Abstract
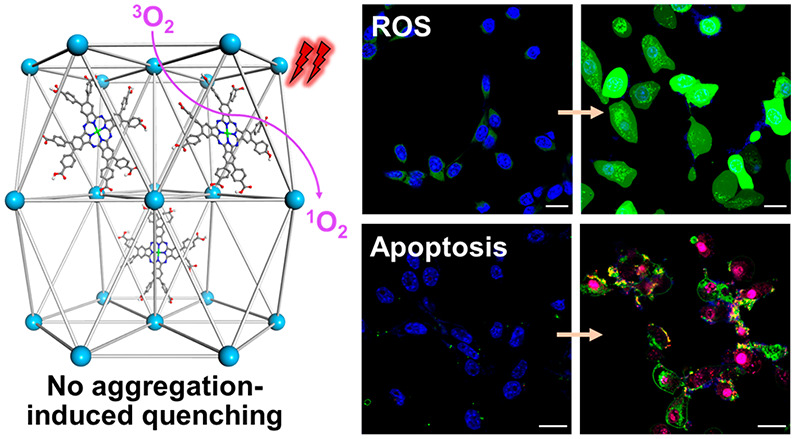
The performance of photodynamic therapy (PDT) depends on the solubility, pharmacokinetic behaviors, and photophysical properties of photosensitizers (PSs). However, highly conjugated PSs with strong reactive oxygen species (ROS) generation efficiency tend to have poor solubility and aggregate in aqueous environments, leading to suboptimal PDT performance. Here, we report a new strategy to load highly conjugated but poorly soluble zinc-phthalocyanine (ZnP) PSs in the pores of a Hf12-QC (QC = 2″,3′-dinitro-[1,1’:4′,1”;4″,1’”-quaterphenyl]-4,4’”-dicarboxylate) nanoscale metal–organic framework to afford ZnP@Hf-QC with spatially confined ZnP PSs. ZnP@Hf-QC avoids aggregation-induced quenching of ZnP excited states to significantly enhance ROS generation upon light irradiation. With higher cellular uptake, enhanced ROS generation, and better biocompatibility, ZnP@Hf-QC mediated PDT exhibited an IC50 of 0.14 μM and achieved exceptional antitumor efficacy with >99% tumor growth inhibition and 80% cure rates on two murine colon cancer models.
Photodynamic therapy (PDT) destroys a malignant tumor while sparing surrounding normal tissues by localizing a photosensitizer (PS) in the tumor and irradiating the tumor with visible or near-infrared light to produce cytotoxic reactive oxygen species (ROS).1−4 The clinical utility of PDT is limited by tissue penetration of light, localization of the PS in the tumor, and the solubility and photophysical properties of the PS.5,6 For example, clinically used porphyrin-based PSs often cause phototoxicity side effects due to their strong absorption in the visible spectrum and retention in the skin.7 Phthalocyanine (Pc) PSs present a promising alternative due to their very strong absorption in 650–800 nm and weak absorption in 400–600 nm, allowing for effective treatment of tumors with low PS doses and reduced phototoxicity.8 Metalation of Pcs with diamagnetic ions (e.g., Zn2+, Si4+, Al3+) increases triplet state yields and lifetimes to enhance the generation of cytotoxic singlet oxygen (1O2).9−11
Despite their improved photophysical properties, Pcs have not been widely used for PDT due to their limited synthetic accessibility and their strong tendency to aggregate in biological media.11 Pcs have been functionalized with ionic or hydrophilic groups in their peripheral positions to increase aqueous solubility or coordinate with bulky metal complexes (axial functionalization) to prevent π–π stacking.12 However, the introduction of ionic or hydrophilic groups to Pcs can adversely impact their cellular uptake while axial functionalization of Pcs is limited to a few nontoxic high-valent metals such as Si4+.13,14
An alternative strategy to address the solubility and aggregation issues of Pcs is through their encapsulation in or conjugation to liposomes, micelles, or other nanoparticles (NPs).15−19 In particular, micelles have been widely investigated as a delivery vehicle for lipophilic conjugated Pcs with superb photophysical properties.20,21 Nanoscale metal–organic frameworks (nMOFs) have recently provided an excellent strategy to deliver porphyrin, chlorin, and bacteriochlorin PSs for PDT.22−28 With structural tunability, rigidity, and porosity, nMOFs can efficiently load PSs via direct incorporation as bridging ligands, postsynthetic ligand exchange, postsynthetic surface modification, and physical loading into pores.29−35 These strategies allow isolation or confinement of lipophilic PSs in rigid nMOF structures to reduce aggregation, improve cellular uptake, and reduce photodegradation.36−41 We hypothesized that nMOFs could also be used to encapsulate Pcs to enhance their PDT efficacy.
Herein, we report the design of a Hf-QC nMOF based on Hf12 secondary building units (SBUs) and QC bridging ligands (QC = 2″,3′-dinitro-[1,1’:4′,1”;4″,1’”-quaterphenyl]-4,4’”-dicarboxylate) for the delivery of zinc(II)-2,3,9,10,16,17,23,24-octa(4-carboxyphenyl)-phthalocyanine (ZnP) PSs for highly efficient type II PDT (Figure 1).42 Postsynthetic loading of ZnP into the pores of the rigid Hf-QC framework afforded ZnP@Hf-QC. The confined PSs in ZnP@Hf-QC efficiently absorbed light and avoided aggregation-induced quenching to significantly enhance 1O2 generation and effectively eradicated/regressed colorectal cancer in mouse models.
