Abstract
BACKGROUND
Evidence regarding the performance of Bipolar Disorder patients (BD) on Emotional Processing (EP) is conflicting, suggesting that heterogeneity within this population may exist. It is not completely understood if this impacts on clinical presentation and functional outcomes.
METHODS
A total of 207 BD patients were recruited. Patients underwent MATRICS Consensus Cognitive Battery as well as a clinical evaluation to detect premorbid traits, comorbidities and clinical features. Performance on each basic emotion on the Emotional Recognition Task (ERT) and Reading the Mind in the Eyes Test were entered into hierarchical cluster analyses in order to determine the number of clusters and to assign subjects to specific clusters. We then compared subgroups on clinical factors and real-world community functioning.
RESULTS
No differences between BD patients as a group and controls were found in EP performance. Two clusters of BD patients were found, one with “intact” performance (71.2%) that performed as healthy controls (HC) and other with “impaired” performance (28.8%) performing worse than HC and schizophrenic patients on basic emotion recognition. Patients in the “impaired group” presented higher rates of childhood trauma, schizotypal traits, lower premorbid IQ and education, poor psychosocial functioning and cognitive performance.
LIMITATIONS
Cross-sectional data which limits our ability to infer directionality of our findings.
CONCLUSION
These results suggest the presence of two subgroups regarding EP performance with unique clinical and neurodevelopmental profiles associated. Next steps will include using these data to identify a homogeneous group of patients to target these disabling symptoms with treatment.
Keywords: Bipolar Disorder, Emotion, Theory of mind, Cluster analysis, cognition, schizophrenia, psychosocial functioning
INTRODUCTION
There is a copious amount of evidence suggesting that patients with Bipolar Disorders (BD) experience neurocognitive deficits even during periods of euthymia(Bourne et al., 2013; Mann-Wrobel et al., 2011), particularly in the domains of attention, memory, and executive function (Bora et al., 2009). Neurocognitive deficits have been described in premorbid stages of the condition(Olvet et al., 2010; Ratheesh et al., 2013), in first-episode patients(Bora and Pantelis, 2015), pediatric populations(Nieto and Castellanos, 2011), and in older adults(Depp et al., 2007). However, a number of reports have suggested that a substantial heterogeneity exists regarding individual performance in BD (Burdick et al., 2014; Martino et al., 2008; Reichenberg et al., 2009). In fact, neurocognitive heterogeneity has been also described in unaffected siblings(Russo et al., 2017), as well as in older adults with BD(Martino et al., 2018). Broadly, several independent studies have identified three distinct cognitive profiles among patients with BD: an intact group, who present a preserved neurocognitive performance; a selectively impaired group, whose neurocognitive performance is only significantly affected in select cognitive domains; and a globally impaired group(Burdick et al., 2014; Jensen et al., 2016; Lewandowski et al., 2014; Van Rheenen et al., 2017).
Relative to this substantial body of research focused on neurocognition in BD, studies of emotional processing are limited and results have been mixed. Specifically, Aparicio et al. (2017) (Aparicio et al., 2017) reported that tasks that assay higher-level social cognitive abilities are impaired in euthymic patients with BD versus controls, while performance on measures of emotional-processing (i.e., lower-level) were relatively conserved, a finding replicated by others(Martino et al., 2011; Robinson et al., 2015). Conversely, Samamé et al. (2015) conducted an individual-task meta-analysis and found a small (d=0.37) but significant deficit in emotional processing among BD patients (Samamé et al., 2015), in line with other reports showing impaired EP performance in BD as a group(David et al., 2014; de Brito Ferreira Fernandes et al., 2016). Taken together, these results point to impaired emotion processing, at least in a subset of patients with BD. Indeed, a recent meta-analysis comparing BD and schizophrenia (SZ) patients reported that SZ patients performed significantly worse than BD patients on tasks of emotion recognition (d = 0.39) but considerable heterogeneity (p for Q < 0.001 and I2 = 61–68%) was found, suggesting that a substantial number of BD patients were performing the same or worse than SZ patients(Bora et al., 2016).
In the current study, we tested the hypothesis that different subgroups of BD patients can be defined by their EP task performance, with some patients showing normal patterns, while others are impaired. We used an empirical approach rather than an arbitrary cut-off to classify patients. Hierarchical clustering was applied to a large cohort of affectively stable BD patients to examine: (1) whether definable subgroups exist; (2) how many subgroups emerge; and (3) the neurocognitive, clinical, and functional profiles associated with these subgroups.
METHODS
Patients
Participants with DSM-IV BD-I and II (N=212) were recruited from the Icahn School of Medicine at Mount Sinai Hospital (ISMMS). Inclusion criteria were: (1) diagnosis of BD I or BD II; (2) age 18–65 years; (3) current affective stability [<12 on the 24-item Hamilton Rating Scale for Depression (HAMD(Hamilton, 1960)); and <8 on the Clinician-Administered Rating Scale for Mania (CARS-M(Altman et al., 1994))]. Exclusion criteria included a history of central nervous system (CNS) trauma, neurological disorder, or attention deficit hyperactivity disorder; diagnosis of recent substance abuse/dependence (past 3 months); electroconvulsive therapy (ECT) in the past 12 months; an active and unstable medical problem that may interfere with cognition; medications with known adverse cognitive effects (i.e., topiramate, tricyclics, anticholinergics); agents that enhance cognition (e.g., amphetamine, dopamine agonists) and IQ below 70. Benzodiazepines were not allowed within 4 hours of testing.
