellular signal-regulated kinase (ERK1/2), and β-Actin were purchased from Proteintech Group (USA). Antibody targeting phospho-JNK and JNK1/2/3 were purchased from MyBioSource.com. Antibodies against NF-kB (p65), p-p65 (ser536), cleaved poly-adenosine diphosphate (ADP)-ribose polymerase (PARP), and phospho-ERK were purchased from Cell Signaling Technology (USA). Anti-collagen type II (Col II) and collagen X antibody was obtained from Arigo biolaboratories Corp (Taiwan). Antibodies against IL-1b, IL-8, and TNF-α were from Boster biological technology (USA). Protease and phosphatase inhibitors were purchased from BIOTOOLS (Taiwan).
Isolation and culture of porcine NP cells
All animal experiments were approved by the Hospital Institutional Review Board (IRB). The lumbar spinal columns (L2-L6) of eight-week-old rats were dissected and lumbar discs were collected. The spinal discs from the same rats were pooled, and the NP tissues were collected separately. After the discs were separated, the attached ligament and connective tissue were removed under a dissecting microscope. The spine was isolated within two hours of death and the NP tissue was placed into an enzymatic solution (0.2% collagenase) (Sigma-Aldrich) for four hours at 37°C. The NP cells were collected and cultured in Dulbecco modified Eagle medium (DMEM)/F12 medium containing 10% fetal bovine serum ((FBS), Sigma-Aldrich) and antibiotics (1% penicillin/streptomycin) under standard conditions (37°C, 5% CO2, and 20% O2). When cell numbers up to 70% to 90% confluence, the cells were harvested by using 0.25% Trypsin-ethylenediaminetetraacetic acid (EDTA) (Gibco, Invitrogen). All experiments in this study used NP cells from the third passage.
MTT assay
The cytotoxic effects of suramin on NP cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. NP cells were seeded in 24-well plates (2 × 104 cells/well), and suramin added at various concentrations of 0, 1, 5, 10, 50, and 100 µM; (Sigma-Aldrich) combined with or without IL-1β (Sino Biological) were added to the wells. The plates were incubated for 72 hours, and then supplemented with 200 μl MTT solution (final concentration 2 mg/ml) and incubated for another four hours. After the supernatant was discarded, each well was added with 150 μl of dimethylsulfoxide (DMSO) was added to each well, and the plates were measured spectrophotometrically at 570 nm.
Western blot
The NP cells were washed three times with cold phosphate-buffered saline (PBS) and cell lysates were extracted on ice. The total protein concentration of different lysates was determined according to the Bio-Rad Bradford Protein Assays kit (ThermoFisher) using bovine serum albumin (BSA) as a standard. Equal amounts (40 μg protein per lane) of proteins were separated on analytical 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidenedifluoride membrane (PerkinElmer) by a semidry apparatus. Primary antibodies against the following proteins were used: cleaved form PARP, aggrecan, IL-1β, IL-8, TNF-α, p-ERK1/2, ERK2, p65, p-p65 (ser536), p-p38, p38, p-JNK1/2/3, JNK, TLR2, MyD88, and β-Actin were employed as primary antibodies. Immunoblot analysis was performed using secondary mouse or rabbit immunoglobulin G (IgG) antibody coupled with horseradish peroxidase and detected by the Millipore ECL kit (Thermo Fisher Scientific, UK). The densities of the immunoblots were determined using an image analysis system installed with a software BIO-ID (VilberLourmat, France).
RNA isolation and real time PCR
Total RNA was extracted using RNAzol; 2 μg of purified total RNA was reverse-transcribed by the Thermo Scientific Maxima First Strand cDNA Synthesis Kit (ThermoFisher, USA) according to the manufacturer’s instructions. Briefly, the solution was incubated at 65°C for five minutes, it was mixed with first-strand buffer, Dithiothreitol (DTT), and RNaseOUT in a final volume of 20 μl. Then, the solution was incubated at 42°C for 60 minutes and then at 70°C for 15 minutes to inactivate the reverse transcriptase activity. Real-time PCR was conducted using the SYBR Green PCR Master Mix (Qiagen, Germany) and was processed on a LightCycler PCR and detection system (Roche Diagnostics, Switzerland). Each reaction (20 μl) was run in duplicate and contained 1 μl of cDNA template along with the following primer sequences: col2a1, forward (ACTCCTGGCACGGATGGTC) and reverse (CTTTCTCACCAACATCGCCC); aggrecan, forward (CCCAACCAGCCTGACAACTT) and reverse (CCTTCTCGTGCCAGATCATCA); col10a1, forward (TGAACTTGGTTCATGGAGTGTTTTA) and reverse (TGCCTTGGTGTTGGATGGT); gapdh, forward (TCACGACCATGGAGAAGGCT) and reverse (CAGGAGGCATTGCTGATGATC); sox5, forward (GGCCAAGCAGCAGCAAGAACAG) and reverse (AGCTGAAGCCTGGAGGAAGGAG); sox6, forward (CAGCCCTGTCAGTCTGCCTAACA) and reverse (GCATCTTCCGAGCCTCCTGAATAGC); sox9, forward (GGCAATCCCAGGGTCCACCAAC) and reverse (TGGTCGAACTCGTTGACGTCGAAG); mmp13, forward (ACCCAGGAGCCCTCATGTTTCC) and reverse (CAGGGTTTCTCCTCGGAGACTG); IL-1β, forward (GTACATGGTTGCTGCCTGAA) and reverse (CTAGTGTGCCATGGTTTCCA); IL-6, forward (GGCAGAAAACAACCTGAACC) and reverse (GTGGTGGCTTTGTCTGGATT); IL-8, forward (TAGGACCAGAGCCAGGAAGA) and reverse (CAGTGGGGTCCACTCTCAAT); TNFa, forward (ACTGCACTTCGAGGTTATCG) and reverse (GCTGGTTGTCTTTCAGCTTC); and macrophage migration inhibitory-1 (MIF), forward (CGTGCGCCCTTTGCAGTCTG) and reverse (TGGCCGCGTTCATGTCGTAG). Cycling parameters were 95°C for 15 minutes to activate DNA polymerase, followed by 40 cycles of 95°C for 15 seconds, 60°C for 20 seconds, and 72°C for 30 seconds. Melting curves were generated at the end of the reaction. Threshold cycles (C t) for each gene tested were normalized to the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene value (ΔCt ) and every experimental sample was referred to its control (ΔΔCt ). Fold change values were expressed as 2−ΔΔCt .
Cell death analysis
Cell death was evaluated by flow cytometry after staining with fluorescein isothiocyanate (FITC)-conjugated Annexin V and 0.5 μg/ml propidium iodide (PI). An Annexin V-FITC apoptosis detection kit (Biovision, USA) was used to detect early apoptosis according to the manufacturer’s instructions, with slight modifications. The NP cells were harvested and washed twice with ice-cold PBS and resuspended in 100 μl of binding buffer. Annexin V (5 μl) and PI (10 μl) were added and the mixture was incubated for 15 minutes in the dark. Finally, 400 μl of binding buffer was added to the cells, the mixture was analyzed with a flow cytometer (FACSCalibur; BD Biosciences) and analyzed with CellQuest (BD Biosciences, USA) software. Results were represented as a percentage of the total cell count.
Transfection and luciferase reporter assay
Disc NP cells were subcultured in a 12-well plate for 24 hours before transfection at a density of 8 × 104 cells in 0.5 ml of fresh culture medium per well. They were then transfected with pNF-κB-luc (Promega, USA), which contains NF-κB binding motifs (GGGAATTTCC), by using Lipofectamine 2000. For use in transfection assay, plasmids were mixed with Lipofectamine 2000 reagent in 0.5 ml of Opti-MEM medium (Thermo Fisher) and then incubated at room temperature for 20 minutes. Cells were incubated in the mixture at 37°C in a humidified atmosphere of air/CO2 (19:1) for another 48 hours. Next, the cells were pretreated with suramin (0, 10 μM) for one hour and stimulated with IL-1β (10 ng/ml) for another two hours, then lysed for the measurement of luciferase activity as described previously by Huang et al. 30 The luciferase activity was determined and normalized with the amount of total protein. Values are means and standard deviations (SDs) for three determinations (p < 0.05, p < 0.05, and p < 0.001 compared with vehicle).