Figure 1.
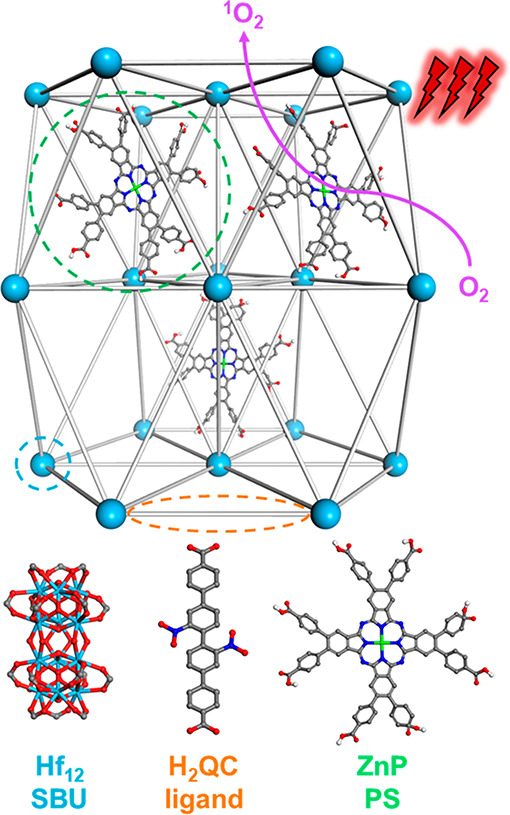
Scheme showing the structure of ZnP@Hf-QC consisting of a 3D framework of Hf12 SBUs and QC bridging ligands and ZnP PSs confined in the pores. ZnP@Hf-QC efficiently generates 1O2 upon 700 nm light irradiation.
Hf-QC was synthesized through a solvothermal reaction between HfCl4 and H2QC in a mixture of N,N-dimethylformamide (DMF), acetic acid, and water at 80 °C (Figure 2a). Transmission electron microscopy (TEM) imaging of Hf-QC revealed a hexagonal nanoplate morphology with a diameter of ∼150 nm while atomic force microscopy (AFM) showed a plate thickness of ∼64 nm (Figure 2b,d). Dynamic light scattering (DLS) measurements of Hf-QC gave a number-averaged size of 167.1 ± 2.9 nm (Figure 3c). Powder X-ray diffraction (PXRD) studies (Figure 3b) showed that Hf-QC adopted the same hcp topology as previously reported Zr12-QPDC (QPDC = para-quaterphenyldicarboxylate).43 High resolution TEM (HRTEM) imaging and fast Fourier transform (FFT) pattern of Hf-QC revealed a lattice point distance of 2.3 nm and displayed a 6-fold symmetry (Figure 2c, Figure S15), which matched well with the modeled structure for Zr12-QPDC. 1H nuclear magnetic resonance (NMR) analysis of digested Hf-QC showed an acetate (OAc) modulator to QC linker ratio of 0.11:1, corresponding to approximately 0.5 missing linkers per SBU (Figure S11). Thermogravimetric analysis (TGA) of Hf-QC showed a weight loss of 39.4% in the 300–800 °C region, matching the expected value of 37.9% for the Hf-QC with a 0.5 linker defect per SBU (Figure S14). On the basis of these results, Hf-QC was formulated as Hf12(μ3-O)8(μ3–OH)8(μ2–OH)6(QC)8.5(OAc).
Figure 2.
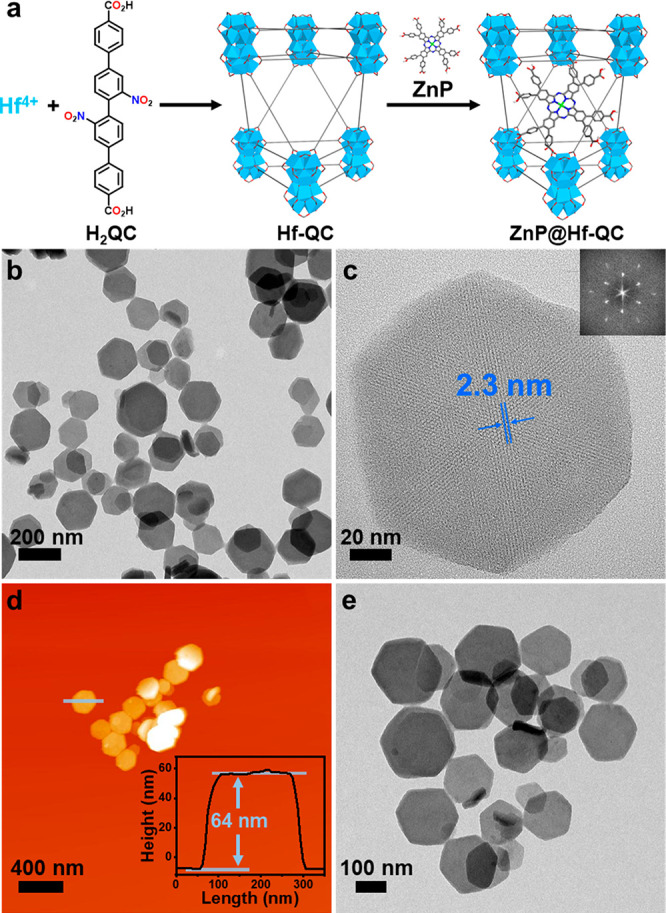
(a) Synthetic scheme of ZnP@Hf-QC. (b) TEM image and (c) HRTEM image and its FFT pattern (inset) of Hf-QC. (d) AFM topography and height profile (inset) of Hf-QC. (e) TEM image of ZnP@Hf-QC.