Healthy Controls (HC) (N=59) with no personal or family history of an Axis-I disorder were recruited through advertisements. All other exclusion criteria for HCs mirrored those for the BD sample, with the exception of an Axis-I disorder. Schizophrenic patients (SZ, n=63) were recruited from the same center (ISMMS) and the same protocol was applied. SZ patients had to be symptomatically stable in order to be included, and the same inclusion and exclusion criteria were applied to them. The study was approved by the ISMMS Institutional Review Board (IRB). BD and HC participants provided written informed consent prior to participating in the study.
Neurocognitive Measures
The MATRICS Consensus Cognitive Battery (MCCB; Matrics Assessment Inc., USA(Nuechterlein et al., 2008)) was used to measure neurocognitive functioning. The MCCB was originally developed to be used in clinical trials targeting cognition in schizophrenia; however, recent studies demonstrated its suitability to effectively capture neurocognitive deficits in BD patients(Burdick et al., 2011). The MCCB is composed of several individual tests that give rise to seven cognitive domains: processing speed, attention and vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. The battery can be completed in a single session of about 70 minutes.
Premorbid intelligence was estimated using the WRAT-3 reading test. Standardized scores were used, where higher scores indicate better performance.
Emotional processing
The CANTAB Emotion Recognition Test (ERT) was used to evaluate facial emotion recognition. It is a computerized test that includes 60 photographs of different individuals displaying facial expressions of six basic emotions (sadness, happiness, anger, fear, disgust, and surprise). The photographs stay on the screen for 5 seconds and the participants choose one of these six basic emotions. Total scores, sub scores for each individual emotion, and mean overall latency response time were obtained. Additionally, we administered the Reading the Mind in the Eyes test(Baron-Cohen et al., 1997) (RMET). RMET is a computerized task in which patients are shown 36 photographs of the eye region of faces and are asked to select among 4 options the best word that describes what the individual in the picture is thinking or feeling, and also to indicate whether the photographed face is a man or a woman. This task is sensitive to subtle emotion processing deficits since it involves recognition of complex mental states. Only total score was obtained.
Clinical, pharmacological, and functional outcomes
Data on current symptoms, history of psychosis, number of mood episodes and lifetime history of co-morbid substance use disorders were collected using the Structured Clinical Interview for the DSM-V (SCID-V) interview and standardized mood ratings (HAMD and CARS-M).
Premorbid adjustment was assessed using the Premorbid Adjustment Scale(Cannon-Spoor et al., 1982) (PAS) which assesses the “degree of achievement of developmental goals” over the course of childhood, adolescence, and where applicable, adulthood. Individual items in the childhood and adolescence categories assess premorbid adjustment by asking about sociability and social withdrawal, peer relationships, scholastic performance, adaptation to school, and ability to form socio-sexual relationships prior to the onset of illness.
A history of childhood trauma was assessed using the Childhood Trauma Questionnaire(Bernstein et al., 1994) (CTQ). The CTQ consists of 28 questions which comprise 5 subscales: Physical Abuse, Physical Neglect, Emotional Abuse, Emotional Neglect, and Sexual Abuse.
Psychosis proneness as a trait was assessed using the Schizotypal Personality Questionnaire(Raine, 1991) (SPQ) that evaluates nine schizotypal traits: ideas of reference, social anxiety, odd beliefs/magical thinking, unusual perceptual experiences, eccentric/odd behaviors, no close friends, odd speech, constricted affect, and suspiciousness/paranoid ideation. Trait impulsivity was evaluated through the Barratt Impulsiveness Scale(Patton et al., 1995) (BIS-11) that describes impulsivity in three main areas: motor, planning, and attentional.
Functional outcomes were evaluated using the Brief UCSD Performance-Based Skills Assessment scale(Patterson et al., 2001) (UPSA-B). This scale was designed to evaluate an individual’s functional capacity in selected domains of basic living skills. These two subtests administered were Financial Skills and Communication. A global score was calculated by summing all items, with greater UPSA scores representing worse functioning.
Statistical analysis
Quantitative variables are presented as mean and standard deviation, or, in case of noticeably skewed data, as median and interquartile range. Case-control differences in demographic, neurocognitive, and emotion processing measures were assessed using chi-square test for categorical variables and Student’s t-test for continuous variables.
To identify homogeneous subgroups of BD patients based on their emotional processing performance, we conducted a hierarchical cluster analysis (HCA). Similarity between cases was computed with the squared Euclidian distance and Ward linkage was used as the agglomeration procedure. We pre-standardized the variables used to construct the clusters (total score for the RMET and each separate emotion from the ERT) prior to conduct the HCA. The inspection of the dendrogram was used as criterion to establish the appropriate number of clusters to retain and also by inspection of the drop in the agglomeration coefficients as described by Sugar et al. (1998)(Sugar et al., 1998). Finally, we inspected the scree plot using Principal Component Analysis (PCA) to further validate the number of appropriate clusters. Once a consensus between the authors was achieved and the appropriate number of clusters provided by the data was identified, in a second step of the analysis, we performed a k-means analysis requesting to provide the previously defined number of clusters and cluster membership was saved as a grouping variable. We compared the emotional processing profiles of the BD clusters with HCs using Analysis of Variance (ANOVA) and to provide additional information on diagnostic specificity, we also compared BD clusters with a sample of 63 patients with SZ collected using the same protocol from the same hospital.
Finally, to begin to understand the clinical and functional correlates of cluster membership, descriptive analyses (ANOVA and χ2 applied as appropriate) were carried out to investigate differences in demographic characteristics, clinical features, neurocognitive functioning, and functional disability between the identified BD clusters. Further, to explore if the variables shown significant in the univariate analysis predicted cluster belonging, we fitted logistic regression models using the resulting clusters as dependent variables adjusting by demographic variables. Secondary analyses to control for general task accuracy were performed, using the grouping variable as an explanatory variable, and the mean overall latency response and premorbid IQ as covariates.