Pellet cultures
The cells were cultured as a 3D pellet using a method described by Kato et al. 40 Briefly, pellet cultures were prepared by adding 1 × 106 cells in 2 ml DMEM-F12 medium to 15 ml conical polypropylene (PE) centrifuge tubes. The cells were pelleted by centrifugation at 400 × g for five minutes at room temperature. The cell pellets were cultured in the same centrifuge tubes, with loosened lids, at 37°C and 5% humidified CO2. The pellets were also maintained in medium without (control) or with the addition of various dosages of suramin and IL-1β.
Total glycosaminoglycans quantification
Total sulfated glycosaminoglycans (GAG) content was determined in cell pellet and media samples by using 1,9-dimethylmethylene blue (DMMB; Polysciences). Chondroitin sulfate C from shark cartilage was used as a reference. Briefly, 100 μl of the digested sample was combined with 1 ml dimethylmethylene blue dye solution, and the absorbance was immediately measured at 656 nm. DNA was measured using Hoechst 33,258 dye (Thermo Fisher). Briefly, 10 μl of the digested sample was combined with 200 μl Hoechst dye solution (0.7 μg/ml). Fluorescence measurements were taken with an excitation wavelength of 340 nm and emission wavelength of 465 nm. A standard curve was generated using calf thymus DNA. The GAG content was normalized against the amount of DNA measured in each sample.
Ex vivo analysis using organ culture model
Ten eight-week-old male Sprague-Dawley rats (n = 10 spines, 20 discs) were euthanized and the total of 20 entire discs including the NP, annulus fibrosus, and vertebral endplates were dissected and harvested under sterile conditions for ex vivo organ culture. Briefly, the lumbar spine was approached through an abdominal midline incision, and further dissection was performed through the posterior abdominal wall to directly visualize the L3-L6 IVDs. The isolated discs showed no signs of degeneration (grade 0). The discs were cultured in DMEM/F-12 medium for seven days supplemented with 10% FBS, and 1% penicillin/streptomycin in the presence or absence of recombinant rat IL-1β (100 ng/ml) or 10 µM suramin. We have included an ARRIVE checklist to show that we have conformed to the ARRIVE guidelines.
Alcian blue staining
After three days in culture, the 3D cell pellets were rinsed with phosphate-buffered saline (PBS), fixed for 20 minutes in 4% paraformaldehyde, and rinsed again with PBS. Pellet cultures were stained overnight with Alcian blue (Sigma-Aldrich Chemie, Germany), pH 1.0, and photographed. Pellets were processed into 10 μm sections, which were then photographed.
Histological analysis
Ten of the spines from Sprague-Dawley rats were harvested. A total of 20 entire discs were divided into four groups. Group 1: control (n = 5), Group 2: disc incubated with IL-1β (n = 5), Group 3: disc incubated with IL-1β combined with suramin (n = 5), and Group 4: disc incubated with suramin (n = 5). After seven days, the harvested discs were fixed in 4% paraformaldehyde and then decalcified in EDTA. The decalcified discs were embedded in paraffin for sectioning. Serial mid-sagittal sections of discs (5 µm-thick) were obtained to prepare slides. Safranin O-Fast green was used to identify presence of proteoglycan matrix and Alcian blue to detect accumulation of GAG. For immunohistochemistry (IHC), paraffin sections were dewaxed in xylene and rehydrated in ethanol baths. Endogenous peroxidases were blocked by incubating the sections in 3% hydrogen peroxide for 30 minutes at room temperature. The sections were incubated with anti-IL-1β, IL-8, TNFα, MMP-3, and ADAMTS-4 primary antibody overnight at 4°C and then incubated with a biotinylated secondary antibody for ten minutes at room temperature. 3,3'-Diaminobenzidine (DAB) chromogen substrate was used to detect antibody binding and sections were counterstained with haematoxylin, dehydrated in xylene, and mounted. The staining intensity was expressed as the integral optical density (IOD), which was analyzed using Image-Pro Plus software (Version 5.1, Media Cybernetics, USA) with the same linear calibration for all sections.
Statistical analysis
A representative result is presented — each sample was independently tested at least three times. Significant differences were evaluated using the independent-samples t-test. All values are displayed as means and standard deviations (SDs) for three determinations (*p < 0.05; **p < 0.01; ***p < 0.001 compared with vehicle).
Results
Suramin counteracted the catabolic effect of IL-1β on proteoglycans accumulation in porcine NP cells
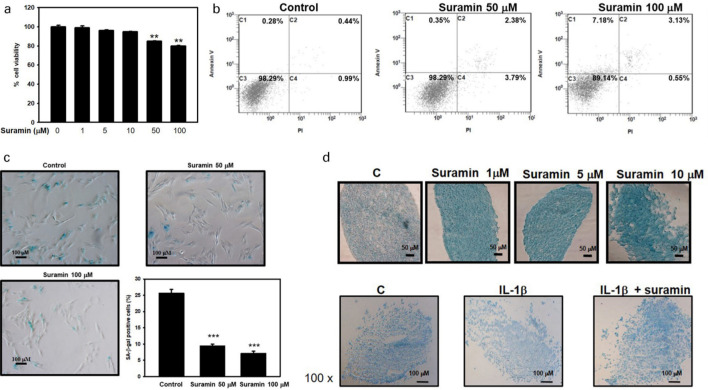
To access the toxic effect of suramin treatment in NP cells, the MTT assay was applied to determine cell survival rate. Treatment with suramin at concentrations under 10 µM appeared to be non-toxic to NP cells after 72 hours of exposure (Figure 1a). We further tested the effects of higher suramin concentrations to determine whether suramin treatment induced cell senescence or cell death. As noted in Figure 1b, suramin had a dose-dependent cytotoxic effect, as demonstrated by the flow cytometry evaluation of apoptosis according to Annexin V and PI staining (1.71% for control, 6.42% for 50 μM suramin, 10.86% for 100 μM suramin). We further investigated the cellular senescence using an acidic beta-galactosidase assay in the NP cells that underwent suramin administration. As displayed in Figure 1c, the proportion of X-gal-positive NP cells (25%) in the control cells was larger than those in NP cells treated with suramin (50 μM and 100 μM, 9.8% and 7.5%, respectively). Based on these results, 10 µM suramin was chosen as the appropriate dose for the following experiments.
Fig. 1.
Effect of suramin on cell viability and proteoglycan and glycosaminoglycan (GAG) expression in nucleus pulposus (NP) cells. a) The viability of suramin-treated NP cells, as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay at 72 hours (n = 3). b) NP cells were treated with or without suramin for 72 hours. The percentage of Annexin V/propidium iodide (PI)-positive cells was shown represented in the boxed region (n = 3). c) The NP cells were treated with various doses of suramin as indicated for 72 hours and the senescence-associated β-galactosidase (SA-β-gal) activity was detected. A representative photomicrograph of the SA-β-gal assay is shown. The percentages of β-galactosidase-positive cells (n each group were compared and are illustrated in the histogram (n = 6 in each experiment). d) Alcian blue staining was used to examine the effects of interleukin (IL)-1β and suramin on glycosaminoglycan expression in pellet culture of NP cells (n = 3). **p < 0.01, ***p < 0.001.
When NP cells with IL-1β were exposed to suramin, the suramin administration was able to increase proteoglycans synthesis (Figure 1d, upper panel). Next, we investigated the biological effects of suramin on suppressing IL-1β-induced ECM degeneration. As noted in Figure 1d, IL-1β inhibited glycosaminoglycan production but the suramin restored ECM production in the NP cells being exposed in IL-1β inhibition by Alcian blue staining (Figure 1d, lower panel). Our results showed that suramin inhibited the matrix catabolism in IL-1β-treated NP cells’ ECM.