Figure 3.
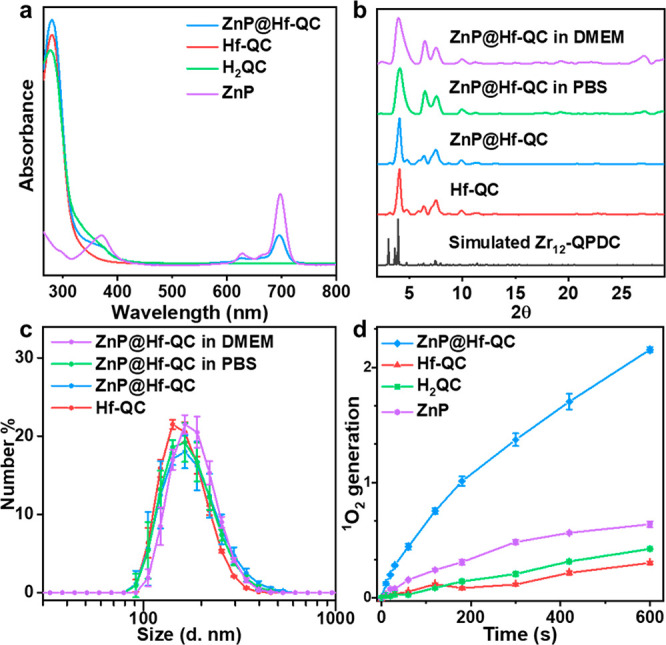
(a) UV–vis spectra of ZnP, H2QC, Hf-QC, and ZnP@Hf-QC in DMSO. (b) PXRD patterns of Hf-QC, ZnP@Hf-QC (as synthesized and after soaking in PBS or DMEM for 24 h), and the simulated pattern for Zr12-QPDC. (c) Number-averaged diameters of Hf-QC and ZnP@Hf-QC (as synthesized and after soaking in PBS or DMEM for 24 h) in ethanol. (d) 1O2 generation of ZnP, H2QC, Hf-QC, and ZnP@Hf-QC detected by SOSG assay (n = 3).
ZnP@Hf-QC was synthesized by heating a mixture of ZnP and Hf-QC in DMF at 70 °C for 24 h. Loading of ZnP in ZnP@Hf-QC was confirmed by the presence of characteristic Ultraviolet–visible (UV–vis) and infrared (IR) peaks for ZnP (Figure 3a, Figure S18). UV–vis spectroscopy and inductively coupled plasma-mass spectrometry (ICP-MS) showed the loading of 13.6 wt % ZnP in ZnP@Hf-QC, corresponding to a ZnP to Hf12 SBU ratio of 0.68:1. 1H NMR analysis of digested ZnP@Hf-QC showed that the OAc modulator to QC linker ratio was maintained after ZnP loading (Figure S19). TGA of ZnP@Hf-QC showed a weight loss of 36.3% in the 300–800 °C region, which matched well with the expected value of 34.1% for physical loading of ZnP in the nMOF pores and confirmed the ratio of ZnP to Hf (Figure S14). On the basis of these results, ZnP@Hf-QC was formulated as (ZnP)0.68@Hf12(μ3-O)8(μ3–OH)8(μ2–OH)6(QC)8.5(OAc).
TEM and DLS showed that ZnP@Hf-QC retained the hexagonal nanoplate morphology and size (175.8 ± 5.6 nm) of Hf-QC (Figures 2e and 3c, Figure S15). HRTEM images (Figure S15) and PXRD patterns (Figure 3b) of ZnP@Hf-QC supported the maintenance of Hf-QC structure after ZnP loading. ZnP@Hf-QC displayed a slightly more negative ζ potential of −24.0 ± 1.5 mV compared to Hf-QC at −22.1 ± 0.7 mV (Figure S16), consistent with loading negatively charged ZnP into the pores of Hf-QC. The presence of QC and ZnP was confirmed by its characteristic UV–vis and 1H NMR signals in digested ZnP@Hf-QC (Figures S11, S19). The stability of ZnP@Hf-QC was demonstrated by PXRD and DLS after incubation in phosphate-buffered saline (PBS) or Dulbecco’s Modified Eagle Medium (DMEM) at 37 °C for 24 h (Figure 3b,c).