RESULTS
Demographic and clinical data
A total of 212 BD patients were included in the present analysis. Of these, 169 were diagnosed with BD I and 43 with BD II. Mean age of the BD patients was 44.1 (±12.1) and mean age at onset was 21.7 (±7.9). 47.2% of the BD sample was white and 48.1% were female. Mean duration of education of patients was 14.5 years (±3.3). Control group (n=55) was comprised by 54% females, with a mean age of 43.8 (±13.8) and 15.5 (±1.8) years of education.
The comparison sample of 63 SZ patients (46% female) had a mean age of 42.2 (±13.3) and 13.6 years of education (±2.3) They were symptomatically-stable (mean BPRS was 25.5 ± 6.2, mean total SANS was 16.5 ± 13.9).
At the group level (all BD patients vs controls), BD patients performed significantly worse than controls on the MCCB composite score; however, we did not observe significant differences on tasks of emotional processing except for sadness recognition (Table 1).
Table 1.
Demographic and Clinical data of the BD and HC groups.
| BD (n=212) | HC (n=55) | p | |
|---|---|---|---|
| Diagnosis, n | |||
| BD I | 166 | - | |
| BD II | 41 | - | |
|
| |||
| Sex, n(%) | |||
| Males | 106 (51.2) | 23 (46) | 0.53 |
| Females | 101 (48.8) | 27 (54) | |
|
| |||
| Race, n(%) | |||
| Caucasian | 97 (46.9) | 34 (68) | 0.08 |
| Non-Caucasian | 110 (53.1) | 16 (32) | |
|
| |||
| Age, ys | 44.1 (12.1) | 39.1 (13.3) | 0.01 |
|
| |||
| Age of onset, ys | 21.7 (7.9) | -- | |
|
| |||
| Duration of education, ys | 14.5 (3.3) | 15.5 (1.8) | 0.03 |
|
| |||
| HDRS score | 7.7 (6.5) | 0.5 (1.2) | < 0.001 |
|
| |||
| CARS-M score | 3.4 (4.4) | 0.2 (0.8) | < 0.001 |
|
| |||
| WRAT-3 score | 102.1 (14.9) | 106.4 (12.4) | 0.03 |
|
| |||
| Cognitive domains, mean (T-Score) | |||
|
| |||
| MCCB composite score | 43.7 (8.3) | 47.0 (7.7) | 0.01 |
|
| |||
| Emotional Processing domains (Z-score) | |||
|
| |||
| RMET total score | −0.25 (0.9) | 0.0 (1.0) | 0.07 |
|
| |||
| Recognition – Happiness (%) | −0.24 (0.9) | 0.0 (1.0) | 0.10 |
|
| |||
| Recognition – Sadness (%) | −0.37 (1.1) | 0.0 (1.0) | 0.02 |
|
| |||
| Recognition – Anger (%) | −0.14 (0.9) | 0.0 (1.0) | 0.32 |
|
| |||
| Recognition – Disgust (%) | −0.25 (0.9) | 0.0 (1.0) | 0.08 |
|
| |||
| Recognition – Fear (%) | −0.15 (0.9) | 0.0 (1.0) | 0.27 |
|
| |||
| Recognition – Surprise (%) | −0.04 (0.8) | 0.0 (1.0) | 0.75 |
Clinical Administered Rating Scale for Mania (CARS-M); Hamilton Depression Rating Scale 24 item(HAMD); Wide Range Achievement Test (WRAT-3). MATRICS Consensus Cognitive Battery (MCCB). Data represented as N(%) or Mean (±SD).
Clustering of BD patients
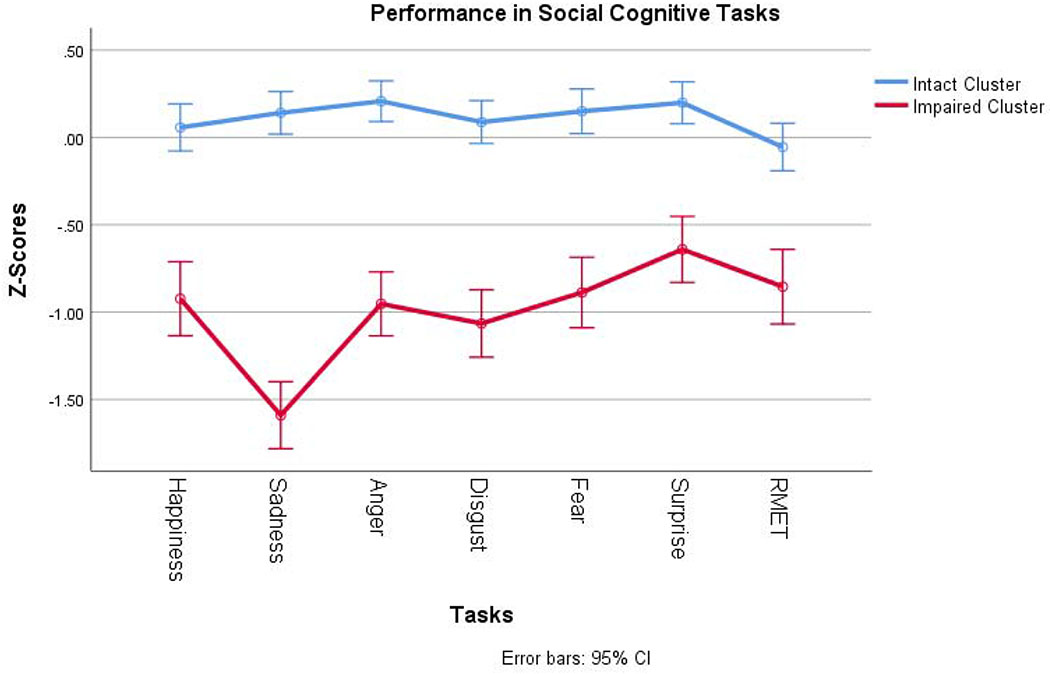
Results from the hierarchical clustering analysis showed that the patient group would be best described by two discrete clusters. First, the agglomeration coefficient showed the largest drop between observation 255 and 256 (last observation). Second, visual inspection of the dendrogram also revealed 2 clusters as the best fit. Confirmatory scree plot from a PCA using standardized emotional processing variables is shown in Supplementary eFigure 1. Once a consensus was reached that two was the optimal number of clusters to describe emotional processing performance among the BD sample, we proceeded to compare relevant variables between the two clusters. The first resulting cluster included 151 patients (71.2%; “intact”), and the second cluster included 61 patients (28.8%; “impaired”). Accuracy in identifying happiness and anger showed the greatest correlation coefficients (0.886 and 0.700, respectively) suggesting that they contributed more than the other emotional processing domains to classify subjects into the final clusters.