Suramin alters the anabolic and catabolic gene expression induced by IL-1β
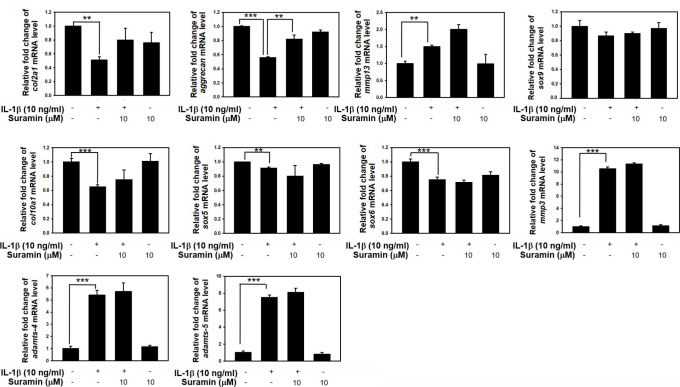
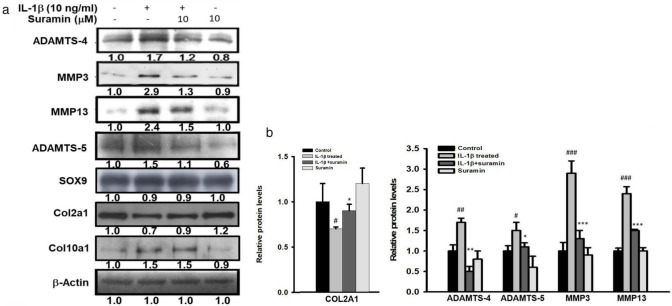
We further investigated the biological effect of suramin treatment on ECM anabolic and catabolic gene expression in the NP cells being exposed to IL-1β. The mRNA and protein levels of aggrecan, collagen 2A (Col2a1), Col10a1, SRY-Box Transcription Factor 5 (Sox5), Sox6, Sox9, MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 transcripts and protein expression levels were determined by using qRT-PCR and western blot analysis, respectively. The mRNA levels of Col2a1, aggrecan, Col10a1, Sox5, Sox6, and Sox9 were significantly reduced in cells exposed to IL-1β (Col2a1, control vs IL-1β-treated: 1 (SD 0.01) vs 0.5 (SD 0.12); aggrecan, control vs IL-1β-treated: 1 (SD 0.02) vs 0.55 (SD 0.03) fold; Col10a1, control vs IL-1β-treated: 1 (SD 0.01) vs 0.61 (SD 0.1); Sox5, control vs IL-1β-treated: 1 (SD 0.01) vs 0.9 (SD 0.01) fold; Sox6, control vs IL-1β-treated: 1 (SD 0.01) vs 0.78 (SD 0.09)). No changes were observed in the mRNA levels of Col2a1, Sox5, Sox6, and Sox9 following suramin treatment in NP cells exposed to IL-1β (Figure 2 and Figure 3a). In contrast, suramin treatment reversed the IL-1β-induced downregulation of aggrecan mRNA expression in NP cells (control vs IL-1β-treated vs IL-1β+ suramin: 1.0 (SD 0.1) vs 0.55 (SD 0.03) vs 0.81 (SD 0.1)) (Figure 3a), and almost completely rescued aggrecan protein expression level in the IL-1β-treated NP cells (control vs IL-1β-treated vs IL-1β+ suramin: 1.0 (SD 0.2) vs 0.4 (SD 0.05) vs 0.8 (SD 0.11)) (Figure 3c). On the other hand, the IL-1β treatment induced ADAMTS-4, ADAMTS-5, MMP-3, and MMP-13 protein expression levels in NP cells, the suramin appeared to effectively blunt IL-1β signalling in downregulation of ADAMTS-4, ADAMTS-5, MMP-3, and MMP-13 in NP cells (ADAMTS-4, IL-1β treated vs IL-1β+ suramin: 1.7 (SD 0.1) vs 1.1 (SD 0.12); ADAMTS-5, IL-1 treated vs IL-1β+ suramin: 1.5 (SD 0.2) vs 1.1 (SD 0.1); MMP-3, IL-1 treated vs IL-1β+ suramin: 2.9 (SD 0.3) vs 1.3 (SD 0.21); MMP-13, IL-1 treated vs IL-1β+ suramin: 2.4 (SD 0.17) vs 1.5 (SD 0.03)) (Figure 3c, Supplementary Figure a). These results clearly demonstrated the biological effects of suramin that can effectively turn down IL-1β signalling associated ECM catabolic proteases expression, and restore ECM components expressions.
Fig. 2.
Effect of suramin on interleukin (IL)-1β-induced anabolic and catabolic factors expression in nucleus pulposus (NP) cells. Sub-confluent NP cells were pretreated with 10 μM suramin for one hour, and then followed by treatment with 10 ng/ml IL-1β for 24 hours. Total RNA were extracted for real-time polymerase chain reaction analysis of collagen 2A (Col2a1), aggrecan, Col10a1, SRY-Box Transcription Factor 5 (Sox5), Sox6, Sox9, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4, ADAMTS-5, matrix metalloproteinase (MMP)-13, and MMP-3 messenger RNA (mRNA) expression. Data represent means and standard deviations (n = 3 to 5) (**p < 0.01, ***p < 0.001 compared with untreated control or IL-1β treated group).
Fig. 3.
Effect of suramin on interleukin (IL)-1β-induced anabolic and catabolic factors expression in nucleus pulposus (NP) cells. a) Western blotting of protein levels of matrix metalloproteinase (MMP)-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4, and ADAMTS-5 in NP cells treated with suramin combined with or without interleukin (IL)-1β (10 ng/ml) for 24 hours (n = 3 to 5). b) Semi-quantitative analysis of the protein levels of collagen 2A (COL2A1), ADAMTS-4, ADAMTS-5, MMP-3, and MMP-13. #p < 0.05, ##p < 0.01, ###p < 0.001 versus control group; *p < 0.05, **p < 0.01, ***p < 0.001 versus IL-1β group. SOX9, SRY-Box Transcription Factor 9.
Suramin protects against IL-1β-induced apoptosis in porcine NP cells
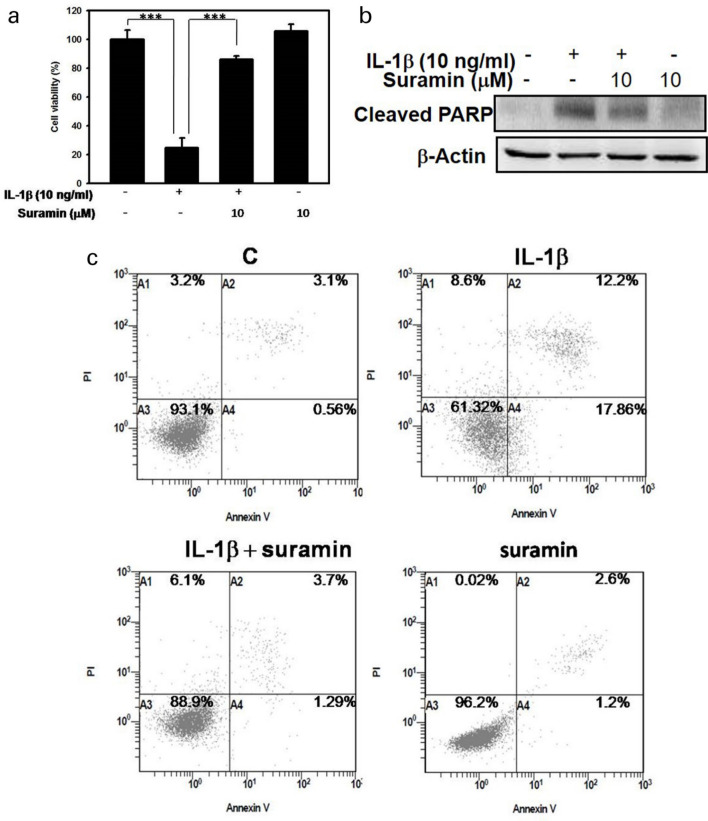
The pharmacological effects of suramin were assessed in terms of the prevention of IL-1β-induced irreversible cell injury and apoptosis. As noted in Figure 4, IL-1β exposure results in an approximately five-fold reduction in cell viability compared with the untreated group, and cell viability was rescued by suramin treatment (Figure 4a). In parallel, flow cytometric analysis by Annexin V/PI was performed to determine whether cell death was due to either necrosis or apoptosis. The administration of IL-1β (10 ng/ml) to NP cells for 72 hours markedly increased apoptosis (to about 38%) while suramin treatment significantly inhibited IL-1β-induced apoptosis (p = 0.049, independent-samples t-test), with only 11% of apoptotic cells detected (Figure 4c). To evaluate the cell-protective effects of suramin against IL-1β-induced apoptosis, activated PARP protein expression was examined by western blot analysis. As shown in Figure 4b, active PARP expression increased after IL-1β induction compared with untreated controls. In contrast, active PARP expression was reduced following suramin treatment. Our data suggested that suramin rescued NP cells from IL-1β-induced apoptosis.
Fig. 4.