ZnP@Hf-QC showed a much higher cellular uptake than free ZnP and accumulated in endo/lysosomes. Confocal laser scanning microscopy (CLSM) revealed that fluorescence signals of ZnP@Hf-QC started to overlap with endo/lysosomes in CT26 cells after incubation for 12 h (Figure 4b–f, Figure S23).44 However, fluorescence signals were barely observed for CT26 cells incubated with free ZnP (Figure 4a, Figure S22). Quantification of cellular uptake by UV–vis spectroscopy showed that ZnP@Hf-QC delivered up to15-fold more ZnP than free ZnP in vitro (Figure 5d).
Figure 4.
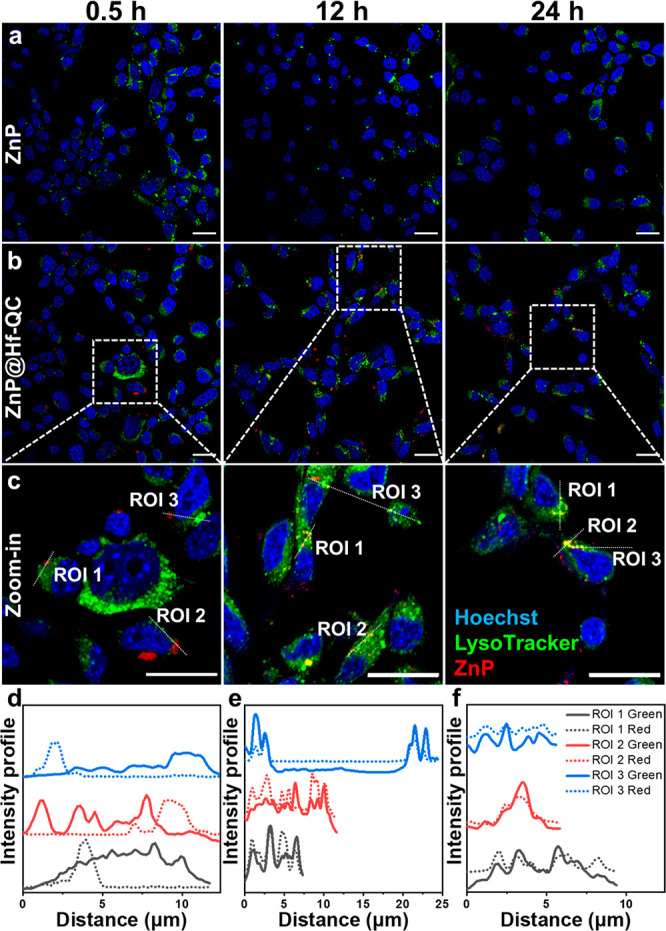
CLSM images showing colocalization of ZnP (a) and ZnP@Hf-QC (b, c) with endo/lysosomes after incubation with CT26 cells for 0.5, 12, and 24 h (yellow = green + red, scale bars are 20 μm). (d–f) Colocalization analysis between endo/lysosomes (green) and ZnP (red) in different ROIs (white dashed lines in Figure 4c).
Figure 5.
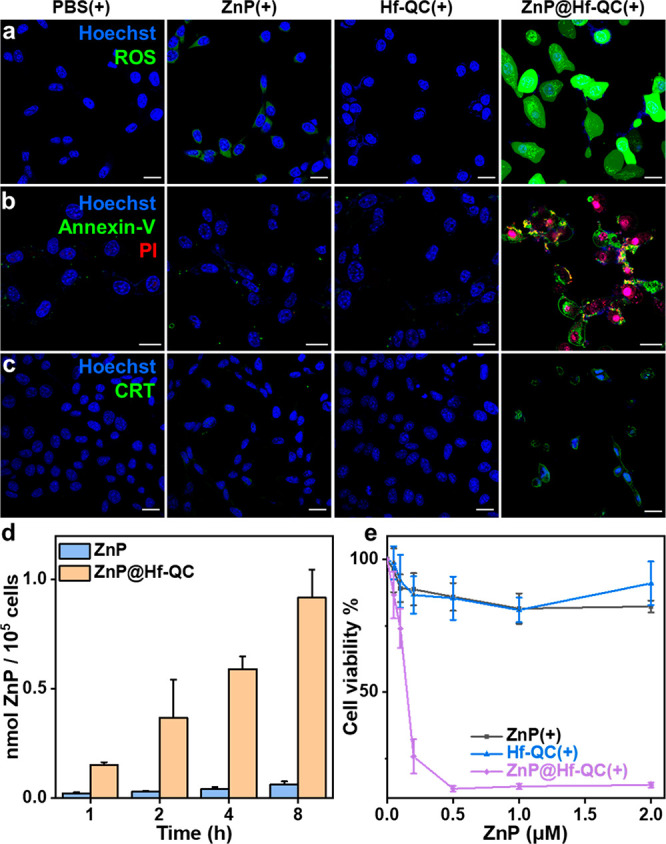
(a) Total ROS generation by DCF-DA assay (green). (b) Cell apoptosis stained by Alexa Fluor 488 Annexin V (green) and PI (red) (pink = red + blue). (c) CRT translocation stained by Alexa Fluor 488 CRT antibody (green) (d) Cellular uptake measured by UV–vis spectroscopy, n = 3. (e) Viability of cells treated with ZnP(+), Hf-QC(+), and ZnP@Hf-QC(+) by MTS assay. Cell nuclei were stained by Hoechst 33342 (blue) in (a-c). CT26 cells were used for all in vitro experiments and a total light dose of 60 J/cm2 was given. All scale bars equal 20 μm.