Figure 1 shows the performance of the two BD clusters, and healthy controls on all emotional processing tasks. Supplementary eFigure 2. shows the performance of the BD clusters and SZ patients. Broadly, one cluster of BD patients outperformed HC while the other cluster performed worse than SZ patients as a group. When ANOVA was performed, we found no differences in the performance of the impaired BD cluster and the SZ patients in Reading the Mind in the Eyes total score (p=0.148) and the recognition of happiness, anger and surprise on the ERT. However, the impaired BD cluster performed significantly worse than SZ patients in the recognition of sadness, disgust, and fear (all p < 0.001). In contrast, the intact BD cluster did not perform significantly different from the healthy controls on any of the emotion processing measures.
Figure 1.
Performance in EP tasks of the two BD clusters.
RMET: Reading the Mind in the Eyes Test
Clinical, neurocognitive, and functional characteristics of the BD clusters
The two resulting clusters presented with similar clinical profiles. No appreciable differences were found between the clusters regarding BD subtype, rapid cycling, psychosis history, or the number of prior mood episodes or hospitalizations. No significant differences were observed regarding the pattern of medication use (Table 2).
TABLE 2.
Demographic, clinical and pharmacological features of BP clusters regarding EP
| Cluster | Statistics | ||||
|---|---|---|---|---|---|
| Characteristic | Poor performance (N=61) | Good Performance (N=151) | df | T or X2 | p value |
| Demographic and Clinical characteristics | |||||
| Gender (n, % female) | 22 (36.1) | 80 (53.0) | 1 | 5.18 | 0.033 |
| Race (n, % white) | 20 (32.8) | 80 (53.7) | 1 | 7.58 | 0.006 |
| Years of education | 13.5 (2.1) | 14.6 (2.6) | 137.9 | 2.94 | 0.004 |
| BD Subtype (n, % BD I) | 46 (75.4) | 123 (82.0) | 1 | 1.18 | 0.341 |
| Psychotic Symptoms (n, %) | 22 (36.1) | 65 (43.3) | 1 | 0.95 | 0.358 |
| Rapid Cycling (n, %) | 6 (9.8) | 18 (12.5) | 1 | 0.29 | 0.812 |
| Age at first manic episode (mean, SD) | 27.4 (11.1) | 23.5 (6.8) | 78.5 | −2.57 | 0.012 |
| Number of psych hospitalizations per year (mean, SD) | 0.2 (0.3) | 0.3 (0.4) | 209 | 0.94 | 0.351 |
| Number of suicide attempts per year (mean, SD) | 0.1 (0.2) | 0.1 (0.1) | 209 | −0.14 | 0.886 |
| Number of mood episodes per year (mean, SD) | 1.2 (1.2) | 1.8 (3.9) | 202 | 1.14 | 0.254 |
| CARS-M score (mean, SD) | 3.4 (4.0) | 3.4 (4.4) | 209 | −0.01 | 0.989 |
| HDRS score (mean, SD) | 6.1 (5.9) | 8.3 (6.8) | 207 | 2.16 | 0.032 |
| Premorbid Traits | |||||
| Premorbid Adjustment – Childhood Score (6-11y) | 0.2 (0.2) | 0.2 (0.2) | 118.6 | −0.80 | 0.424 |
| Premorbid Adjustment – Early Adolescence Score (12-15y) | 0.2 (0.2) | 0.2 (0.2) | 201 | −1.35 | 0.179 |
| SPQ Ideas of Reference Score | 4.0 (3.1) | 2.9 (2.6) | 203 | 2.75 | 0.007 |
| SPQ Unusual Perceptual Experiences Score | 3.0 (2.9) | 2.4 (2.4) | 144.4 | 2.30 | 0.02 |
| SPQ – No Close Friends Score | 4.4 (3.0) | 3.6 (2.7) | 204 | 1.95 | 0.02 |
| SPQ – Odd Speech Score | 3.6 (2.7) | 4.3 (2.6) | 202 | −1.91 | 0.05 |
| SPQ – Suspiciousness Score | 3.8 (2.8) | 3.7 (2.8) | 202 | 0.09 | 0.922 |
| Childhood Trauma Total Score | |||||
| CTQ Physical Abuse subscore | 9.8 (5.1) | 8.6 (4.3) | 204 | −1.75 | 0.08 |
| CTQ Physical Neglect subscore | 11.5 (3.5) | 10.2 (2.5) | 205 | −2.64 | 0.009 |
| CTQ Sexual Abuse subscore | 9.2 (5.7) | 7.3 (4.3) | 86.9 | −2.36 | 0.02 |
| Neurocognitive functioning | |||||
| MCCB composite T-score | 38.6 (8.2) | 44.7 (6.9) | 204 | 5.42 | < 0.001 |
| WRAT-3 standard score | 93.4 (15.0) | 104.2 (13.9) | 209 | 4.97 | < 0.001 |
| Pharmacological Exposure | |||||
| Use of antipsychotics (%) | 31 (50.8) | 75 (50.7) | 1 | 0.00 | 1.00 |
| Use of lithium (%) | 7 (11.5) | 17 (11.5) | 1 | 0.00 | 1.00 |
| Use of anticonvulsants (%) | 23 (37.7) | 63 (42.6) | 1 | 0.42 | 0.54 |
| Use of benzodiazepines [%) | 4 (6.6) | 16 (10.8) | 1 | 0.90 | 0.44 |
| Global and Financial Functioning | |||||
| UPSA total score | 16.3 (2.4) | 17.2 (2.0) | 95.5 | 2.70 | 0.008 |
| UPSA finance skills | 8.8 (1.8) | 9.4 (1.4) | 94.4 | 2.38 | 0.02 |
| UPSA communication skills | 7.5 (1.1) | 7.8 (1.1) | 206 | 1.89 | 0.06 |
| SAS total score | 2.0 (0.5) | 2.4 (0.6) | 209 | 3.49 | 0.001 |
| Comorbid Substance Use | |||||