Effect of suramin on interleukin (IL)-1β-induced apoptosis in nucleus pulposus (NP) cells. a) The NP cells were treated with 10 ng/ml IL-1β, 10 μM suramin, or suramin plus IL-1β for 72 hours and cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Values are expressed as the mean and standard deviation of triplicate measurements (***p < 0.001 compared with untreated control). b) Western blot was used to measure the protein levels of cleaved polyadenosine diphosphate-ribose polymerase (PARP) in NP cells treated with 10 ng/ml IL-1β, 10 µM suramin, or suramin plus IL-1β for 72 hours. c) NP cells were cultured in the absence or presence of IL-1β with or without the addition of suramin. The percentages of Annexin V/propidium iodide (PI)-positive cells are shown, represented in the boxed regions (n = 3). c, control.
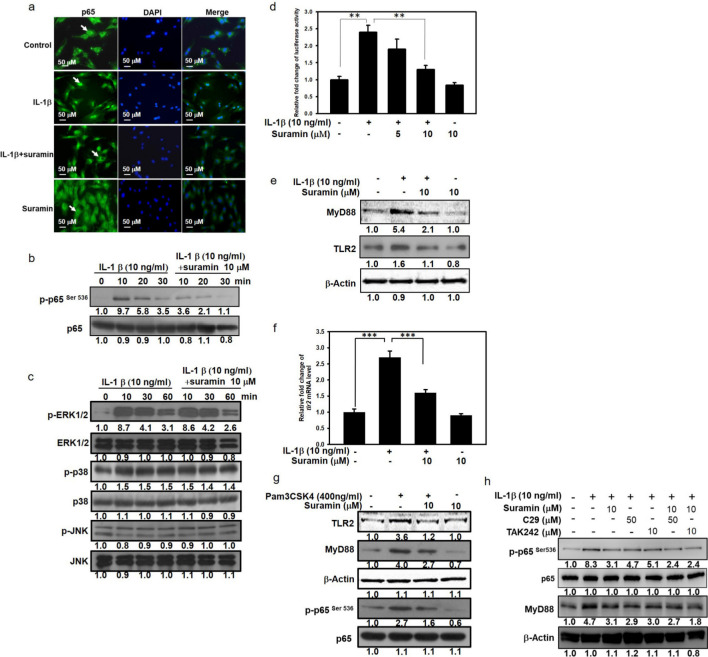
Suramin inhibits the IL-1β-induced activation of the NF-κB signalling pathway in porcine NP cells
To verify the molecular mechanism through which suramin disrupted IL-1β-induced pathological signalling in NP cells, we examined the MAPK and NF-κB signalling pathway, which are both known to be activated by IL-1β induction. In the NP cells, the IL-1β-induced phosphorylation of ERK and p38 started ten minutes after IL-1β stimulation, and this activated state was sustained for at least 60 minutes. In contrast, the JNK signalling pathway was not activated by IL-1β stimulation. Suramin was unable to attenuate the IL-1β-stimulated phosphorylation of ERK and p38 (Figure 5c). In contrast, suramin treatment was able to reduce the IL-1β-activated phosphorylation of NF-κB at ser536 (Figure 5b), suggesting that suramin interrupted the IL-1β-mediated activation of NF-κB signalling, but not MAPK signalling. Because the translocation of NF-κB into nucleus is a prominent characteristic of NF-κB activation, the effects of suramin on IL-1β-induced nuclear translocation of NF-κB were examined. In the NP cells without IL-1β treatment, NF-κB was predominantly distributed in the cytoplasm, and the IL-1β induction resulted in the translocation of the NF-κB to the nucleus. Instead of nuclear localization of NF-κB under IL-1β induction, the suramin exposure resulted in the absence of NF-κB in the nucleus (Figure 5a). To verify the biological effect of suramin on the suppression of the IL-1β-induced NF-κB activation, NF-κB-dependent transcription was examined using the luciferase activity assay. The IL-1β-stimulated luciferase-reporter activity was also reduced by suramin treatment in a dose-dependent manner (Figure 5d). In addition to the IL-1β-associated inflammatory reaction, IL-1β activates the toll-like receptor (TLR)-2/MyD88/NF-κB signalling cascade which drives the expression of inflammatory cytokines. 41 Thus, we examined the effects of suramin on the TLR2/MyD88 signalling pathway in IL-1β-treated NP cells. As shown in Figure 5f, compared with the protein expression levels of TLR2 and MyD88 in the control group, the protein expression level of TLR2 and MyD88 were upregulated in the IL-1β-treated NP cells; however, the IL-1β signalling to upregulation of TLR2 and MyD88 in NPCs was abolished after suramin administration. In contrast, the upregulation of TLR2 and MyD88 was abolished by suramin administration (Figure 5f). The IL-1β-mediated increase in TLR2 mRNA expression was also attenuated by suramin treatment (Figure 5f). To further corroborate the protective effects of suramin on the suppression of the TLR2/NF-κB signalling pathway in NP cells, we examined MyD88, a downstream effector of TLR2/NF-κB signalling, following the administration of the TLR2-specific agonist Pam3CSK4. As shown in Figure 5g, Pam3CSK4 effectively increased the expression levels of TLR2, MyD88, and p-p65 in NP cells compared with the levels in the control group. However, suramin treatment inhibited the Pam3CSK4-mediated upregulation of TLR2, MyD88, and p-p65 in NP cells. In addition, C29 (50 µM) and TAK 242 (10 µM), which are TLR2 and TLR4 inhibitors, respectively, were used to dissect the effects of suramin treatment on TLR2 and TLR4 signalling. Treatment with both C29 and TAK 242 markedly decreased the MyD88 and p-p65 expression levels compared with the levels in control cells (Figure 5h). Consistently, suramin administration had a similar effect, reducing MyD88 and p-p65 protein expression in NP cells treated with IL-1β. The inhibitory effects of suramin against the TLR2/NF-κB signalling pathway ameliorate IL-1β-induced NP cell degeneration.
Fig. 5.
Effect of suramin on interleukin (IL)-1β-induced mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-kB signalling pathway activation in nucleus pulposus (NP) cells. a) Cells were treated with or without 10 μM suramin for one hour before IL-1β treatment (10 ng/ml) for ten minutes. NF-kB localization was determined by immunofluorescence analysis. b) The NP cells were treated with 10 ng/ml IL-1β, 10 μM suramin, or suramin plus IL-1β for ten, 20, or 30 minutes. The total protein and phosphorylation levels of NF-κB in NP cells were detected using western blotting (n = 3 to 5). c) NP cells cultured in the absence or presence of IL-1β, with or without the addition of suramin for ten, 30, or 60 minutes. Cells were then collected and total protein extracted. The expressions of phosphorylated extracellular signal-regulated kinase (p-ERK), p-p38, phosphorylated c-Jun N-terminal kinase (p-JNK), ERK, p38, and JNK were detected by immunoblotting. Antibody to β-Actin was used as loading control (n = 3 to 5). d) Cells were transfected with NF-κB luciferase plasmid by using lipofection method as described in the Methods section. Cells were pretreated with different doses of suramin for 60 minutes, and then incubated with 10 ng/ml IL-1β for four hours. The luciferase activity was determined and normalized with the amount of total protein. Values are means and standard deviations of triplicate measurements (**p < 0.01 compared with untreated control or IL-β treated group). e) Sub-confluent cells were pretreated with 10 μM of suramin for one hour, and then followed by treatment with 10 ng/ml IL-1β for 24 hours. Total cell lysates were analyzed by immunoblot using anti-Toll-like receptor 2 (TLR2) and MyD88 antibody (n = 3). f) Cells were pretreated with 10 μM of suramin for one hour followed by treatment with 10 ng/ml IL-1β for 24 hours. Total RNA were extracted for real-time polymerase chain reaction analysis of TLR2 messenger RNA (mRNA) expression. The level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA verified loading of an equivalent amount of RNA (n = 5) (***p < 0.001 compared with untreated control or IL-β treated group). g) NP cells were pretreated with 10 µM of suramin for one hour, followed by treatment with 400 ng/ml Pam3CSK4 for 24 hours. Total cell lysates were analyzed by immunoblot using TLR2, p65, p-p65(ser536), and MyD88 antibodies (n = 3). h) NP cells were pretreated with 10 µM of suramin for one hour, followed by co-treatment with 400 ng/ml Pam3CSK4, 50 μM C29, or 10 μM transforming growth factor-beta-activated kinase for 24 hours. Total cell lysates were analyzed by immunoblot using TLR2, p65, p-p65(ser536), and MyD88 antibodies (n = 3). DAPI, 4′,6-diamidino-2-phenylindole.