1O2 generation by ZnP and ZnP@Hf-QC was determined by singlet oxygen sensor green (SOSG) assay. ZnP@Hf-QC generated 3.4-fold as much 1O2 as free ZnP (Figure 3d), indicating that the entrapment of ZnP PSs in MOF pores prevented aggregation-induced quenching of ZnP excited states and enhanced 1O2 generation in a type II PDT process. CLSM imaging (Figure 5a, Figure S29) and flow cytometry analysis (Figure S28) showed a ROS burst in CT26 cells incubated with ZnP@Hf-QC after light irradiation (denoted as “+”, 100 mW/cm2, 10 min, “-” denotes no light treatment) by 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) assay, confirming the enhanced ROS generation by ZnP@Hf-QC in vitro. MTS assays showed that ZnP(+) exhibited minimal toxicity at concentrations up to 2 μM, while ZnP@Hf-QC(+) was highly cytotoxic with an IC50 of 0.14 μM (Figure 5e, Figure S24). No obvious toxicity or morphological changes were observed for CT26 cells treated with Hf-QC(−), Hf-QC(+), or ZnP@Hf-QC(−). Live cell imaging confirmed significant growth inhibition of CT26 cells by ZnP@Hf-QC(+) (Figure S25, Movies S1, S2).
We then examined apoptosis and immunogenic cell death of CT26 cells after PDT by CLSM and flow cytometry. CT26 cells treated with ZnP@Hf-QC(+) showed upregulation of phosphatidylserine by Annexin V staining on cell membranes and colocalization of propidium iodine (PI) and Hoechst 33342 (Figure 5b, Figures S26, S27). These results indicated apoptosis and compromised membrane functions for ZnP@Hf-QC(+) treated CT26 cells, which were absent in control groups. Calreticulin (CRT) staining revealed enhanced immunogenic cell death and surface translocation of CRT signals in ZnP@Hf-QC(+) group (Figure 5c, Figures S30, S31). Taken together, ZnP@Hf-QC(+) not only killed cancer cells more effectively but also induced immunogenic cell death to expose tumor antigens and danger signals for immune activation.45,46
We evaluated antitumor efficacy of ZnP@Hf-QC(+) on two subcutaneous murine colon cancer models with CT26 tumors on BALB/c mice and MC38 tumors on C57BL/6 mice. Hf-QC and ZnP@Hf-QC were pegylated before intravenous administration. The mice were injected with PBS, ZnP, Hf-QC, or ZnP@Hf-QC via tail veins at an equivalent ZnP dose of 50 nmol (equivalent Hf dose of 0.88 μmol). Twelve hours post injection, the mice were anesthetized, and tumor areas were irradiated with 700 nm LED with a total light flux of 60 J/cm2 (100 mW/cm2).
Compared to PBS(+), Hf-QC(+) had little effect on tumor growth with minimal tumor growth inhibition indices (TGIs) of 17.8% and 7.4% for CT26 and MC38 tumors, respectively. ZnP(+) moderately slowed tumor growth with TGI values of 41.3% and 41.4% for CT26 and MC38 tumors, respectively. ZnP@Hf-QC(+) treatment showed excellent antitumor efficacy with >99% TGIs and 80% cure rates for both CT26 and MC38 tumors (Figure 6a,b, Figures S32, S33, S38, S39). H&E and TUNEL staining revealed severe apoptosis/necrosis and infiltration of inflammatory cells in tumor regions in the ZnP@Hf-QC(+) group (Figure 6c, Figure S43). Several mice in the ZnP(+) and ZnP(−) groups showed weight loss, pulmonary edema, and local liver inflammation (Figures S35, S36), likely caused by aggregation of ZnP into large particles in vivo. In comparison, although ZnP@Hf-QC were observed to accumulate in spleens and livers similar to other nanoparticles47,48 (Figures S36, S37) by tumor tissue sections, mice treated with ZnP@Hf-QC with or without light irradiation showed steady body weights (Figures S41, S42). ZnP@Hf-QC and its aggregate were not observed in lungs and minimal abnormities were observed in the major organs of ZnP@Hf-QC treated mice compared to PBS control (Figures S34, S40). The different in vivo behaviors between ZnP and ZnP@Hf-QC showed that the nMOF pore loading strategy provides an efficient, safe, and biocompatible approach to deliver PSs with unfavorable solubility and pharmacokinetic properties.
Figure 6.
