Abstract
Background
Recent data suggest that human epidermal growth factor receptor 2 (HER2)-low breast cancer may represent a distinct entity. We aimed to compare disease characteristics and outcomes between HER2-low and HER2-0 in estrogen receptor (ER) positive, early-stage breast cancer.
Methods
A single center retrospective study comprising all women with ER positive, HER2 negative early breast cancer, for whom an Oncotype DX test was performed between 2005 and 2012. Women were grouped to HER2-low (immunohistochemistry +1 or +2 and in situ hybridization not amplified) or HER2-0. Clinico-pathological features and Oncotype recurrence score (RS) were collected. Data on overall-survival (OS), disease-free survival (DFS) and distant disease-free survival (DDFS) were evaluated according to HER2 expression status.
Results
608 women were included, of which 304 women had HER2-0 and 304 had HER2-low disease. Lobular subtype was significantly more common in HER-0 compared to HER2-low disease (17% vs. 8%, p = 0.005). The prevalence of other clinic-pathological characteristics and long-term prognosis were comparable between both groups. For women with high genomic risk (RS > 25), HER2-low expression was associated with significantly favorable OS (HR = 0.31, 95% CI 0.11–0.78, p = 0.01), DFS (HR = 0.40, 95% CI 0.20–0.82, p = 0.01) and DDFS (HR = 0.26, 95% CI 0.11–0.63, P = 0.002) compared to women with HER2-0. For women with low genomic risk (RS ≤ 25), long-term prognosis was unrelated to HER2 expression.
Conclusion
The prognostic impact of HER2-low expression in early-stage luminal disease varies across the genomic risk, with significant favorable outcomes of HER2-low expression compared to HER2-0 in women with high genomic risk.
Keywords: Breast cancer, HER2-Low, Oncotype DX RS, Genomic risk, Prognosis
Highlights
-
•
The dichotomous categorization of HER2-positive and HER2-negative breast cancer has been challenged.
-
•
The prognostic impact of HER2-low expression differs between Oncotype-DX risk groups.
-
•
High risk (RS > 25) HER2-low disease is associated with significantly favorable outcomes compared to HER2-0.
-
•
Outcomes for low genomic risk (RS < 25) HER2-low vs. HER2-0 were similar.
-
•
These novel findings may point on a new prognostic feature of early-stage, high genomic risk luminal disease.
1. Introduction
Estrogen-receptor (ER) positive, human epidermal growth factor receptor 2 (HER2)–negative, early-stage breast cancer is the most common subtype of breast carcinoma [1]. Adjuvant chemotherapy after surgery is often guided by genomic assays [2,3]. The 21-gene recurrence-score (RS) assay (Oncotype DX, Genomic Health) is a well validated assay that provides both prognostic and predictive information regarding the benefits of adjuvant chemotherapy [[4], [5], [6]]. Histopathological features such as tumor size, nodal involvement and grade have important prognostic value independently to genomic risk, and are also used to tailor treatment decisions [[7], [8], [9]].
HER2 is a tyrosine kinase receptor belonging to the human epidermal receptor family. Encoded by the ERBB2 gene, it is considered as an important proto-oncogene in the biology of breast carcinoma. ERBB2 amplifications leads to HER2 receptor overexpression on cell membrane and upon homo/hetro dimerization of HER2 receptor, triggering a signal transduction cascade that leads to cell proliferation, migration, invasion, and survival which translates into an aggressive and rapidly spreading disease [10,11]. According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) 2018 guidelines [12], HER2 status is determined by either a +3 score by immunohistochemistry (IHC) assay, or ERBB2 amplification by situ hybridization (ISH) for tumors with +2 by IHC. Tumors with IHC results of 0, +1 or +2 with negative ISH are considered HER2 negative.
For tumors with IHC results of +1 or +2/ISH negative, defined as “HER2-low”, the addition of trastuzumab, an anti-HER2 targeted therapy, to adjuvant chemotherapy in high-risk patients did not demonstrate a clinical benefit [13]. Nevertheless, new clinical data on potential benefit of novel anti-HER2 therapies in HER2-low population with metastatic disease yielded encouraging results. Trastuzumab-deruxtecan, an antibody-drug conjugate targeting HER2, have shown in a phase 1b study, a confirmed objective response rate of 37% with a median duration of response of 10.4 months, in heavily pre-treated patients with HER2-low breast cancer [14], leading to an ongoing randomized control trials investigating Trastuzumab-deruxtecan in this population [15]. This new definition of breast carcinoma challenges the traditional dichotomous classification of HER2-positive versus HER2-negative tumors.
Approximately 55–60% of breast carcinomas are considered as HER2-low, of which 80% are luminal-like tumors and 15–20% are triple-negative breast cancer, depending on hormone receptor status [11,16,17]. While about 65% of luminal disease have HER-low expression, only third of all patients with triple negative breast cancer have HER2-low tumors [18]. Several recent studies have extensively characterized the clinicopathological and molecular features of HER2-low tumors, supporting potential biological differences between ER positive, HER2-low and ER positive, HER2-0 disease. Schettini et al. [18] reported on an analysis utilizing PAM50 assay of more than 3600 patients showing ER positive, HER2-low tumors had a higher ERBB2 and luminal-related gene expression levels than those with HER2-0 disease. In another study using PAM50 assay on primary breast cancer from the Cancer Genome Atlas dataset, HER2-enriched subtype was more frequent in HER-low compared to HER2-0 tumors, regardless of ER expression [17]. A recently published analysis on more than 2300 patients with HER2 non-amplified disease who were treated with neoadjuvant chemotherapy has shown that HER2-low breast cancer is significantly different from HER2-0 breast cancer with regard to hormone receptor status, tumor proliferation, grading, and response to neoadjuvant chemotherapy [19].