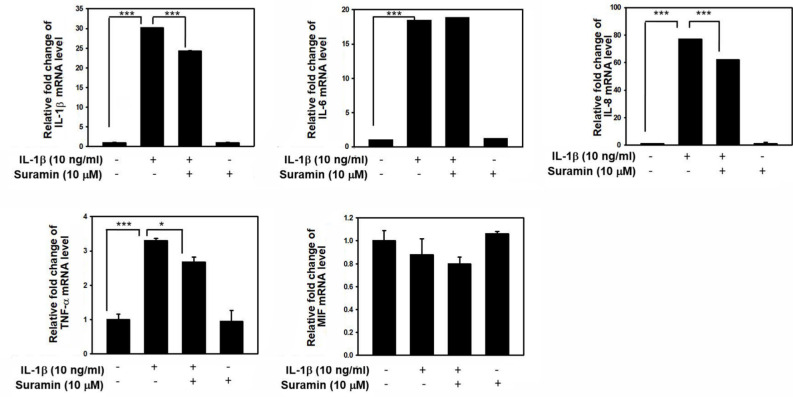
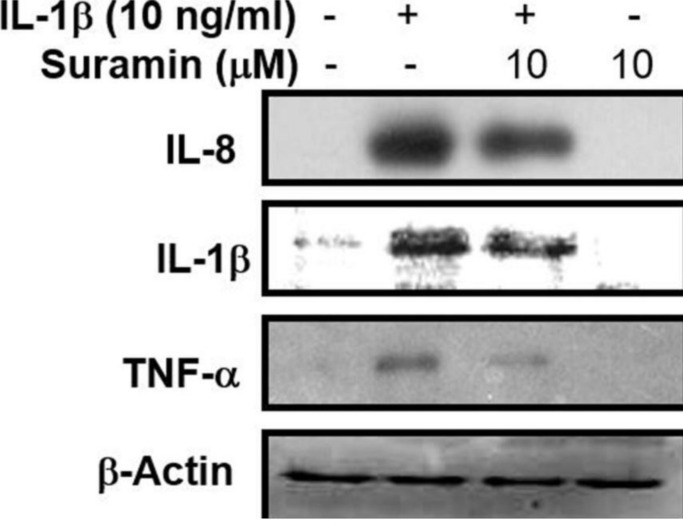
Suramin exerts an anti-inflammatory effect. An abnormal inflammatory reaction is a pathological feature observed during the pathological progression of IDD, characterized by an increase in proinflammatory cytokine secretion by NP and AF cells and the presence of infiltrating immune cells in the IVD. These inflammatory cytokines, especially TNF-α and IL-1β, can promote abnormal ECM remodelling through the downregulation of Col2 and aggrecan expression and the upregulation of Col1. 42-44 To validate the anti-inflammatory effects of suramin against IL-1β-mediated inflammatory reactions, as shown in Figure 6, the expression levels of IL-1β, IL-6, IL-8, and TNF-α were examined. These proinflammatory mediators were upregulated after IL-1β stimulation, whereas the expression levels of IL-1β, IL-8, and TNF-α were reduced by suramin administration. The western blotting analysis showed that suramin treatment could partially reduce the IL-1β-induced increase in IL-1β, IL-8, and TNF-α protein expression in NP cells (Figure 7). These data were consistent with the qRT-PCR results. Therefore, we suggested that suramin protected against IDD through the inhibition of inflammatory cytokine release by NP cells.
Fig. 6.
Effect of suramin on interleukin (IL)-1β-induced proinflammatory cytokines production. The nucleus pulposus (NP) cells were treated with or without 10 ng/ml IL-1β for 24 hours, following pretreatment with vehicle or suramin for 10 μM suramin for 24 hours. Total RNA were extracted for real-time polymerase chain reaction analysis of IL-1β, IL-6, IL-8, tumour necrosis factor alpha (TNF-α), and macrophage migration inhibitory-1 (MIF) messenger RNA (mRNA) expression. *p < 0.05, ***p < 0.001. All data are shown as the mean and standard deviation (n = 3 to 5), and were analyzed by independent-samples t-test.
Fig. 7.
Effect of suramin on interleukin (IL)-1β-induced proinflammatory cytokines production. Nucleus pulposus (NP) cells were treated with 10 ng/ml IL-1β for 24 hours, following pretreatment with vehicle or 10 μM suramin for one hour. The cell lysates were collected for western blot to measure the IL-1, IL-8, and tumour necrosis factor alpha (TNF-α), respectively. The expression of glyceraldehyde 3-phosphate dehydrogenase (gapdh) or β-actin was used as an internal control of real-time polymerase chain reaction or Western blot, respectively.
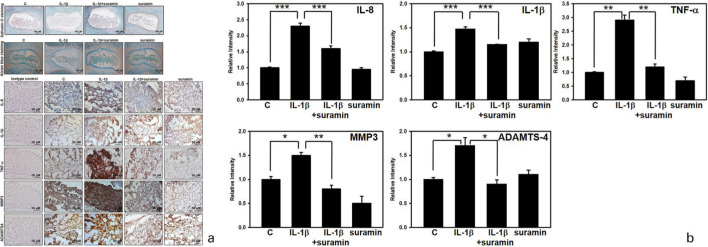
Suramin prevented NP degeneration in ex vivo disc organ culture
To determine the pharmacological effects of suramin on the NP cells, an ex vivo IVD organ culture was generated and the Safranin O-Fast green staining was used to evaluate the density of PGs in the discs. As noted in Figure 8a (upper panel), PGs content in the group treated with IL-1β was markedly reduced compared with that in the control group. However, PG expression was markedly increased by treatment with suramin. Furthermore, the alcian blue staining analysis revealed a deep staining pattern in the control group; however, in the IL-1β treatment groups, the blue staining faded. In the group co-incubated with IL-1β and suramin, the IVD maintained the loss of staining intensity induced by IL-1β, which indicated a significant improvement in the PG content (Figure 8a, middle panel). Next, an IHC analysis was used to investigate the matrix-degrading enzymes expressed in the NP tissues from the rat ex vivo model. Consistent with the in vitro results, immunostaining for IL-1β, TNF-α, IL-8, ADAMTS-4, and MMP-3 in the IL-1β-treated group was increased compared with that in the control group, whereas staining decreased in the suramin group (Figure 8a, lower panel, and Figure 8b). Thus, we speculated that the suramin-mediated inhibition of ECM destruction, which was associated with changes in protein expression and the blockade of IL-1β, TNF-α, and IL-8 release by NP cells, represents the molecular mechanism through which suramin effectively prevented IDD in the rat ex vivo model.
Fig. 8.
Effect of suramin on nucleus pulposus (NP) matrix degeneration in an ex vivo rat NP model. a) The rat discs were dissected and incubated with vehicle (n = 5) or interleukin (IL)-1β (100 ng/ml) (n = 5), combined with or without suramin for seven days. Harvested discs were fixed in 4% paraformalin, and then decalcified followed by paraffin embedment. Serial disc sections of exactly 5 μm thickness were prepared for slides, and Safranin O-fast green (upper panel) and alcian blue (middle panel) staining was performed. Immunocytochemical analysis of IL-1β, IL-8, tumour necrosis factor alpha (TNF-α), matrix metalloproteinase (MMP)-3, and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 in NP sections of discs (lower panel). b) Relative staining intensity in the disc NP matrix of mice that were treated with vehicle (n = 5) or IL-1β (100 ng/ml) (n = 5) combined with (n = 5) or without (n = 5) suramin for seven days. *p < 0.05, **p < 0.01, ***p < 0.001. c, control.