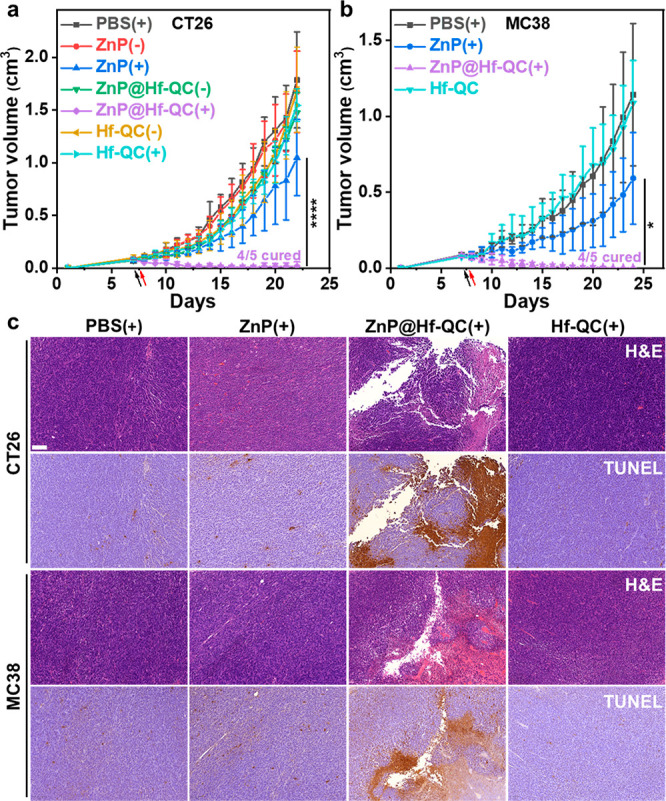
Antitumor efficacy of ZnP@Hf-QC(+) on subcutaneous CT26 tumor-bearing BALB/c (a) and MC38 tumor-bearing C57BL/6 (b) mouse models, n = 5. (c) Representative images of H&E staining and TUNEL IHC staining of excised CT26 and MC38 tumors. Scale bars are 100 μm, *p < 0.05 and ****p < 0.0001 by ANOVA with Tukey’s tests.
In summary, we developed an nMOF confinement strategy to isolate ZnP PSs and prevent their aggregation and excited state quenching. As a result, the isolated PSs in ZnP@Hf-QC efficiently absorbed light to significantly enhance 1O2 generation and efficiently kill cancer cells. ZnP@Hf-QC mediated PDT effectively eradicated/regressed colorectal cancers in two mouse models. The confinement of photosensitizers in nMOF pores provides a new strategy to unleash the potential of poorly soluble, highly conjugated PSs in PDT.
Acknowledgments
We thank Jianming Mao, Michael Kaufmann, and Yingjie Fan for experimental help. We also thank Dr. Christine Labno and Shirley Bond for help with confocal imaging and whole slide scanning, Dr. Shihong Li for help with histology study, and Dr. Carman Ka Man Ip for help with live cell imaging. We acknowledge the National Cancer Institute (U01-CA198989 and 1R01CA253655) and the University of Chicago Medicine Comprehensive Cancer Center (NIH CCSG: P30 CA014599) for funding support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c07379.
Author Contributions
$ T.L. and G.N. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Lovell J. F.; Liu T. W. B.; Chen J.; Zheng G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110 (5), 2839–2857. 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- Li X.; Lovell J. F.; Yoon J.; Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17 (11), 657–674. 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Liu J.; Fan J.; Chao H.; Peng X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021, 50 (6), 4185–4219. 10.1039/D0CS00173B. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Ni K.; Deng H.; Chen X. Dancing with reactive oxygen species generation and elimination in nanotheranostics for disease treatment. Adv. Drug Delivery Rev. 2020, 158, 73–90. 10.1016/j.addr.2020.06.006. [DOI] [PubMed] [Google Scholar]
- Lim C.-K.; Heo J.; Shin S.; Jeong K.; Seo Y. H.; Jang W.-D.; Park C. R.; Park S. Y.; Kim S.; Kwon I. C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334 (2), 176–187. 10.1016/j.canlet.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Mallidi S.; Anbil S.; Bulin A.-L.; Obaid G.; Ichikawa M.; Hasan T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6 (13), 2458–2487. 10.7150/thno.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96 (3), 506–516. 10.1111/php.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detty M. R.; Gibson S. L.; Wagner S. J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47 (16), 3897–3915. 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- Machacek M.; Cidlina A.; Novakova V.; Svec J.; Rudolf E.; Miletin M.; Kučera R.; Simunek T.; Zimcik P. Far-Red-Absorbing Cationic Phthalocyanine Photosensitizers: Synthesis and Evaluation of the Photodynamic Anticancer Activity and the Mode of Cell Death Induction. J. Med. Chem. 2015, 58 (4), 1736–1749. 10.1021/jm5014852. [DOI] [PubMed] [Google Scholar]
- Kobayashi N.; Ogata H.; Nonaka N.; Luk’yanets E. A. Effect of Peripheral Substitution on the Electronic Absorption and Fluorescence Spectra of Metal-Free and Zinc Phthalocyanines. Chem. - Eur. J. 2003, 9 (20), 5123–5134. 10.1002/chem.200304834. [DOI] [PubMed] [Google Scholar]
- Lo P.-C.; Rodríguez-Morgade M. S.; Pandey R. K.; Ng D. K. P.; Torres T.; Dumoulin F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49 (4), 1041–1056. 10.1039/C9CS00129H. [DOI] [PubMed] [Google Scholar]
- Dumoulin F.; Durmuş M.; Ahsen V.; Nyokong T. Synthetic pathways to water-soluble phthalocyanines and close analogs. Coord. Chem. Rev. 2010, 254 (23), 2792–2847. 10.1016/j.ccr.2010.05.002. [DOI] [Google Scholar]
- Roguin L. P.; Chiarante N.; García Vior M. C.; Marino J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. 10.1016/j.biocel.2019.105575. [DOI] [PubMed] [Google Scholar]
