Abstract
Background and Aims
Root proliferation is a response to a heterogeneous nutrient distribution. However, the growth of root hairs in response to heterogeneous nutrients and the relationship between root hairs and lateral roots remain unclear. This study aims to understand the effects of heterogeneous nutrients on root hair growth and the trade-off between root hairs and lateral roots in phosphorus (P) acquisition.
Methods
Near-isogenic maize lines, the B73 wild type (WT) and the rth3 root hairless mutant, were grown in rhizoboxes with uniform or localized supply of 40 (low) or 140 (high) mg P kg−1 soil.
Results
Both WT and rth3 had nearly two-fold greater shoot biomass and P content under local than uniform treatment at low P. Significant root proliferation was observed in both WT and rth3 in the nutrient patch, with the WT accompanied by an obvious increase (from 0.7 to 1.2 mm) in root hair length. The root response ratio of rth3 was greater than that of WT at low P, but could not completely compensate for the loss of root hairs. This suggests that plants enhanced P acquisition through complementarity between lateral roots and root hairs, and thus regulated nutrient foraging and shoot growth. The disappearance of WT and rth3 root response differences at high P indicated that the P application reduced the dependence of the plants on specific root traits to obtain nutrients.
Conclusions
In addition to root proliferation, the root response to a nutrient-rich patch was also accompanied by root hair elongation. The genotypes without root hairs increased their investment in lateral roots in a nutrient-rich patch to compensate for the absence of root hairs, suggesting that plants enhanced nutrient acquisition by regulating the trade-off of complementary root traits.
Keywords: Maize (Zea mays L.), rth3, root hairs, heterogeneous nutrient supply, phosphorus acquisition, root proliferation
INTRODUCTION
Phosphorus (P) has strong fixation and poor mobility in soil, resulting in its low availability in soil and low P-acquisition efficiency by plants (Hinsinger, 2001; Lambers and Plaxton, 2015; Shen et al., 2019). Plants have evolved diverse root traits to enhance P acquisition (Shen et al., 2011; Wang and Lambers, 2019), such as morphologically exploring larger soil volumes through lateral roots and root hairs (Postma and Lynch, 2012; Wang and Shen, 2019), and physiologically increasing the secretion of protons and carboxylates to dissolve insoluble soil P (Hinsinger et al., 2011; Wang and Lambers, 2019; Wen et al., 2019). Among these adaptive strategies, root hairs are considered a cheap, ‘cost-effective’ strategy because the direct cost of carbon allocation to root hair is basically considered to be minimal (Bates and Lynch, 2000; Lynch and Ho, 2005; Brown et al., 2013). Root hairs that extend outward from the roots expand the consuming area around the roots, allowing P to be absorbed by the plant from a larger volume of soil (Brown et al., 2013; Pausch et al., 2016; Ma et al., 2021). Studies have shown that the diameter of the root hair cylinder around the root is about 10 times greater than that of the root itself, directly expanding the soil volume that the root can explore by almost 100 times (Dittmer, 1937; Rongsawat et al., 2021).
Root hair formation and growth have been demonstrated to be regulated by soil P availability (Ma et al., 2001a; Miguel et al., 2015). Alterations in root hair traits in response to P deficiency include root hair length and density as well as the size and location of the root hair zone (Bates and Lynch, 1996; Ma et al., 2001a; Brown et al., 2013). Generally, root hairs tend to be short and sparse in P-sufficient plants, but increase in length and density as plants sense P deficiency (Gahoonia et al., 1997; Zhu et al., 2010; Nestler et al., 2016). For instance, Arabidopsis thaliana developed longer and denser root hairs under low P conditions in comparison with high P, and the enhancement of these root hair traits was demonstrated to take up more P (Gahoonia et al., 1997; Bates and Lynch, 2001; Ma et al., 2001a). In addition, studies with bald barley (brb) mutants have provided an overview of the importance of root hairs to P acquisition, rhizosphere extension (expand the rhizosphere size) and crop growth (Gahoonia et al., 2001; Dodd and Diatloff, 2016; Holz et al., 2018). However, most of these experiments were carried out in medium or soil with uniform nutrients, and there is a lack of systematic understanding of root hair formation and response in heterogeneous nutrient environments.
Nutrient distribution in natural soil environments is highly heterogeneous, both spatially and temporally. To capture more nutrients, plant roots exhibit morphological or physiological plasticity to the heterogeneous nutrient environment (Hodge, 2004; Hodge, 2006; Mommer et al., 2012; Li et al., 2014). A classic study by Drew (1975) showed that the length and number of primary and secondary lateral roots of barley were greatly stimulated by localized supply of phosphate, ammonium (NH4+-N) or nitrate (NO3−-N). Subsequent studies have shown that root proliferation in some crops is one of the most important root response characteristics in nitrogen-rich or P-rich soil patches (Robinson, 1994; Hodge, 2004; Officer et al., 2009; Jing et al., 2010; Li et al., 2014; Ma et al., 2014). For instance, in the field, localized application of NH4+-N and P significantly stimulated the proliferation of maize lateral roots and promoted maize growth as well as P uptake (Jing et al., 2010; Ma et al., 2014). The underlying mechanism is that NH4+-N acidifies the rhizosphere soil environment and enhances the bioavailability of P on calcareous soil, while root proliferation improves P-acquisition efficiency by plants (Jing et al., 2010; Shen et al., 2013). So far, the response of roots to heterogeneous soil environments has been widely reported and clearly understood, but how the root hairs respond in this process has never been reported.