Clinical data on the prognostic significance of HER2-low expressing tumors are inconsistent. Several studies have shown an association with a worse outcome, in both early and advanced stages [[20], [21], [22], [23], [24]]. Other studies reported HER2-low disease was associated with similar or improved clinical outcomes compared to HER2-0 disease [17,19]. HER2-low status is currently not considered as an independent prognostic or predictive factor for potential benefit from adjuvant chemotherapy in ER positive disease [8,25].
The aim of this study is to investigate whether HER2 expression in early-stage, ER positive, HER2 negative disease has an impact on clinico-pathological characteristics and to examine the prognostic role of HER2 expression in the whole population and by genomic risk.
1.1. Patients and methods
The study was approved by the institutional ethics committees in accordance with the declaration of Helsinki. This single center retrospective study included all newly diagnosed women with ER positive, HER2 negative early-stage breast cancer who were treated in our institute between January 2005 and March 2012. Women with more than 3 lymph nodes involved or T4 disease were excluded. Medical charts and pathology reports were reviewed for pre-specified parameters, including: demographics, tumor size, nodal status, histologic grade, HER2 expression, ER and progesterone receptor (PR) staining, Ki-67 staining, presence of lympho-vascular (LVI) or perineural (PNI) tumor cell invasion and Oncotype DX RS results.
High-risk Oncotype DX RS patients were defined as RS of 26 or higher, and low risk patients were categorized with scores of 25 and lower, consistent with the TailorX study [6]. HER2-low tumors were defined by IHC as +1 or IHC +2/ISH negative. HER2-0 were defined as IHC-0 [16]. Women for whom the exact IHC staining was not reported were excluded. ER and PR staining are presented with a score range from 0 to 3, according to the modified version of the H-score method [26]. Accordingly, when the intensity of hormone receptors was reported as percentages it was converted by the H-score method: [(1˟ % cells +1) + (2 ˟ % cells +2) + (3 ˟ % cells +3)]/100 [23]. The intensity of hormone receptor staining was classified into 3 categories: weak (0 < ER/PR ≤ 1), intermediate (1 < ER/PR ≤ 2) and strong (ER/PR > 2). The histologic grade was determined using the Nottingham Histologic Scoring system [27]. Data on initial adjuvant hormonal therapy and administration of adjuvant chemotherapy were also extracted.
Long term outcomes on recurrence and survival were also collected, with data-lock at 18/06/2020. Patients' vital status was ascertained through Israel's ministry of interior database. Overall-survival (OS) was defined as the interval between date of diagnosis to the date of death from any cause or date of data-lock. Disease-free survival (DFS) was defined as the time from surgery to disease recurrence or death from any cause. Loco-regional recurrence was defined as disease recurrence confirmed by tissue biopsy in the ipsilateral breast, contralateral breast or confirmed involvement of ipsilateral axillary nodes. Distant recurrence was determined by imaging suggestive for metastatic disease with or without confirmation by biopsy. Time from surgery to the first event of distant metastasis were defined as distant disease-free survival (DDFS). When available, upon disease recurrence data on HER2 expression from site of recurrence were collected from the pathology reports.
1.2. Statistical analyses
The statistical analysis was generated using SAS software, version 9.4 (SAS System for PC, SAS Institute, Cary, NC). Data were reported descriptively for each of the pre-specified categories as described above. Categorical variables were presented as proportions and continuous variables were presented as mean ± SD or median (IQR) as appropriate. Chi-Square test or Fisher's exact test were utilized to analyze differences in the frequency of categorical variables between the HER-0 and HER2-low groups. T-test was used to compare the normally distributed continuous variables and the Mann Whitney test was used for non-normal variables.
OS, DFS and DDFS rates were calculated by the Kaplan–Meier method with the log-rank test, and the differences in outcomes by HER2 expression were evaluated. Hazard ratios (HRs) and associated 95% confidence intervals (CI) for time-to-event endpoints were analyzed using a Cox proportional hazards regression model. First, the differences in outcomes by HER2 status were evaluated for the entire study cohort. Subsequently the impact of HER2 expression on outcomes was investigated according to genomic risk group. Additionally, multivariate analyses for DFS, DDFS and OS were conducted, including HER2 expression and the following prognostic characteristics: age, tumor size (using the categories: T ≤ 1, 1<T ≤ 2 and T > 2 cm), lymph nodes involvement, grade (grade 3 compared to grade 1,2) and Oncotype DX recurrence score. Two-sided p-values less than 0.05 were considered statistically significant.
2. Results
A total of 705 patients were screened, and 608 patients were included in the final analysis, comprising 304 patients with HER2-0 disease and 304 with HER2-low disease. Reasons for exclusion included: unavailable HER2 expression status (n = 65), HER2 positive disease by IHC (n = 13), male gender (n = 9), ER negative (n = 4), more than 3 lymph nodes involved (n = 2), metastatic disease (n = 2) and patients who did not proceed with surgery (n = 2). Baseline clinical and pathological characteristics according to HER2 expression status are summarized in Table 1. Invasive lobular carcinoma was significantly more prevalent in HER2-0 disease compared to HER-low disease (17% vs. 8%, respectively, p = 0.005). Other histopathological features including tumor size, nodal involvement, grade, proliferation index Ki-67% and intensity of hormone receptor staining, angiolymphatic invasion and Oncotype DX RS were similar between both groups.