- Kollar J.; Machacek M.; Halaskova M.; Lenco J.; Kucera R.; Demuth J.; Rohlickova M.; Hasonova K.; Miletin M.; Novakova V.; Zimcik P. Cationic Versus Anionic Phthalocyanines for Photodynamic Therapy: What a Difference the Charge Makes. J. Med. Chem. 2020, 63 (14), 7616–7632. 10.1021/acs.jmedchem.0c00481. [DOI] [PubMed] [Google Scholar]
- Macháček M.; Carter K. A.; Kostelanský F.; Miranda D.; Seffouh A.; Ortega J.; Šimunek T.; Zimčík P.; Lovell J. F. Binding of an amphiphilic phthalocyanine to pre-formed liposomes confers light-triggered cargo release. J. Mater. Chem. B 2018, 6 (44), 7298–7305. 10.1039/C8TB01602J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Carter K. A.; Lovell J. F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. 10.1016/j.biomaterials.2019.119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nostrum C. F. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv. Drug Delivery Rev. 2004, 56 (1), 9–16. 10.1016/j.addr.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Setaro F.; Wennink J. W. H.; Mäkinen P. I.; Holappa L.; Trohopoulos P. N.; Ylä-Herttuala S.; van Nostrum C. F.; de la Escosura A.; Torres T. Amphiphilic phthalocyanines in polymeric micelles: a supramolecular approach toward efficient third-generation photosensitizers. J. Mater. Chem. B 2020, 8 (2), 282–289. 10.1039/C9TB02014D. [DOI] [PubMed] [Google Scholar]
- Lucky S. S.; Soo K. C.; Zhang Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115 (4), 1990–2042. 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- Gao D.; Lo P.-C. Polymeric micelles encapsulating pH-responsive doxorubicin prodrug and glutathione-activated zinc(II) phthalocyanine for combined chemotherapy and photodynamic therapy. J. Controlled Release 2018, 282, 46–61. 10.1016/j.jconrel.2018.04.030. [DOI] [PubMed] [Google Scholar]
- Yu W.; Ye M.; Zhu J.; Wang Y.; Liang C.; Tang J.; Tao H.; Shen Y. Zinc phthalocyanine encapsulated in polymer micelles as a potent photosensitizer for the photodynamic therapy of osteosarcoma. Nanomedicine 2018, 14 (4), 1099–1110. 10.1016/j.nano.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Lismont M.; Dreesen L.; Wuttke S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27 (14), 1606314. 10.1002/adfm.201606314. [DOI] [Google Scholar]
- Zeng J.-Y.; Zou M.-Z.; Zhang M.; Wang X.-S.; Zeng X.; Cong H.; Zhang X.-Z. π-Extended Benzoporphyrin-Based Metal–Organic Framework for Inhibition of Tumor Metastasis. ACS Nano 2018, 12 (5), 4630–4640. 10.1021/acsnano.8b01186. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Wang L.; Pei Q.; He S.; Liu S.; Xie Z. Metal–Organic Framework@Porous Organic Polymer Nanocomposite for Photodynamic Therapy. Chem. Mater. 2017, 29 (5), 2374–2381. 10.1021/acs.chemmater.7b00228. [DOI] [Google Scholar]
- Park J.; Jiang Q.; Feng D.; Mao L.; Zhou H.-C. Size-Controlled Synthesis of Porphyrinic Metal–Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138 (10), 3518–3525. 10.1021/jacs.6b00007. [DOI] [PubMed] [Google Scholar]
- Song Y.; Wang L.; Xie Z. Metal–Organic Frameworks for Photodynamic Therapy: Emerging Synergistic Cancer Therapy. Biotechnol. J. 2021, 16 (2), 1900382. 10.1002/biot.201900382. [DOI] [PubMed] [Google Scholar]
- Gao P.; Chen Y.; Pan W.; Li N.; Liu Z.; Tang B. Antitumor Agents Based on Metal–Organic Frameworks. Angew. Chem., Int. Ed. 2021, 60 (31), 16763–16776. 10.1002/anie.202102574. [DOI] [PubMed] [Google Scholar]
- Wan X.; Zhong H.; Pan W.; Li Y.; Chen Y.; Li N.; Tang B. Programmed Release of Dihydroartemisinin for Synergistic Cancer Therapy Using a CaCO3 Mineralized Metal–Organic Framework. Angew. Chem., Int. Ed. 2019, 58 (40), 14134–14139. 10.1002/anie.201907388. [DOI] [PubMed] [Google Scholar]
- Xu W.; Tu B.; Liu Q.; Shu Y.; Liang C.-C.; Diercks C. S.; Yaghi O. M.; Zhang Y.-B.; Deng H.; Li Q. Anisotropic reticular chemistry. Nat. Rev. Mater. 2020, 5 (10), 764–779. 10.1038/s41578-020-0225-x. [DOI] [Google Scholar]
- Helal A.; Yamani Z. H.; Cordova K. E.; Yaghi O. M. Multivariate metal-organic frameworks. Natl. Sci. 2017, 4 (3), 296–298. 10.1093/nsr/nwx013. [DOI] [Google Scholar]