| Current alcohol abuse (n, %) | 9 (14.8) | 49 (32.5) | 1 | 8.5 | 0.014 |
| Lifetime substance abuse (n, %) | 32 (52.5) | 65 (44.5) | 1 | 1.27 | 0.288 |
| Impulsivity Traits | |||||
| BIS total score | |||||
| Impulsivity Motor sub-score | 24.1 (5.3) | 25.0 (5.5) | 208 | 0.99 | 0.32 |
| Impulsivity Attentional sub-score | 17.0 (3.3) | 17.9 (4.6) | 151.0 | 1.50 | 0.136 |
| Impulsivity Non-Planning sub-score | 26.5 (4.8) | 26.7 (5.5) | 208 | 0.24 | 0.808 |
SD: Standard Deviation, BD: Bipolar Disorder, CARS-M: Clinical Administered Rating Scale for Mania, HDRS: Hamilton Depression Rating Scale 24 item, SPQ: Schizotypal Personality Questionnaire, CTQ: Childhood Trauma Questionnaire, MCCB: MATRICS Consensus Cognitive Battery, WRAT-3: Wide Range Achievement Test, UPSA: University Of California, San Diego Performance-Based Skills Assessment, AUDIT: Alcohol Use Disorders Identification Test, BIS: Barratt Impulsivity Scale. Data represented as % or Mean (±SD).
However, interesting differences were noted on demographic factors, premorbid features, and neurocognitive performance. Overall, we found that the impaired cluster had a lower proportion of females, a lower proportion of Caucasians, fewer years of education, and lower premorbid IQ than did the intact cluster. BD patients in the impaired cluster were more likely to have suffered childhood trauma (specifically, physical and sexual abuse as well as physical neglect). Perhaps not surprisingly, the impaired cluster had more severe neurocognitive impairment and lower psychosocial functioning than did the intact cluster (Table 2). Finally, the impaired cluster had a higher number of psychosis-like traits as evidenced by a significantly higher score in the following subscales: ideas of reference, unusual perceptual experiences, no close friends, and suspiciousness (Table 2). Finally, patients in the impaired cluster exhibited a lower proportion of current alcohol abuse, as compared to intact patients (14.8% vs 32.5%).
After adjusting for demographic variables, of all the variables shown significant in Table 2, only childhood sexual abuse (OR=2.24, p=0.02), composite neurocognitive score (OR for a one-unit increase in Z-score = 0.90, p< 0.001), and premorbid IQ (OR for a one-unit increase in standardized score = 0.96, p<0.001) predicted cluster membership.
Finally, we explored whether clusters based on EP performance still predicted functional and cognitive outcomes after accounting by the a priori selected confounders, namely, sex, race, premorbid IQ and mean overall response latency (i.e., as a proxy of overall EP task proficiency). We observed that, despite an attenuation in the point estimate, the observed clusters still predicted clinically relevant outcomes for BD patients such as psychosocial and financial functioning and neurocognitive status (Table 3). Moving from the “intact” to the “impaired” cluster was associated with a deterioration in UPSA total scores, UPSA financial scores, SAS total scores and the composite z-scores for global neurocognitive functioning.
Table 3.
Crude and Adjusted coefficient for cluster membership and selected outcomes
| Outcome | Crude coefficient for cluster | 95% CI | Adjusted coefficient for cluster# | 95% CI |
|---|---|---|---|---|
| Psychosocial Functioning (UPSA total score) | −0.94 | −1.56 to −0.31 | −0.60 | −1.28 to 0.09 |
| Psychosocial Functioning (SAS total score) | −0.29 | −0.46 to −0.13 | −0.31 | −0.48 to −0.12 |
| Finance Skills (UPSA score) | −0.61 | −1.10 to −0.15 | −0.32 | −0.80 to 0.16 |
| MCCB composite score | −6.12 | −8.35 to −3.90 | −2.36 | −4.56 to −0.17 |
Cluster coefficient reflects the change in the outcome associated with being in the impaired cluster as compared to the intact cluster.
β coefficient adjusted by gender, premorbid IQ level (standardized WRAT-3 vocabulary) and by mean response latency to complete EP tasks.
DISCUSSION
To our knowledge, this is the first study to use an empirical approach to classify BD patients based upon emotional processing performance profiles. Using validated measures of emotion recognition (RMET and individual basic emotion recognition from the ERT), we classified patients based upon their objective performance, identifying two discrete subgroups – one with emotion processing deficits and the other with intact emotion processing.