Table 1.
Patients clinicopathologic characteristics at baseline.
| Characteristic | HER2-0 (n = 304) | HER2-Low (n = 304) | P-Value |
|---|---|---|---|
| Age | |||
| Median (range)-yr. | 61 (34–84) | 60 (35–85) | 0.36 |
| Age <50 | 53 (17%) | 63 (21%) | 0.12 |
| Ethnicity | 0.58 | ||
| Ashkenazi Jews | 151 (52%) | 133 (47%) | |
| Sephardi Jews | 119 (40%) | 120 (43%) | |
| Arab | 5 (2%) | 6 (2%) | |
| Other | 17 (6%) | 23 (8%) | |
| Postmenopausal | 234 (80%) | 220 (76%) | 0.32 |
| Tumor size | 0.12 | ||
| ≤2 cm | 226 (74%) | 242 (80%) | |
| >2 cm | 78 (26%) | 61 (20%) | |
| Node negative | 257 (85%) | 245 (81%) | 0.23 |
| Grade | 0.83 | ||
| Low | 43 (18%) | 42 (16%) | |
| Intermediate | 154 (64%) | 174 (66%) | |
| High | 43 (18%) | 46 (18%) | |
| Proliferation Index –Ki67% < 20% | 150 (70%) | 161 (71%) | 0.36 |
| Histologic typea | 0.005 | ||
| IDC | 232 (76%) | 259 (86%) | |
| ILC | 51 (17%) | 25 (8%) | |
| Other | 21 (7%) | 19 (6%) | |
| Estrogen receptor intensity | 0.103 | ||
| Weak | 4 (1%) | 6 (2%) | |
| Intermediate | 76 (25%) | 55 (18%) | |
| Strong | 224 (74%) | 243 (80%) | |
| Progesterone receptor positive | 260 (86%) | 262 (86%) | 0.90 |
| Progesterone receptor intensity | 0.83 | ||
| Weak | 79 (30%) | 88 (33%) | |
| Intermediate | 79 (30%) | 80 (31%) | |
| Strong | 102 (40%) | 94 (36%) | |
| Lympho-vascular invasion present | 20 (7%) | 14 (5%) | 0.37 |
| Perineural invasion present | 15 (5%) | 12 (4%) | 0.69 |
| Oncotype DX recurrence score | |||
| ≤ 25 | 252(83%) | 245 (81%) | 0.52 |
| < 25 | 52 (17%) | 59 (19%) | |
| <10 | 57 (19%) | 55 (18%) | 0.76 |
| 11-25 | 195 (64%) | 190 (63%) |
Data were missing on: Ethnicity = 34, Menopausal status = 25, Tumor size = 1, nodal status = 3, histologic grade = 106, Ki67 = 167, histologic type = 1, Lympho vascular invasion = 23, PNI = 23, adjuvant chemotherapy = 5, adjuvant hormonal therapy = 21.
3 Intensity hormone receptor was defined as follows: Weak- 0 < ER/PR ≤ 1, Intermediate – 1 < ER/PR ≤ 2, Strong – ER/PR > 2.
IDC- Infiltrating Ductal Carcinoma: includes IDC only or IDC and DCIS (Ductal Carcinoma In Situ), Other histology included: Tubular, Medullary and Mucinous.
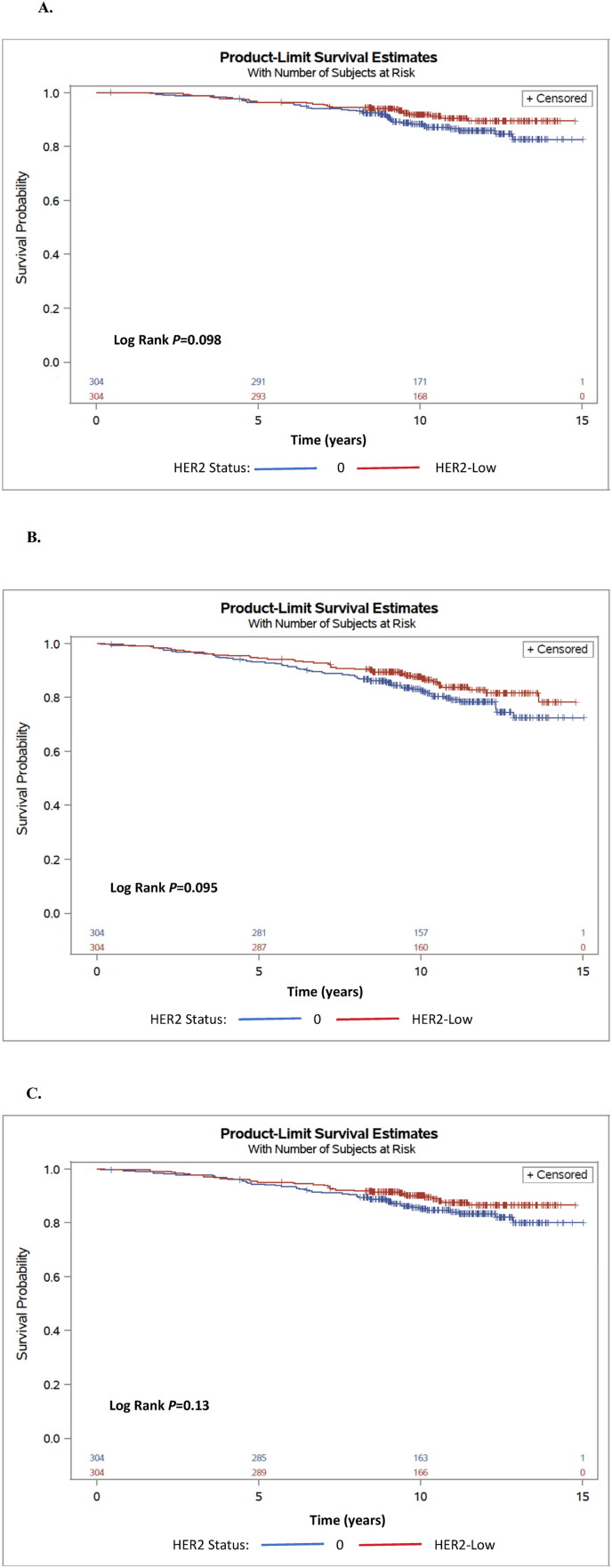