- Della Rocca J.; Liu D.; Lin W. Nanoscale Metal–Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44 (10), 957–968. 10.1021/ar200028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G.; Ni K.; Lin W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. 10.1016/j.ccr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F.; Mao D.; Kenry; Wang Y.; Wu W.; Zhao D.; Kong D.; Liu B. Metal–Organic Framework as a Simple and General Inert Nanocarrier for Photosensitizers to Implement Activatable Photodynamic Therapy. Adv. Funct. Mater. 2018, 28 (19), 1707519. 10.1002/adfm.201707519. [DOI] [Google Scholar]
- Cai X.; Xie Z.; Ding B.; Shao S.; Liang S.; Pang M.; Lin J. Monodispersed Copper(I)-Based Nano Metal–Organic Framework as a Biodegradable Drug Carrier with Enhanced Photodynamic Therapy Efficacy. Adv. Sci. 2019, 6 (15), 1900848. 10.1002/advs.201900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.; He C.; Lin W. Supramolecular metal-based nanoparticles for drug delivery and cancer therapy. Curr. Opin. Chem. Biol. 2021, 61, 143–153. 10.1016/j.cbpa.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T.; Ni K.; Culbert A.; Lan G.; Li Z.; Jiang X.; Kaufmann M.; Lin W. Nanoscale Metal–Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142 (16), 7334–7339. 10.1021/jacs.0c02129. [DOI] [PubMed] [Google Scholar]
- Yu W.; Zhen W.; Zhang Q.; Li Y.; Luo H.; He J.; Liu Y. Porphyrin-Based Metal–Organic Framework Compounds as Promising Nanomedicines in Photodynamic Therapy. ChemMedChem 2020, 15 (19), 1766–1775. 10.1002/cmdc.202000353. [DOI] [PubMed] [Google Scholar]
- Bao Z.; Li K.; Hou P.; Xiao R.; Yuan Y.; Sun Z. Nanoscale metal–organic framework composites for phototherapy and synergistic therapy of cancer. Materials Chemistry Frontiers 2021, 5 (4), 1632–1654. 10.1039/D0QM00786B. [DOI] [Google Scholar]
- Liu J.; Huang J.; Zhang L.; Lei J. Multifunctional metal–organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50 (2), 1188–1218. 10.1039/D0CS00178C. [DOI] [PubMed] [Google Scholar]
- Zeng J.-Y.; Wang X.-S.; Song W.-F.; Cheng H.; Zhang X.-Z. Metal-Organic Framework Mediated Multifunctional Nanoplatforms for Cancer Therapy. Adv. Ther. 2019, 2 (2), 1800100. 10.1002/adtp.201800100. [DOI] [Google Scholar]
- Wang Y.; Wu W.; Liu J.; Manghnani P. N.; Hu F.; Ma D.; Teh C.; Wang B.; Liu B. Cancer-Cell-Activated Photodynamic Therapy Assisted by Cu(II)-Based Metal–Organic Framework. ACS Nano 2019, 13 (6), 6879–6890. 10.1021/acsnano.9b01665. [DOI] [PubMed] [Google Scholar]
- Nash G. T.; Luo T.; Lan G.; Ni K.; Kaufmann M.; Lin W. Nanoscale Metal–Organic Layer Isolates Phthalocyanines for Efficient Mitochondria-Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143 (5), 2194–2199. 10.1021/jacs.0c12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai R.; Peng F.; Ji P.; Lu K.; Wang C.; Sun J.; Lin W. Electron Crystallography Reveals Atomic Structures of Metal–Organic Nanoplates with M12(μ3-O)8(μ3-OH)8(μ2-OH)6 (M = Zr, Hf) Secondary Building Units. Inorg. Chem. 2017, 56 (14), 8128–8134. 10.1021/acs.inorgchem.7b00845. [DOI] [PubMed] [Google Scholar]
- Brilkina A. A.; Dubasova L. V.; Sergeeva E. A.; Pospelov A. J.; Shilyagina N. Y.; Shakhova N. M.; Balalaeva I. V. Photobiological properties of phthalocyanine photosensitizers Photosens, Holosens and Phthalosens: A comparative in vitro analysis. J. Photochem. Photobiol., B 2019, 191, 128–134. 10.1016/j.jphotobiol.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Krysko D. V.; Garg A. D.; Kaczmarek A.; Krysko O.; Agostinis P.; Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12 (12), 860–875. 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Castano A. P.; Mroz P.; Hamblin M. R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6 (7), 535–545. 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-N.; Poon W.; Tavares A. J.; McGilvray I. D.; Chan W. C. W. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Controlled Release 2016, 240, 332–348. 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S. K.; Jittiwat J.; Manikandan J.; Ong C.-N.; Yu L. E.; Ong W.-Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 2010, 31 (8), 2034–2042. 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