When comparing the full sample of affectively stable BD patients (n=212) to healthy controls, performance was comparable on both of the emotion processing tasks. These results are consistent with some prior studies that have reported intact emotion processing in BD(Aparicio et al., 2017; Martino et al., 2011; Robinson et al., 2015) but are in contrast with several other studies that have reported impaired performance in BD(Barrera et al., 2013; David et al., 2014; de Brito Ferreira Fernandes et al., 2016; Samamé et al., 2015). These conflicting findings may be due to the presence of within-group heterogeneity, as has been previously shown on measures of neurocognitive functioning(Burdick et al., 2014; Russo et al., 2017; Van Rheenen et al., 2017).
Indeed, using hierarchical clustering, we found empirical evidence supporting the existence of two distinct clusters among BD patients. One “intact” cluster represented 71.2% of the sample and performed similarly to HCs and even outperformed controls on anger and surprise recognition (Figure 1). The other cluster (“impaired”) performed significantly worse than HCs on all emotion processing measures. The impaired cluster had deficits that were as severe or even more severe than those seen in a sample of SZ patients who were tested on the same battery (Supplementary eFigure 2).
When characterizing these BD clusters on demographic, clinical, and cognitive features, we found that the impaired cluster was characterized by lower cognitive reserve (based upon education level and premorbid IQ), higher rates of childhood trauma, and more salient psychosis-like traits (e.g. schizotypy; but not psychosis history). These characteristics - which tend to be early life features or ‘traits’ rather than ‘state’ characteristics - might act as markers to distinguish this subgroup of patients as one with a more neurodevelopmentally-abnormal course. The impaired cluster had worse outcomes than did the intact cluster including poorer psychosocial functioning, financial skills, and neurocognitive impairment. However, we observed that “intact” patients presented an earlier age at onset (i.e., age at first manic episode). This should not be readily interpreted as suggesting that these patients present a more severe course, since it could point that “intact” patients might be suffering from a more ‘classical’ form of BD characterized by full-blown episodes and return to euthymia while the “impaired” cluster could be experiencing a more subsyndromal and chronic symptoms course disease and thus, making it easier to diagnose BD for the “intact” subgroup, reflected by the earlier age at diagnosis. Interestingly, these clusters could not be differentiated by using other typical clinical measures, such as BD subtype, rapid cycling, psychosis history, or number of prior mood episodes. In sum, these clusters do not simply recapitulate traditional diagnostic subtypes, rather they may represent a different and clinically meaningful classification of patients.
Our results could have direct clinical implications. First, our findings suggest that not all BD patients are alike, a fact that is perhaps obvious but one that is often overlooked in studies that focus on BD at the diagnostic group-level. We show empirical evidence that reveals that there are indeed some BD patients with significantly impaired emotional processing and some BD patients who are intact or even highly skilled in this domain, which may speak to the need for personalized treatment approaches for the subgroup of patients that might benefit most from them. For example, the impaired cluster of BD patients might optimally benefit from a specific type of intervention targeting this deficit, such as attentional bias training techniques. Furthermore, these subgroups could aid clinicians in identifying patients at higher risk for poorer outcomes and potentially be a target for a functional remediation therapy(Bonnin et al., 2016; Sanchez-Moreno et al., 2017). Finally, given that many of the markers for being in the impaired cluster were early life risk factors, intervention efforts should focus on high-risk settings (i.e., having first-degree relative affected with BD) during childhood and adolescence to prevent its development(Miklowitz et al., 2017). Furthermore, we found consistent evidence linking childhood trauma with impaired emotion recognition both in samples of psychiatric patients(Aas et al., 2017) and healthy adults(Chu et al., 2016). Although this line of research is particularly interesting as it could raise potential therapeutic implications from our findings, such as the recent evidence linking oxytocin administration to improve emotion processing abilities in adults with a prior history of childhood adversity(Schwaiger et al., 2018), these results should also be appraised with caution. Since childhood adversity and emotional processing were measured at the same interview, the directionality of this association remains to be unraveled by further studies.
Further limitations such as the cross-sectional nature of the study should be acknowledged. Furthermore, it remains to be tested if these emotion processing deficits are primary in their nature or secondary to more general neurocognitive impairment, use of psychotropic medications, or other illness-related factors. We might conclude that there is an effect of cluster on global functioning outcomes that might be direct or mediated by neurocognitive impairment. To test this idea, we ran a mediation analysis to determine the direct effect of cluster belonging on psychosocial functioning and to determine the proportion of the effect that was mediated through neurocognitive impairment. We found that more than half of the total effect of EP clusters on psychosocial functioning is mediated through neurocognitive functioning as measured by the composite score of the MCCB. Intervening to improve neurocognitive impairments could remove more than half of the deleterious effects of poor emotional processing abilities on psychosocial functioning in patients with BD. This is in line with neural network models of emotion regulation, which point to a core regulatory role for cognitive control in top-down processing of emotional information. In fact, it is hypothesized that cognitive remediation strategies that target cognitive control would lead to improvements in emotion recognition and emotional processing (Hooker et al., 2012; Mendella et al., 2015; Peña et al., 2018)., as both processes depend on the same regulatory circuits (e.g. salience networks) (Hooker et al., 2012).
As suggested by prior research, the influences of psychotropic medication on emotional recognition should be carefully appraised(Bilderbeck et al., 2017). Although we collect information regarding the number of meds and the type, we do not collect the dose nor duration of treatment. Another limitation is the use of Caucasian male faces in the CANTAB ERT -- research using ERT would benefit from having a more diverse choice of faces, as there are known effects of both race and sex on emotion recognition. Despite this limitation of the test, we have made every effort to control for race, sex and other relevant demographic confounders in our analyses.