The median follow-up was 10.3 years. OS, DFS and DDFS of the entire study cohort and by genomic risk are presented in Table 2. HER2 expression was not associated with outcomes in the studied cohort. Estimated 10-year OS rate was 91% for HER2-low and 88% for HER2-0 disease (Fig. 1A), with HR = 0.66, 95% CI 0.40–1.08, P = 0.10. Estimated 10-year DFS was 87% and 82%, for HER2-low and HER2-0, respectively (Fig. 1B), HR = 0.72, 95% CI 0.49–1.06 P = 0.09; and estimated 10-year DDFS was 90% and 85%, for HER2-low and HER2-0, respectively (Fig. 1C), HR = 0.71, 95% CI 0.45–1.1 P = 0.13.
Table 2.
OS, DFS and DDFS by HER2 expression level and genomic risk.a.
| HER2 Status, Genomic Risk | Estimated 10-year OS (95% CI) | OS HR, 95% CI, p value | Estimated 10-year DFS (95% CI) | DFS HR, 95% CI, p value | Estimated 10-year DDFS (95% CI) | DDFS HR, 95% CI, p value | |
|---|---|---|---|---|---|---|---|
| All population | HER2-0 | 88% (0.83–0.91) | 0.66 (0.4–1.08), P = 0.10 | 82% (0.77–0.86) | 0.72 (0.49–1.06) P = 0.09 | 85% (0.8–0.88) | 0.71 (0.45–1.1) P = 0.13 |
| n = 304 | |||||||
| HER2-low | 91% (0.87–0.94) | 87% (0.83–0.90) | 90% (0.85–0.92) | ||||
| n = 304 | |||||||
| Low Genomic Risk Oncotype RS ≤ 25 | HER2-0 | 92% (0.88–0.95) | 0.91 (0.5–1.67) P = 0.77 | 87% (0.82–0.91) | 0.89 (0.55–1.42), P = 0.65 | 90% (0.86–0.93) | 1.05 (0.61–1.79) P = 0.85 |
| n = 252 | |||||||
| HER2-low | 92% (0.87–0.95) | 88% (0.84–0.92) | 90% (0.85–0.93) | ||||
| n = 245 | |||||||
| High Genomic Risk Oncotype RS ≥ 26 | HER2-0 | 68% (0.53–0.80) | 0.30 (0.11–0.78) P = 0.01 | 59% (0.43–0.71) | 0.4 (0.2–0.82), P = 0.01 | 59% (0.43–0.71) | 0.26 (0.11–0.63) P = 0.002 |
| n = 52 | |||||||
| HER2-low | 89% (0.78–0.95) | 81% (0.68–0.89) | 89% (0.78–0.95) | ||||
| n = 59 | |||||||
Abbreviations: CI – confidence interval, DFS – disease free survival, DDFS – distant disease-free survival, HR – hazard ratio, OS – overall survival, RS – recurrence score.
OS, DFS and DDFS rates were calculated by the Kaplan–Meier method with the long-rank test, HR and associated 95% CI were analyzed using a Cox proportional hazards regression model.
Fig. 1.
Kaplan-Meier analyses for all study population, according to HER2 expression status: 10-year overall survival (A), 10-year disease-free survival (B), and 10-year distant disease-free survival (C).