Discussion
The anti-inflammatory and anti-apoptotic pharmacological effects of suramin have been demonstrated in a variety of cell types. 45-47 In this study, we examined the therapeutic effects of suramin on IDD. Our in vitro experimental data suggested that NP cells induced towards pathological tendencies by IL-1β administration could be rescued by suramin treatment, including the downregulation of ECM-destructive proteins, such as ADAMTS-4, ADAMTS-5, MMP‐3, and MMP-13, and the upregulation of aggrecan (Figure 3c). Consequently, disc degeneration could be alleviated by suramin treatment. Several studies have documented that IL-1β signalling serves as the pathological pathway associated with IDD by increasing protease expression in NP cells, and that the inactivation of NF-κB signalling could reverse the IL-1β-mediated upregulation of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5. 48-50 In the present study, we demonstrated that suramin was able to disrupt the IL-1β-activated the NF-κB pathway in disc NP cells. Additionally, the IL-1β-induced protein expression of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 was also mitigated by co-treatment with suramin, although the mRNA levels of these components were not affected. These findings implied that suramin did not directly affect the transcriptional regulation of these tissue destruction-associated genes. The observed changes in protein expression levels might be regulated by certain post-transcriptional mechanisms, instead of increasing their transcripts.
We also proposed the possibility that catabolic proteins, including MMPs and ADAMTS, can be deposited outside of the cells. In particular, the western blot and real-time PCR can detect these proteins and transcripts in the cell lysate but cannot precisely confirm that these proteins are inside or outside of cells. Our results are consistent with documented literature that has shown that the suramin treatment results in ECM accumulation in chondrocytes without affecting the mRNA levels of MMP-13, ADAMTS-4, and MMP-9. 33 In other literature, suramin was able to inactivate heparanase, which functions in the degradation of extracellular matrix heparan sulfate proteoglycans (ECM HSPG). 51 Whether the enzymatic activities of TIMP-3 or heparanase in NP cells are inhibited by the suramin treatment remains unclear.
We demonstrated that suramin treatment markedly attenuated the IL-1β-induced upregulation of IL-1β, IL-8, and TNF-α transcription and translation in the NP cells (Figures 6 and 7). A variety of proinflammatory cytokines, such as IL-1β, IL-6, IL-8, and TNF-α, have been found to be significantly elevated in IDD. 52 These proinflammatory cytokines mediate ECM catabolism and trigger inflammation, finally resulting in IVD deterioration. 2 The suppression of proinflammatory cytokine production is one potential method for preventing or attenuating IDD. We have demonstrated that cytokine stimulation in NP cells promotes macrophage infiltration through the p38 MAPK signalling pathway activation of chemokine ligand 3 (CCL3) expression. 15 Infiltrated macrophage might progress severe inflammatory condition in the disc cells, by which the cells might progress apoptosis and disc may progress to degeneration. 53 Indeed, the activated macrophages can be categorized into two distinct phenotypes, namely classically activation M1 and the alternative activation M2. The macrophages with M1 activation produce a high level of proinflammatory factors such as IL-6, IL-12, and TNF-α, whereas the macrophages with M2 activation secreted the cytokines IL-10 and CCL18. The degenerated NP cells can produce TNF-α, IL-1β, and monocyte chemotactic protein-1 (MCP-1) to direct macrophage upon M1 actibation in IDD. 43 It has been reported that suramin blocks extracellular ATP-mediated autocrine signalling in the macrophages that underwent stimulation of lipopolysaccharide (LPS), and secrete cytokine (IL-1β) and chemokine (CCL-2). 54,55 Those results hint that suramin might regulate the innate immune response and switch the macrophage polarization in IDD. Besides, NF-κB signalling is evitable of regulating several proinflammatory cytokine genes expression. Without cytokine stimulation, NF-κB is sequestered in the cytoplasm by interaction of NF-κB-inhibitory protein, IκB with NF-κB. 56,57 Upon cytokine stimulation, IκB kinase (IKK) phosphorylates IκB, subjecting IκB to proteasomal degradation. 58 The unbound NF-κB translocates to the nucleus, where it actively regulates a wide spectrum of target gene expression. To gain insight into the molecular mechanisms underlying the anti-inflammatory effects of suramin in degenerating NP cells, we investigated the nuclear translocation of NF-κB. Our results showed that NP cells co-treated with suramin showed the substantial suppression of IL-1β-induced NF-κB nuclear translocation (Figure 5a). We then examined the phosphorylation of NF-κB at the IKK phosphorylation site serine 536, which is known to be required for the activation and nuclear translocation of NF-κB. 59 NF-κB phosphorylation at serine 536 increased in NP cells exposed to IL-1β and decreased when the cells were treated with suramin (Figure 5b). The protective anti-inflammatory effects of suramin against NP degeneration were partially due to the disruption of NF-κB signalling. In this study, suramin mitigated the IL-1β-induced transcription and translation of IL-1β, IL-8, and TNF-α. We further surveyed the transcription factor database (TRANSFAC) and identified several consensus sequences for NF-κB binding in the IL-1β, IL-8, and TNF-α promoters, suggesting that NF-κB might regulate the transcription of IL-1β, IL-8, and TNF-α.
Suramin treatment mitigated the pathological effects of IL-1β on cell death in NP cells (Figure 4). A variety of NP cells synergistically adapt their physiological activities to meet the physiological demands of IVDs; therefore, when cell death occurs in IVDs, the population-based harmony might be disrupted, resulting in IDD. 60,61 Mounting evidence has suggested that excessive mechanical stress, oxidative stress, and disordered cytokines can induce cell death in IVDs. 20,62 In this study, we demonstrated that suramin could downregulate proinflammatory cytokine production and abolish the NF-κB pathway (Figure 5). These data characterized the molecular mechanism through which suramin suppressed the pathological induction of apoptosis by IL-1β. In addition to inflammation, suramin also acts as a reactive oxygen species (ROS) scavenger and an inhibitor of nitric oxide, which is characterized in a variety of tissues, such as the joints, liver, spleen, and kidneys. 28 In addition, suramin has been shown to display antioxidative effects and increase cell survival in NP cells exposed to H2O2, which suffered from cell death through both intrinsic and extrinsic apoptotic pathways without suramin treatment. 63 Nitric oxide is the typical source of ROS in NP cells, generated by excessive mechanical force and the proinflammatory cytokine IL-1β. 64,65 In the present study, we also found that suramin inhibited IL-1β-induced ROS production in porcine NP cells. Intriguingly, the IL-1β-induced ROS production was not effectively eliminated by suramin treatment in porcine NP cells (data not shown).
Suramin is known to act as a potent blocker of the P2X and P2Y purinergic receptors, including P2X 2, P2X 3, P2X 5, P2X 7, and P2Y2. 66 Members of the P2X receptor family are largely expressed in the central nervous system and in the periphery, where they serve in essential physiological and pathophysiological roles associated with a variety of biological processes. For instance, neurophysiological studies have demonstrated that ATP can cause pain by directly improving neuronal excitability through the activation of the P2X 3 and P2X 2/3 receptors. 67 In addition, ATP activates glial cells in the central nervous system (e.g. microglia), corresponding to persistent nociceptive stimulation and resulting in inflammatory pain associated with various receptor-mediated signalling pathways, such as P2X 4, P2X 7, and P2Y12. 68,69 P2 receptor inhibitors (antagonists) have been shown to be beneficial in preclinical trials for the treatment of pathological pain. In this study, we found that suramin inhibits proinflammatory cytokine production. Taken together, these results suggested that the attenuation of neuropathic pain in the spinal cord mediated by suramin treatment might be associated with the suppression of inflammation. Similarly, suramin may act to mitigate inflammation in IDD. Notably, suramin blocks the interaction of several growth factors with their corresponding receptors, and abolishes their downstream signalling effects. The growth factors platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), and basic fibroblast growth factor (bFGF) regulate intense proliferative activities in both AF and NP cells. 70 PDGF, IGF-1, and bFGF also play regulatory roles in the differentiation of stem cells into NP cells. 71,72 VEGF is highly expressed in IDD and has been implicated in the pathological progression of IDD. 73 Determining whether the biological effects of suramin on IDD act through the interruption of these growth factor-induced signalling pathways requires untangling a complicated network, which we were unable to do with our limited experimental data; however, this remains an important issue that should be addressed in the future.
This study has several experimental limitations. We collected the NP cells from several lumbar spine segments of multiple animals, and the cells from different animals may feature biological variations between individuals that might offer identical data. Ageing and IDD might induce the disappearance of notochordal cells (NCs), 74 which are thought to be important cells necessary for the regeneration of degenerative discs. In this study, the younger discs (eight weeks) that we collected are unable to mimic true IDD condition, as the loss of NC cells in the younger disc is unlikely to occur. We cannot be certain based on our current data of whether suramin would have similar pharmacological effects on cells derived from older discs. AF cell apoptosis is also a key factor associated with IVD. Whether suramin has similar effects in AF and CEP cells as those observed in NP cells must be clarified in future research.