Our data suggest the existence of different profiles of emotional processing in patients diagnosed with bipolar disorder. Importantly, these profiles were proven to be independent of the premorbid IQ, and the basic neurocognitive demands of the emotion processing tasks. In this sense, emotion processing performance may emerge as a potential marker of poor outcome and neurodevelopmental insults. Further studies with larger samples and longitudinal designs are needed to confirm our results, to establish the stability of the obtained clusters, and to elucidate whether these EP deficits are primary or secondary in their nature.
Supplementary Material
HIGHLIGHTS.
We show that patients with BD cluster in two in terms of their Emotional Processing performance.
Patients with poor EP presented: childhood trauma, schizotypal traits, lower premorbid IQ and education, poor psychosocial functioning and cognitive performance.
Patients with poor EP might represent a subgroup of BD patients with neurodevelopmental abnormalities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
None
REFERENCES
- Aas M, Kauppi K, Brandt CL, Tesli M, Kaufmann T, Steen NE, Agartz I, Westlye LT, Andreassen OA, Melle I, 2017. Childhood trauma is associated with increased brain responses to emotionally negative as compared with positive faces in patients with psychotic disorders. Psychol Med 47, 669–679. 10.1017/S0033291716002762 [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM, 1994. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol. Psychiatry 36, 124–134. [DOI] [PubMed] [Google Scholar]
- Aparicio A, Santos JL, Jiménez-López E, Bagney A, Rodríguez-Jiménez R, Sánchez-Morla EM, 2017. Emotion processing and psychosocial functioning in euthymic bipolar disorder. Acta Psychiatr Scand 135, 339–350. 10.1111/acps.12706 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M, 1997. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry 38, 813–822. [DOI] [PubMed] [Google Scholar]
- Barrera A, Vázquez G, Tannenhaus L, Lolich M, Herbst L, 2013. Theory of mind and functionality in bipolar patients with symptomatic remission. Rev Psiquiatr Salud Ment 6, 67–74. 10.1016/j.rpsm.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151, 1132–1136. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Bilderbeck AC, Atkinson LZ, Geddes JR, Goodwin GM, Harmer CJ, 2017. The effects of medication and current mood upon facial emotion recognition: findings from a large bipolar disorder cohort study. J. Psychopharmacol. (Oxford) 31, 320–326. 10.1177/0269881116668594 [DOI] [PubMed] [Google Scholar]
- Bonnin CM, Torrent C, Arango C, Amann BL, Solé B, González-Pinto A, Crespo JM, Tabarés-Seisdedos R, Reinares M, Ayuso-Mateos JL, García-Portilla MP, Ibañez Á, Salamero M, Vieta E, Martinez-Aran A, CIBERSAM Functional Remediation Group, 2016. Functional remediation in bipolar disorder: 1-year follow-up of neurocognitive and functional outcome. Br J Psychiatry 208, 87–93. 10.1192/bjp.bp.114.162123 [DOI] [PubMed] [Google Scholar]
- Bora E, Bartholomeusz C, Pantelis C, 2016. Meta-analysis of Theory of Mind (ToM) impairment in bipolar disorder. Psychol Med 46, 253–264. 10.1017/S0033291715001993 [DOI] [PubMed] [Google Scholar]
- Bora E, Pantelis C, 2015. Meta-analysis of Cognitive Impairment in First-Episode Bipolar Disorder: Comparison With First-Episode Schizophrenia and Healthy Controls. Schizophr Bull 41, 1095–1104. 10.1093/schbul/sbu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C, 2009. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord 113, 1–20. 10.1016/j.jad.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Bourne C, Aydemir Ö, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh JTO, Clark L, Cubukcuoglu Z, Dias VV, Dittmann S, Ferrier IN, Fleck DE, Frangou S, Gallagher P, Jones L, Kieseppä T, Martínez-Aran A, Melle I, Moore PB, Mur M, Pfennig A, Raust A, Senturk V, Simonsen C, Smith DJ, Bio DS, Soeiro-de-Souza MG, Stoddart SDR, Sundet K, Szöke A, Thompson JM, Torrent C, Zalla T, Craddock N, Andreassen OA, Leboyer M, Vieta E, Bauer M, Worhunsky PD, Tzagarakis C, Rogers RD, Geddes JR, Goodwin GM, 2013. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr Scand 128, 149–162. 10.1111/acps.12133 [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Cornblatt BA, Keefe RS, Gopin CB, Derosse P, Braga RJ, Malhotra AK, 2011. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology 36, 1587–1592. 10.1038/npp.2011.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, Malhotra AK, 2014. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol Med 44, 3083–3096. 10.1017/S0033291714000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Potkin SG, Wyatt RJ, 1982. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 8, 470–484. [DOI] [PubMed] [Google Scholar]
- Chu DA, Bryant RA, Gatt JM, Harris AWF, 2016. Failure to differentiate between threat-related and positive emotion cues in healthy adults with childhood interpersonal or adult trauma. J Psychiatr Res 78, 31–41. 10.1016/j.jpsychires.2016.03.006 [DOI] [PubMed] [Google Scholar]
- David DP, Soeiro-de-Souza MG, Moreno RA, Bio DS, 2014. Facial emotion recognition and its correlation with executive functions in bipolar I patients and healthy controls. J Affect Disord 152–154, 288–294. 10.1016/j.jad.2013.09.027 [DOI] [PubMed] [Google Scholar]