In some studies, the root morphology of wild-type and root hairless mutants was compared under stress conditions. In barley, brb shows enhanced root growth in dry soil, which compensated for the surface loss of root hairs (Dodd and Diatloff, 2016). Maize root hairless mutant rth2 had more fine roots and increased specific root length (SRL) in both P-deficient and dry soil, which also largely compensated for the reduced root–soil contact due to rth2 shorted root hairs (Klamer et al., 2019). Similarly, maize lacking root hairs (rth3) increased mycorrhizal colonization in P-limited soil to obtain more P (Kumar et al., 2019; Ma et al., 2021). All these results demonstrate that there is a coordination and trade-offs among root functional traits (lateral roots, root hairs and arbuscular mycorrhizal fungi) to enhance the acquisition of soil nutrients. Fine roots with a diameter of <0.2 mm are generally considered as the roots with nutrient absorption function and are essential for obtaining soil nutrients. Plants, especially maize, generally increase the proportion of fine roots in response to heterogeneous nutrients (Lyu et al., 2016; Wen et al., 2017). Considering the characteristics of lateral roots and root hairs to obtain soil nutrients, it is worth determining whether a lack of root hairs affects root responses to heterogeneous nutrients. In addition, whether plants can increase lateral root growth to compensate for the absence of root hairs in nutrient-rich patches remains to be further studied.
Therefore, the present study was aimed at examining the responses of maize root hairless mutant rth3 and wild type under heterogeneous nutrient supply, and at testing the following hypotheses: (1) the absence of root hairs represses plant growth and increases the severity of P deficiency in maize; localized application of nutrients could alleviate the deficiency of P, especially in low P soil; and (2) for WT (with root hairs), root proliferation will be also accompanied by enhanced root hair growth in the nutrient-rich patch; for the rth3 mutant (without root hairs), maize growth will be facilitated by complementary functional traits (more intense root proliferation) in the nutrient-rich patch; functional compensation effects of root traits are expected to be repressed at high P supply in comparison to P deficiency.
MATERIALS AND METHODS
Experimental set-up
A long-term stored calcareous subsoil (air-dried, sieved to 2 mm) with low nutrient levels was used. Soil properties were as follows: pH 7.6 (1 : 5, soil : water), Olsen-P 2.6 mg kg−1, organic carbon 11.5 g kg−1, total N 0.72 g kg−1 and exchangeable K 32.3 mg kg−1. The use of this carbonate-rich subsoil is due to its low basal nutrient content and especially its low P availability. Mycorrhization was previously checked across a wide array of plant species samples (including maize) over several years in the same subsoil and confirmed to be absent, eliminating the effect of mycorrhiza on P acquisition (Neumann, 2007; Klamer et al., 2019). Before treatments were established, the following basal nutrients were uniformly added to the soil in the form of nutrient solution (mg kg−1 soil): 100 N (Ca(NO3)2.4H2O), 300 K (K2SO4), 100 Mg (MgSO4), 1.9 Fe (EDTA-FeNa), 2.6 Zn (ZnSO4.7H2O) and 1.0 Cu (CuSO4.5H2O). Plants were grown in semicircular PVC-rhizoboxes (height 48 cm; diameter 10 cm, Fig. 1A) as described by Mpanga et al. (2019). Every rhizobox was filled with 2.4 kg soil-sand substrate (1.68 kg soil was mixed well with 0.72 kg quartz sand to improve soil structure).
Fig. 1.
(A) Minirhizobox with root observation window based on semicircular PVC tubes. (B) Schematic representation of the local treatment. (C) Shoot growth under different treatments (just before harvest). (D) Root growth along the observation plane. (E) Lateral roots proliferate in the nutrient-rich patch (after washing the soil). (F) The root of wild type maize was accompanied by dense root hairs, while no root hairs were observed in the rth3 mutant.