One previous study has reported the effects of suramin as a therapeutic agent for the treatment of various diseases. 26 Numerous early oncology applications of suramin used the continuous infusion of the drug to establish a target concentration. However, the initially reported half-life of suramin was two days. Therefore, maintenance doses would be necessary to achieve target concentrations more rapidly than a continuous infusion. 75 For example, suramin is administered at 1 g (reconstituted to 100 mg/ml = 77 µM), which is injected intravenously on days 1, 3, 7, 14, and 21, to treat trypanosomiasis. In other clinical trials, a continuous infusion method was used to target a suramin plasma concentration between 100 and 350 µg/ml. Subsequently, concentrations below 100 µg/ml were found to have no significant biological activity, whereas concentrations above 350 µg/ml resulted in significant neurotoxicity. 76 In a previous study, that intraperitoneal (IP) administration of suramin at a dose of 10 mg/kg for 20 days was able to protect against collagen-induced arthritis in mice. 28 Another study has reported that papain-induced knee joint damage can be treated by the intra-articular injection of 0.1 mg suramin in mice. 33 Therefore, further research is necessary to evaluate the utility of an intradiscal injection of suramin to prevent IDD in an animal model.
Author contributions
Z-M. Liu: Investigation, Formal analysis, Writing - original draft.
C-C. Lu: Writing - review & editing.
P-C. Shen: Conceptualization, Writing - review & editing.
S-H. Chou: Writing - review & editing.
C-L. Shih: Writing - review & editing.
J-C. Chen: Writing - review & editing.
Y. C. Tien: Conceptualization, Writing - original draft.
Funding statement
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 108-2314-B-037 -060-) (open access funding) and Kaohsiung Medical University Hospital (No. KMUH-107-7R52 and KMU-108-8M51). No benefits in any form have been recieved or will be recieved from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
All authors declare no conflicts of interest.
Ethical review statement
This study was approved by the Institutional Animal Care and Use Committees (IACUC) (KMU-104246).
Supplementary material
Two figures including the Western blots of protein levels and the ARRIVE checklist.
References
- 1. Blanquer SBG, Grijpma DW, Poot AA. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev. 2015;84:172–187. [DOI] [PubMed] [Google Scholar]
- 2. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feng H, Danfelter M, Stromqvist B, Heinegard D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88-A(Suppl 2):25–29. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed K, Shaw HV, Koval A, Katanaev VL. A second Wnt for old drugs: drug repositioning against WNT-Dependent cancers. Cancers. 2016;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology. 2008;47(6):809–814. [DOI] [PubMed] [Google Scholar]
- 7. Deng X, Zhao F, Kang B, Zhang X. Elevated interleukin-6 expression levels are associated with intervertebral disc degeneration. Exp Ther Med. 2016;11(4):1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwon W-K, Moon HJ, Kwon T-H, Park Y-K, Kim JH. Influence of rabbit notochordal cells on symptomatic intervertebral disc degeneration: anti-angiogenic capacity on human endothelial cell proliferation under hypoxia. Osteoarthritis Cartilage. 2017;25(10):1738–1746. [DOI] [PubMed] [Google Scholar]
- 9. Shah BS, Burt KG, Jacobsen T, et al. High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via Toll-like receptor 4-dependent pathway. J Orthop Res. 2019;37(1):220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krock E, Currie JB, Weber MH, Ouellet JA, Stone LS, Rosenzweig DH. Nerve growth factor is regulated by Toll-like receptor 2 in human intervertebral discs. J Biol Chem. 2016;291(7):3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sims JE. Il-1 and IL-18 receptors, and their extended family. Curr Opin Immunol. 2002;14(1):117–122. [DOI] [PubMed] [Google Scholar]
- 12. Krock E, Rosenzweig DH, Currie JB, Bisson DG, Ouellet JA, Haglund L. Toll-Like receptor activation induces degeneration of human intervertebral discs. Sci Rep. 2017;7(1):17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phillips KLE, Jordan-Mahy N, Nicklin MJH, Le Maitre CL. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013;72(11):1860–1867. [DOI] [PubMed] [Google Scholar]
- 14. Tian Y, Yuan W, Fujita N, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am J Pathol. 2013;182(6):2310–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286(46):39738–39749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniels J, Binch AAL, Le Maitre CL. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J Orthop Res. 2017;35(1):74–85. [DOI] [PubMed] [Google Scholar]
- 17. Yu CD, Miao WH, Zhang YY, Zou MJ, Yan XF. Inhibition of miR-126 protects chondrocytes from IL-1β induced inflammation via upregulation of Bcl-2. Bone Joint Res. 2018;7(6):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang C-C, Zhou J-S, Hu J-G, et al. Effects of IGF-1 on IL-1β-induced apoptosis in rabbit nucleus pulposus cells in vitro. Mol Med Rep. 2013;7(2):441–444. [DOI] [PubMed] [Google Scholar]
- 19. Wang F, Cai F, Shi R, Wang X-. H, Wu X-. T. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24(3):398–408. [DOI] [PubMed] [Google Scholar]
- 20. Yang D, Wang D, Shimer A, Shen FH, Li X, Yang X. Glutathione protects human nucleus pulposus cells from cell apoptosis and inhibition of matrix synthesis. Connect Tissue Res. 2014;55(2):132–139. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Cheng S, Wu Y, et al. Functional self-assembled peptide scaffold inhibits tumor necrosis factor-alpha-induced inflammation and apoptosis in nucleus pulposus cells by suppressing nuclear factor-κB signaling. J Biomed Mater Res A. 2018;106(4):1082–1091. [DOI] [PubMed] [Google Scholar]
- 22. Peng X, Zhang C, Bao JP, Zhu L, Shi R, Xie ZY. A20 of nucleus pulposus cells plays a self-protection role via the nuclear factor-kappa B pathway in the inflammatory microenvironment. Bone Joint Res. 2020;9(5):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Z, Yin Z, Liu C, Liang H, Jiang M, Tian J. IL-1β promotes ADAMTS enzyme-mediated aggrecan degradation through NF-κB in human intervertebral disc. J Orthop Surg Res. 2015;10:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voogd TE, Vansterkenburg EL, Wilting J, Janssen LH. Recent research on the biological activity of suramin. Pharmacol Rev. 1993;45(2):177–203. [PubMed] [Google Scholar]
- 25. Koval A, Ahmed K, Katanaev VL. Inhibition of Wnt signalling and breast tumour growth by the multi-purpose drug suramin through suppression of heterotrimeric G proteins and Wnt endocytosis. Biochem J. 2016;473(4):371–381. [DOI] [PubMed] [Google Scholar]
- 26. Kakuguchi W, Nomura T, Kitamura T, Otsuguro S, Matsushita K, Sakaitani M. Suramin, screened from an Approved drug library, inhibits HuR functions and attenuates malignant phenotype of oral cancer cells. Cancer Med. 2018;7(12):6269–6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGeary RP, Bennett AJ, Tran QB, Cosgrove KL, Ross BP. Suramin: clinical uses and structure-activity relationships. Mini Rev Med Chem. 2008;8(13):1384–1394. [DOI] [PubMed] [Google Scholar]
- 28. Sahu D, Sharma S, Singla RK, Panda AK. Antioxidant activity and protective effect of suramin against oxidative stress in collagen induced arthritis. Eur J Pharm Sci. 2017;101(9):125–139. [DOI] [PubMed] [Google Scholar]
- 29. Veeranarayanan S, Poulose AC, Sheikh Mohamed M, et al. Fitc/suramin harboring silica nanoformulations for cellular and embryonic imaging/anti-angiogenic theranostics. Journal of Materials chemistry B. 2015;3(41). [DOI] [PubMed] [Google Scholar]
- 30. Huang H-S, Liu Z-M, Ding L, et al. Opposite effect of ERK1/2 and JNK on p53-independent p21waf1/cip1 activation involved in the arsenic trioxide-induced human epidermoid carcinoma a431 cellular cytotoxicity. Journal of Biomedical Science. 2005;13(1). [DOI] [PubMed] [Google Scholar]
- 31. Takano S, Gately S, Neville ME, Herblin WF, Gross JL, Engelhard H. Suramin, an anticancer and angiosuppressive agent, inhibits endothelial cell binding of basic fibroblast growth factor, migration, proliferation, and induction of urokinase-type plasminogen activator. Cancer Res. 1994;54(10):2654–2660. [PubMed] [Google Scholar]