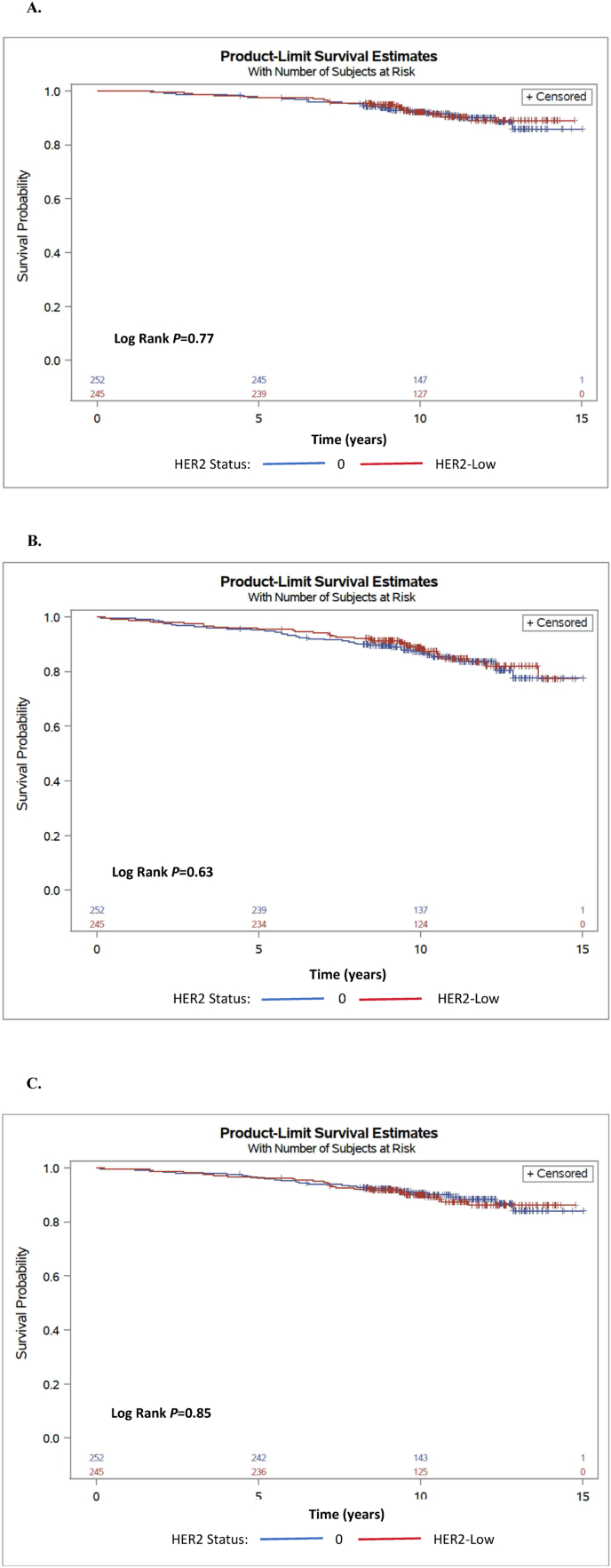
Overall, 497 patients (82%) had low genomic risk (RS of 0–25), among them 245 patients with HER2-low and 252 patients with HER2-0 disease. Analyses for women with low genomic risk showed similar OS, DFS and DDFS, see Table 2 and Fig. 2A–C.
Fig. 2.
Kaplan-Meier analyses for overall survival (A), disease-free survival (B) and distant disease-free survival (C) for low risk Oncotype DX Recurrence Score (0-25) according to HER2 expression status.
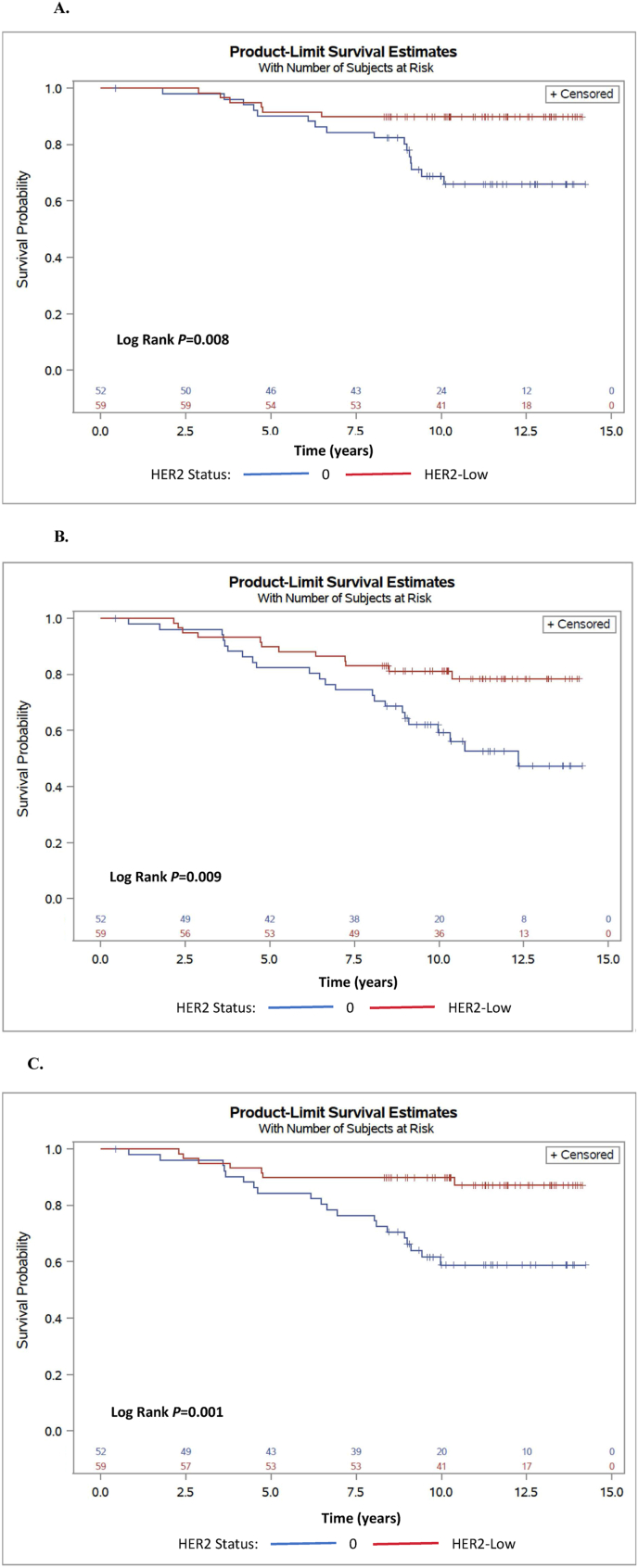
111 patients had a recurrence score of 26 or higher, including 52 who had HER2-0 and 59 with HER2 low disease. Clinical-pathological features for women with high genomic risk were similar between HER2-low and HER2-0 disease, see Supplementary Table 1. Compared to HER2-0 disease, HER2-low was associated with statistically significant improved OS (10-year rates 89% vs. 68%, HR = 0.31, 95% CI 0.11–0.78, P = 0.01), DFS)10-year rates 80% vs. 59%, HR = 0.40, 95% CI 0.20–0.82; P = 0.01) and for DDFS (10-year rates 86% vs. 59%, respectively, HR = 0.26 95% CI 0.11–0.63, P = 0.002), see Fig. 3A–C. Most women with high genomic risk received adjuvant chemotherapy: 40 (78.4%) women with HER2-0 and 45 (78.9%) women with HER-2 low disease. Compared to HER-0 disease, favorable outcomes were seen in HER2-low disease whether adjuvant chemotherapy was given or not (Supplementary Figs. 1A–C). The vast majority (97%) of these patients received initial adjuvant hormonal therapy, with 55 (98%) of patients with HER2-low and 45 (96%) with HER2-0. An analysis of the interaction between tumor subtype, genomic risk and outcomes was not undertaken due to the low number (n = 8) of patients with high genomic risk and invasive lobular carcinoma. However, the signal in favor improved outcomes remained for HER2-low disease, regardless to histological subtype (Supplementary Fig. 2A-B). In multivariate analysis, HER2-low, younger age, smaller tumors and low genomic risk were all independently associated with statistically significant improved DFS, see Supplementary Table 2. Multivariate analysis for DDFS has shown similar findings, but the difference between HER2-low to HER2-0 did not meet the threshold for statistical significance (p = 0.084), see Supplementary Table 2.
Fig. 3.