- de Brito Ferreira Fernandes F, Gigante AD, Berutti M, Amaral JA, de Almeida KM, de Almeida Rocca CC, Lafer B, Nery FG, 2016. Facial emotion recognition in euthymic patients with bipolar disorder and their unaffected first-degree relatives. Compr Psychiatry 68, 18–23. 10.1016/j.comppsych.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Depp CA, Moore DJ, Sitzer D, Palmer BW, Eyler LT, Roesch S, Lebowitz BD, Jeste DV, 2007. Neurocognitive Impairment in Middle-Aged and Older Adults with Bipolar Disorder: Comparison to Schizophrenia and Normal Comparison Subjects. J Affect Disord 101, 201–209. 10.1016/j.jad.2006.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Fisher M, Verosky SC, Miyakawa A, Vinogradov S, 2012. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophr. Res 139, 53–59. 10.1016/j.schres.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Knorr U, Vinberg M, Kessing LV, Miskowiak KW, 2016. Discrete neurocognitive subgroups in fully or partially remitted bipolar disorder: Associations with functional abilities. J Affect Disord 205, 378–386. 10.1016/j.jad.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongür D, 2014. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med 44, 3239–3248. 10.1017/S0033291714000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D, 2011. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disord 13, 334–342. 10.1111/j.1399-5618.2011.00935.x [DOI] [PubMed] [Google Scholar]
- Martino DJ, Marengo E, Igoa A, Strejilevich SA, 2018. Neurocognitive heterogeneity in older adults with bipolar disorders. Psychiatry Res 262, 510–512. 10.1016/j.psychres.2017.09.035 [DOI] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Fassi G, Marengo E, Igoa A, 2011. Theory of mind and facial emotion recognition in euthymic bipolar I and bipolar II disorders. Psychiatry Res 189, 379–384. 10.1016/j.psychres.2011.04.033 [DOI] [PubMed] [Google Scholar]
- Martino DJ, Strejilevich SA, Scápola M, Igoa A, Marengo E, Ais ED, Perinot L, 2008. Heterogeneity in cognitive functioning among patients with bipolar disorder. J Affect Disord 109, 149–156. 10.1016/j.jad.2007.12.232 [DOI] [PubMed] [Google Scholar]
- Mendella PD, Burton CZ, Tasca GA, Roy P, St Louis L, Twamley EW, 2015. Compensatory cognitive training for people with first-episode schizophrenia: results from a pilot randomized controlled trial. Schizophr. Res 162, 108–111. 10.1016/j.schres.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Schneck CD, Walshaw PD, Garrett AS, Singh MK, Sugar CA, Chang KD, 2017. Early intervention for youth at high risk for bipolar disorder: A multisite randomized trial of family-focused treatment. Early Interv Psychiatry. 10.1111/eip.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto RG, Castellanos FX, 2011. A meta-analysis of neuropsychological functioning in patients with early onset schizophrenia and pediatric bipolar disorder. J Clin Child Adolesc Psychol 40, 266–280. 10.1080/15374416.2011.546049 [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165, 203–213. 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- Olvet DM, Stearns WH, McLaughlin D, Auther AM, Correll CU, Cornblatt BA, 2010. Comparing clinical and neurocognitive features of the schizophrenia prodrome to the bipolar prodrome. Schizophr. Res 123, 59–63. 10.1016/j.schres.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV, 2001. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull 27, 235–245. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Peña J, Ibarretxe-Bilbao N, Sánchez P, Uriarte JJ, Elizagarate E, Gutierrez M, Ojeda N, 2018. Mechanisms of functional improvement through cognitive rehabilitation in schizophrenia. J Psychiatr Res 101, 21–27. 10.1016/j.jpsychires.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Raine A, 1991. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull 17, 555–564. [DOI] [PubMed] [Google Scholar]
- Ratheesh A, Lin A, Nelson B, Wood SJ, Brewer W, Betts J, Berk M, McGorry P, Yung AR, Bechdolf A, 2013. Neurocognitive functioning in the prodrome of mania--an exploratory study. J Affect Disord 147, 441–445. 10.1016/j.jad.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E, 2009. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull 35, 1022–1029. 10.1093/schbul/sbn044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Gray JM, Burt M, Ferrier IN, Gallagher P, 2015. Processing of Facial Emotion in Bipolar Depression and Euthymia. J Int Neuropsychol Soc 21, 709–721. 10.1017/S1355617715000909 [DOI] [PubMed] [Google Scholar]
- Russo M, Van Rheenen TE, Shanahan M, Mahon K, Perez-Rodriguez MM, Cuesta-Diaz A, Larsen E, Malhotra AK, Burdick KE, 2017. Neurocognitive subtypes in patients with bipolar disorder and their unaffected siblings. Psychol Med 47, 2892–2905. 10.1017/S003329171700143X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samamé C, Martino DJ, Strejilevich SA, 2015. An individual task meta-analysis of social cognition in euthymic bipolar disorders. J Affect Disord 173, 146–153. 10.1016/j.jad.2014.10.055 [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno J, Bonnín C, González-Pinto A, Amann BL, Solé B, Balanzá-Martínez V, Arango C, Jimenez E, Tabarés-Seisdedos R, Garcia-Portilla MP, Ibáñez A, Crespo JM, Ayuso-Mateos JL, Vieta E, Martinez-Aran A, Torrent C, CIBERSAM Functional Remediation Group, 2017. Do patients with bipolar disorder and subsyndromal symptoms benefit from functional remediation? A 12-month follow-up study. Eur Neuropsychopharmacol 27, 350–359. 10.1016/j.euroneuro.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Schwaiger M, Heinrichs M, Kumsta R, 2018. Oxytocin administration and emotion recognition abilities in adults with a history of childhood adversity. Psychoneuroendocrinology 99, 66–71. 10.1016/j.psyneuen.2018.08.025 [DOI] [PubMed] [Google Scholar]
- Sugar CA, Sturm R, Lee TT, Sherbourne CD, Olshen RA, Wells KB, Lenert LA, 1998. Empirically defined health states for depression from the SF-12. Health Serv Res 33, 911–928. [PMC free article] [PubMed] [Google Scholar]
- Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, Gurvich C, Pantelis C, Malhotra AK, Rossell SL, Burdick KE, 2017. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med 47, 1848–1864. 10.1017/S0033291717000307 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.