- 32. Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/AKT pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61(6). [PubMed] [Google Scholar]
- 33. Guns LA, Monteagudo S, Kvasnytsia M, Kerckhofs G, Vandooren J, Opdenakker G. Suramin increases cartilage proteoglycan accumulation in vitro and protects against joint damage triggered by papain injection in mouse knees in vivo. RMD Open. 2017;3(2):e000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beindl W, Mitterauer T, Hohenegger M, Ijzerman AP, Nanoff C, Freissmuth M. Inhibition of receptor/G protein coupling by suramin analogues. Mol Pharmacol. 1996;50(2):415–423. [PubMed] [Google Scholar]
- 35. Chen S, Liu S, Ma K, Zhao L, Lin H, Shao Z. TGF-β signaling in intervertebral disc health and disease. Osteoarthritis Cartilage. 2019;27(8):1109–1117. [DOI] [PubMed] [Google Scholar]
- 36. Wu Q, Mathers C, Wang EW, Sheng S, Wenkert D, Huang JH. Tgf-Beta initiates beta-catenin-mediated CTGF secretory pathway in old bovine nucleus pulposus cells: a potential mechanism for intervertebral disc degeneration. JBMR Plus. 2019;3(2):e10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Osada R, Ohshima H, Ishihara H, et al. Autocrine/Paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14(5):690–699. [DOI] [PubMed] [Google Scholar]
- 38. Binch ALA, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16(5):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chanalaris A, Doherty C, Marsden BD, et al. Suramin inhibits osteoarthritic cartilage degradation by increasing extracellular levels of chondroprotective tissue inhibitor of metalloproteinases 3. Mol Pharmacol. 2017;92(4):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kato Y, Iwamoto M, Koike T, Suzuki F, Takano Y. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors. Proc Natl Acad Sci U S A. 1988;85(24):9552–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang H, Tian W, Wang S, et al. Tsg-6 secreted by bone marrow mesenchymal stem cells attenuates intervertebral disc degeneration by inhibiting the TLR2/NF-κB signaling pathway. Laboratory Invest. 2018;98(6):755–772. [DOI] [PubMed] [Google Scholar]
- 42. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9(4):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li P, Gan Y, Xu Y, Song L, Wang L, Ouyang B. The inflammatory cytokine TNF-alpha promotes the premature senescence of rat nucleus pulposus cells via the PI3K/Akt signaling pathway. Scientific Rep. 2017;7:42938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang A, Wang J, Wu J, Deng X, Zou Y. Suramin protects hepatocytes from LPS-induced apoptosis by regulating mitochondrial stress and inactivating the JNK-Mst1 signaling pathway. J Physiol Sci. 2019;69(3):489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dupre TV, Doll MA, Shah PP, et al. Suramin protects from cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2016;310(3):F248–F258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alyoussef A. Suramin attenuated inflammation and reversed skin tissue damage in experimentally induced atopic dermatitis in mice. Inflamm Allergy Drug Targets. 2015;13(6):406–410. [DOI] [PubMed] [Google Scholar]
- 48. Bai X, Guo A, Li Y. Protective effects of calcitonin on IL-1 stimulated chondrocytes by regulating MMPs/TIMP-1 ratio via suppression of p50-NF-κB pathway. Biosci Biotechnol Biochem. 2019;83(4):598–604. [DOI] [PubMed] [Google Scholar]
- 49. Zhang L, Chen Q, Wang H, Yang J, Sheng S. Andrographolide mitigates IL‑1β‑induced human nucleus pulposus cells degeneration through the TLR4/MyD88/NF‑κB signaling pathway. Mol Med Rep. 2018;18(6):5427–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tu J, Li W, Zhang Y, Wu X, Song Y, Kang L. Simvastatin inhibits IL-1beta-induced apoptosis and extracellular matrix degradation by suppressing the NF-kB and MAPK pathways in nucleus pulposus cells. Inflammation. 2017;40(3):725–734. [DOI] [PubMed] [Google Scholar]
- 51. Marchetti D, Reiland J, Erwin B, Roy M. Inhibition of heparanase activity and heparanase-induced angiogenesis by suramin analogues. Int J Cancer. 2003;104(2):167–174. [DOI] [PubMed] [Google Scholar]
- 52. Lee S, Moon CS, Sul D, Lee J, Bae M, Hong Y. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clinical Biochem. 2009;42(15):1504–1511. [DOI] [PubMed] [Google Scholar]
- 53. Yang H, Liu B, Liu Y, He D, Xing Y, An Y. Secreted factors from intervertebral disc cells and infiltrating macrophages promote degenerated intervertebral disc catabolism. Spine. 2019;44(9):E520–E9. [DOI] [PubMed] [Google Scholar]
- 54. Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PloS one. 2013;8(4):e59778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren H, Teng Y, Tan B, Zhang X, Jiang W, Liu M. Toll-Like receptor-triggered calcium mobilization protects mice against bacterial infection through extracellular ATP release. Infect Immun. 2014;82(12):5076–5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12(8):695–708. [DOI] [PubMed] [Google Scholar]
- 57. Smale ST. Hierarchies of NF-kappaB target-gene regulation. Nat Immunol. 2011;12(8):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shirane M, Hatakeyama S, Hattori K, Nakayama K, Nakayama K. Common pathway for the ubiquitination of IkappaBalpha, IkappaBbeta, and IkappaBepsilon mediated by the F-box protein FWD1. J Biol Chem. 1999;274(40):28169–28174. [DOI] [PubMed] [Google Scholar]
- 59. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–96. [DOI] [PubMed] [Google Scholar]
- 60. Jiang L, Zhang X, Zheng X, et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: implications for diabetic intervertebral disc degeneration. J Orthop Res. 2013;31(5):692–702. [DOI] [PubMed] [Google Scholar]
- 61. Zhao C-Q, Jiang L-S, Dai L-Y. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11(12):2079–2088. [DOI] [PubMed] [Google Scholar]
- 62. Zhang C-C, Cui G-P, Hu J-G, et al. Effects of adenoviral vector expressing hIGF-1 on apoptosis in nucleus pulposus cells in vitro. Int J Mol Med. 2014;33(2):401–405. [DOI] [PubMed] [Google Scholar]
- 63. Kim KW, KY H, Lee JS, Rhyu KW, HS A, Woo YK. The apoptotic effects of oxidative stress and antiapoptotic effects of caspase inhibitors on rat notochordal cells. Spine. 2007;32(22):2443–2448. [DOI] [PubMed] [Google Scholar]
- 64. Liu GZ, Ishihara H, Osada R, Kimura T, Tsuji H. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine. 2001;26(2):134–141. [DOI] [PubMed] [Google Scholar]
- 65. Rannou F, Richette P, Benallaoua M, et al. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90(1):148–157. [DOI] [PubMed] [Google Scholar]
- 66. Abbracchio MP, Burnstock G, Boeynaems JM, et al. International Union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33(1):48–57. [DOI] [PubMed] [Google Scholar]
- 68. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. [DOI] [PubMed] [Google Scholar]
- 69. Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. Embo J. 2010;29(14):2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pratsinis H, Kletsas D. Organotypic cultures of intervertebral disc cells: responses to growth factors and signaling pathways involved. Biomed Res Int. 2015;2015:427138–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ehlicke F, Freimark D, Heil B, Dorresteijn A, Czermak P. Intervertebral disc regeneration: influence of growth factors on differentiation of human mesenchymal stem cells (hMSC). Int J Artif Organs. 2010;33(4):244–252. [PubMed] [Google Scholar]
- 72. Tao Y, Zhou X, Liang C, Li H, Han B, Li F. Tgf-Beta3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling. Growth factors. 2015;33(5-6):326–336. [DOI] [PubMed] [Google Scholar]
- 73. Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Creemers LB. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015;17:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yurube T, Hirata H, Kakutani K, et al. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther. 2014;16(1):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Collins JM, Klecker RW, Yarchoan R, et al. Clinical pharmacokinetics of suramin in patients with HTLV-III/LAV infection. J Clin Pharmacol. 1986;26(1):22–26. [DOI] [PubMed] [Google Scholar]
- 76. Stein CA, LaRocca RV, Thomas R, McAtee N, Myers CE. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989;7(4):499–508. [DOI] [PubMed] [Google Scholar]