Kaplan-Meier analyses for overall survival (A), disease-free survival (B) and distant disease-free survival (C) for high risk Oncotype DX Recurrence Score (RS ≥ 26) according to HER2 expression status.
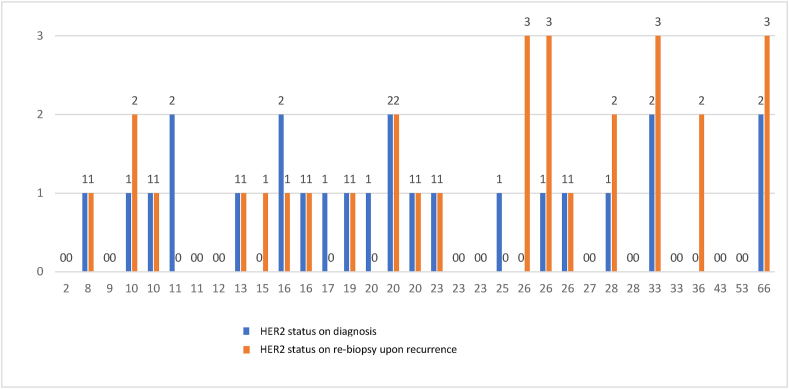
Data on HER2 expression from biopsies at loco-regional or metastatic recurrence by HER2 status and RS at presentation, are presented in Fig. 4. Overall, there were 68 events of recurrence, of which 33 were biopsy proven. HER2 expression in the biopsy from the recurrence site was identical to the expression at baseline in 10/14 (71%) of the women with HER2-0 at presentation, and 12/19 (63%) of the women with HER2-low at presentation. Four (12%) of recurrences were HER2 positive by IHC (+3) or by ISH upon recurrence. Tumors with higher Oncotype DX RS tended to overexpress the HER2 receptor upon recurrence, compared to lower Oncotype DX scores that tended to preserve the original level of HER2 expression.
Fig. 4.
HER2 status upon disease recurrence; 0; HER2 IHC-0, 1; HER2 IHC 1+, 2; HER2-IHC +2∖ISH negative, 3- HER2-Positive tumor: IHC +3/HER2 +2, ERBB2 amplified by ISH).
3. Discussion
The dichotomous classification of HER2 positive and HER2 negative disease has been recently challenged by emerging data on antibody-drug conjugate targeting HER2 among patients with metastatic HER2-low disease [15,28,29]. Several recent studies using PAM-50 analysis have identified differences between HER2-low and HER2-0 disease [17,18], suggesting that in contrast to current classification of HER2 negative disease, which comprises both HER2-0 and HER-low disease, HER2-low tumors represent a specific nosological entity. In this study, there were no differences in all of the prognostic pathological features between HER2-0 and HER2-low. Furthermore, Oncotype DX RS, a well-established prognostic and predictive feature that is not a subject for inter-laboratory heterogeneity [4], was comparable between HER2-0 and HER2-low disease. Despite this resemble, we found that among patients with high genomic risk, HER2-low expression was associated with significant and consistent improvement in OS, DFS and DDFS compared to HER2-0. Interestingly, in patients with low genomic risk, no difference was identified in outcomes between HER2-0 and HER2-low. We are not aware of previous reports of these findings.
While HER2-positive disease is chemo-sensitive [[30], [31], [32]], whether HER2-low expression is predictive to chemotherapy is unclear. Consistent with guidelines [25], most women with high genomic risk received adjuvant chemotherapy in this cohort. As only few women with high genomic risk did not receive chemotherapy, the conclusions that can be drawn from the predictive role of HER2-low in this population are limited. However, the trend for favorable outcomes was noted in HER-low disease with high genomic score both is women who were treated with chemotherapy and in women who forgo chemotherapy.
Lobular subtype was significantly more common in HER2-0 compared to HER2 low disease. Lobular subtype is the most common subtype after ductal carcinoma and represents approximately 10–15% of all invasive breast cancers [33,34]. The distribution of HER2-0 compared to HER2 low in this subtype is not well described. A recent large-scale study which included both ER positive and ER negative disease, reported similar distribution of histological subtypes [18]. It is possible that our results are different because of the more homogenous patient population, as classical lobular carcinoma is almost exclusively ER positive, HER2 negative disease [31]. This finding may further support the postulation that HER2-low and HER2-0 disease represent two different clinical entities.
Other confounders could possibly affect patients’ outcomes. Adherence to adjuvant endocrine therapy is often incomplete [35], and poor adherence is associated with worse outcomes [36,37]. While adherence is not expected to be affected by HER2 expression, data on adherence were not documented. Additionally, variability in the type of adjuvant hormonal therapy that was given may exist. Tamoxifen monotherapy, sequence of tamoxifen followed by aromatase inhibitors or an upfront aromatase inhibitors therapy were prescribed. Tamoxifen monotherapy in post-menopausal women is inferior to treatment that comprises aromatase inhibitors [38] and extending adjuvant hormonal treatment beyond 5 years has also shown to improve outcome [39], but data on duration of treatment were not collected. However, as extending aromatase inhibitors beyond 5 years is not expected to improve OS [40] and the absolute benefit in reducing recurrence is relatively negligible in node negative disease (approximately 1%) [41], variability in duration of adjuvant hormonal treatment is unlikely to explain the remarkable differences in outcomes that were identified by HER2 expression among women with high genomic risk in this cohort. The comparable outcomes in women with low genomic risk further support no meaningful confounders exists between HER2-0 and HER2-low, suggesting true difference between these groups in high genomic risk disease.
HER2-low tumors comprised of HER2-IHC +1 and + 2 by Immunohistochemistry, overexpress the HER2 receptor at a range of 100,0000–500,000 molecules per cell [42]. Therefore, HER2-low status represents a spectrum of HER2 overexpressing tumors that may represent several distinct biological entities with different prognostic characteristics and different response to various treatment strategies. As the number of women with HER2 +2 is relatively limited, whether the intensity of HER2 expression has clinical implication in HER2-low disease could not be inferred from this study.
This study has several limitations. First, HER2 IHC interpretation is observer dependent, and interpretation of tumor sample may vary. Of note, most tumors samples were reviewed by the same team of pathologists in our center, which is a referral hospital, however some tumor specimens were reviewed by pathologists from different centers. Second, as this is a single center retrospective study it is vulnerable to unknown bias. Third, data on the adherence and duration of treatment as well as complete data on the type of endocrine therapy during all period were not documented. Finally, as the number of women with high genomic risk who were not treated with chemotherapy was small, it remains unclear whether HER2-low is independent prognostic factor or is associated with more chemo-sensitive disease, compared to HER2-0.
This study comprises more than 600 women, however the statistically significant differences in outcomes were identified only for 111 women with high genomic risk. While the latter subgroup represents relatively small cohort, differences in outcome achieved statistical significance, suggesting the differences between HER2-low and HER2-0 may be attributed to unique entities and not to a statistical fluke. In is possible that differences were identified in this study despite relatively small sample size due to the long follow-up period.
In conclusion, our study shows that the prognostic significance of HER2-low expression varies across the Oncotype DX risk groups, with favorable outcomes among HER2-low tumors only in high genomic risk patients. Larger studies are warranted to clarify whether HER2-low is an independent prognostic marker in luminal disease or whether it represents a biomarker that may impact treatment decisions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All other authors have no conflicts of interest.
Acknowledgments
The authors would like to thank Prof. Daniel Shepshelovich for his critical contribution of linguistic revision. His involvement improved significantly the quality of our manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.08.016.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Howlader N, Altekruse SF, Li CI, et al US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106 (5), 2014. [DOI] [PMC free article] [PubMed]
- 2.Curigliano G., Burstein H.J., Winer E.P., et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer. Ann Oncol. 2017;28:1700–1712. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 4.Paik S., Shak S., Tang G., et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 5.Albain K.S., Barlow W.E., Shak S., et al. Prognostic and predictive value of the 21 gene recurrence score assay in postmenopausal women with node-positive, estrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomized trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparano J.A., Gray R.J., Makower D.F., et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–112. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparano J.A., Gray R.J., Ravdin P.M., et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein H.J., Curigliano G., Loibl S., Members of the St. Gallen international consensus panel on the primary therapy of early breast cancer 2019,Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen international consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 9.Sparano J.A., Crager M.R., Tang G., et al. Development and validation of a tool integrating the 21-gene recurrence score and clinical-pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J Clin Oncol. 2020 doi: 10.1200/JCO.20.03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes D.F. HER2 and breast cancer - a phenomenal success story. N Engl J Med. 2019;381:1284–1286. doi: 10.1056/NEJMcibr1909386. [DOI] [PubMed] [Google Scholar]
- 11.Marchiò C., Annaratone L., Marques A. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Canc Biol. 2020 doi: 10.1016/j.semcancer.2020.02.016. 0-1. [DOI] [PubMed] [Google Scholar]
- 12.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol; 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 13.Fehrenbacher L., Cecchini R., Geyer C., et al. NSABP B-47 (NRG oncology): phase III randomized trial comparing adjuvant chemotherapy with Adriamycin (A) and Cyclophosphamide (C)→weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-Low IBC) J Clin Oncol. 2019;38:444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi S., Park H., Murthy R.K., et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-Low-Expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Library of Medicine (U.S. Study of trastuzumab deruxtecan (T-DXd) vs investigator's choice chemotherapy in HER2-low, hormone receptor positive. Metastat Breast Canc. July 2020 https://clinicaltrials.gov/ct2/show/NCT04494425 (DB-06) -NCT04494425. [Google Scholar]
- 16.Tarantino P., Hamilton E., Tolaney S.M., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020 doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 17.Agostinetto E., Rediti M., Fimereli D., Debien V., Piccart M. HER2-Low breast Cancer : molecular characteristics. Cancers. 2021;1–16 doi: 10.3390/cancers13112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schettini F., Chic N., Brasó-maristany F., et al. Clinical , pathological , and PAM50 gene expression features of HER2-low breast cancer. npj Breast Canc. 2021;7(1) doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denkert C., Seither F., Schneeweiss A., et al. Articles Clinical and molecular characteristics of HER2-low-positive breast cancer : pooled analysis of individual patient data from four prospective , neoadjuvant clinical trials. Lancet Oncol. 2021;2045(21):1–11. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 20.Rossi V., Sarotto I., Maggiorotto F., et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncol. 2012;17:1418–1425. doi: 10.1634/theoncologist.2012-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggemann H., Ignatov T., Burger E., et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Canc. 2015;22:725–733. doi: 10.1530/ERC-15-0335. [DOI] [PubMed] [Google Scholar]
- 22.Gilcrease M.Z., Woodward W.A., Nicolas M.M., et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. AJ Surg Pathol. 2009;33:759–767. doi: 10.1097/PAS.0b013e31819437f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulakaki N., Makris G.M., Battista M.J., et al. Hormonal receptor status, Ki-67 and HER2 expression: prognostic value in the recurrence of ductal carcinoma in situ of the breast? Breast. 2016;25:57–61. doi: 10.1016/j.breast.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Camp R.L., Dolled-FilhartM, King B.L., et al. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Canc Res. 2003;63:1445–1448. [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network Clinical practice guidelines in oncology. Breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf
- 26.Weidner N., Cote R.J., Suster S., et al. second ed. Saunders Elsevier; Philadelphia: 2009. Modern surgical pathology. [Google Scholar]
- 27.Bloom H.J., Richardson W.W. Histological grading and prognosis in breast cancer. Br J Canc. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerji U., van Herpen C.M.L., Saura C., et al. Trastuzumab duocarmazine in locally advanced andmetastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 29.Eleonora Nicolo, Paola Zagami, Curigliano Giuseppe, et al. Antibody-drug conjugates in breast cancer: the chemotherapy of the future? Curr Opin Oncol. 2020;32(5):494–502. doi: 10.1097/CCO.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 30.Guarneri V., Broglio K., Kau S.W., et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 31.Romond E.H., Perez E.A., Bryant J., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 32.Ménard S., Balsari A., Tagliabue E., et al. Biology, prognosis and response to therapy of breast carcinomas according to HER2 score. Ann Oncol. 2008;19(10):1706–1712. doi: 10.1093/annonc/mdn369. [DOI] [PubMed] [Google Scholar]
- 33.Rakha Emad A., Ellis Ian O. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27(1):49–61. doi: 10.1053/j.semdp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Arpino G., Bardou V.J., Clark G.M., et al. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hershman D.L., Kushi L.H., Shao, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowan T. Chlebowski, Jisang Kim and Reina Haque adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Canc Prev Res. 2014;7(4):378–387. doi: 10.1158/1940-6207.CAPR-13-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chirgwin J.H., Giobbie-Hurder A., Coates A.S., et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;3(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. 386. [DOI] [PubMed] [Google Scholar]
- 39.Gray R.D., Rea D.W., Handley K., Marshall A., Pritchard M.G., Perry P., et al. aTTom (adjuvant tamoxifen—to offer more?): randomized trial of 10 versus 5 years of adjuvant tamoxifen among 6,934 women with estrogen receptor-positive (ER+) or ER untested breast cancer—preliminary results. J Clin Oncol. 2008 [Google Scholar]
- 40.Erik J Blok, Judith R Kroep, Elma Meershoek-Klein Kranenbarg, Cornelis J H van de Velde, on behalf of the IDEAL Study Group Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006-05) JNCI: J Natl Cancer Inst. 2018;110(1):40–48. doi: 10.1093/jnci/djx134. [DOI] [PubMed] [Google Scholar]
- 41.R Gray and Early Breast Cancer Trialists' Collaborative Group . 2019. Effects of prolonging adjuvant aromatase inhibitor therapy beyond five years on recurrence and cause-specific mortality: an EBCTCG meta-analysis of individual patient data from 12 randomized trials including 24,912 women; SABCS18-GS3-03. Published February. [Google Scholar]
- 42.Onsum M.D., Geretti E., Paragas V., et al. Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am J Pathol. 2013;183:1446–1460. doi: 10.1016/j.ajpath.2013.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.