Two maize (Zea mays L.) genotypes were used to test the root morphological and physiological differences in response to heterogeneous P supply: inbred line B73 as the wild type (WT) and the rth3 mutant, which was backcrossed to B73 more than seven times and is therefore nearly isogenic (Wen and Schnable, 1994). The rth3 mutant, isolated from Mutator transposon stocks, can initiate root hair primordia but fails to elongate them (Wen and Schnable, 1994). Subsequent studies demonstrated that the rth3 gene encodes a COBRA-like protein (Hochholdinger et al., 2008). Each maize genotype was tested at uniform P (homogeneous) and localized P (heterogenous) supply, respectively (two nutrient supply methods). There were two P application rates: 40 mg P kg−1 soil as low P and 140 mg P kg−1 soil as high P. For the uniform treatment, P (Ca(H2PO4)2.H2O) and NH4+-N (NH4Cl, 100 mg N kg−1 soil) was spread uniformly throughout the soil column in the form of nutrient solution. For the local treatment, the same amount of P and NH4+-N as in the uniform treatment was applied to the middle–upper part of the rhizobox in a 11-cm-high nutrient-rich patch (as shown in Fig. 1B). The uniform and local P treatments had the same amount of N and P added, but the positioning of that amount varied. The Olsen-P of uniformly nutrient-treated soil after fertilization was 6.6 mg kg−1 for low P and 60.2 mg kg−1 for high P. There were eight treatments (2 genotypes × 2 nutrient supply methods × 2 P levels), with four replicates for each treatment.
Plant growth conditions
The experiment was conducted in a naturally lit glasshouse at the university of Hohenheim, Stuttgart (48°42′44″N, 9°12′32.4″E). The seeds were surface-sterilized with 10 % (v/v) H2O2 for 15 min, washed clean with deionized water, imbibed in a 10 mm CaSO4 solution for 12 h, and germinated with a dark and humid surrounding (seeds were put between two layers of filter paper soaked with a 4 mm CaSO4 solution) in a growth chamber at 25 °C. About 3 d later, uniformly germinated seeds were selected and sown in each rhizobox. All rhizoboxes were arranged in a completely randomized design. During the growing period, each rhizobox was watered by weighting to keep soil moisture at 70 % of field water capacity. The temperature of the glasshouse was maintained at 23–28 °C during the day and 12–20 °C at night.
Measurement of root hair length and density
Before harvest, the transparent resin plate of the rhizobox was removed, and the roots were photographed in situ using a video macroscope (Stemi 200-c; Zeiss, Oberkochen, Germany) to obtain images. The criteria for randomly selected shooting positions were as follows: multiple positions were randomly selected from the top, middle and bottom layers of the rhizobox (two positions were selected from each layer for uniform treatment, and a total of six images were taken; for local treatment, a total of nine images were taken at three locations in the nutrient-rich patch, the upper and lower layers of the nutrient-rich area). The images obtained uniformly covered the primary and lateral roots. The images were then analysed to determine root hair length and density. Axio Vision software Version 3.1.2.1 (Zeiss) was used to measure the length of five randomly selected root hairs in each image. For root hair density, roots with a length of 3 mm were randomly measured on each image with ImageJ to obtain the number of root hairs, and the corresponding number of root hairs per millimetre was then obtained.
Harvest and measurements
All plants were harvested at 42 d after planting and separated into shoots and roots. The semicircular soil column was cut into four layers (0–11, 11–22, 22–33, 33–44 cm), and roots in each layer were collected after washing the soil from the roots and were kept in a refrigerator at −20 °C. To determine root traits, cleaned root samples were scanned at a resolution of 400 dpi. Root images were analysed with WinRHIZO (Regent Instruments Inc., Quebec, Canada) software to obtain the root length, root surface area and root length in different root diameter classes. Roots were then dried at 75 °C for 3 d and weighed.
Shoots were oven-dried at 105 °C for 30 min and then at 75 °C for 3 d, and weighed. Then, dry shoots were ground into powder. To determine shoot P concentrations, the powder shoot sample was incinerated at 580 °C for 4 h, and then dissolved in 2 mL of 1 : 1 (v,v) HNO3 and 18 mL of deionized water and filtered through filter paper. Shoot P concentration was assayed using the vanadate–molybdate method (Johnson and Ulrich, 1959).
Root response ratio and fine root response ratio
Root morphological plasticity was estimated by the root response ratio (RRS) to local nutrient enrichment, which was defined as the extent of root proliferation in the nutrient-enriched patch (11–22 cm layer) in comparison to the same 11–22 cm layer in the uniform nutrient supply treatment (Valladares et al., 2006; Li et al., 2014). Calculation of RRS was based on the following formula,
where RRS is the root response ratio to the localized nutrient patches; Xi is the root length in the nutrient-enriched patch (11–22 cm) and Xi′ is the root length in the 11–22 cm layer in the uniform nutrient treatment. Here i and i′ are two randomly selected individuals (in the present study, i = 1, 2, 3, 4 and i′ = 1, 2, 3, 4, because each treatment had four replicates) of the same genotype belonging to two different nutrient-supplied treatments (localized and uniform) but the same P level (low P or high P); and n = 16 because of each pair of nutrient supply treatments had four replicates. For fine root (<0.2 mm) response ratios, the calculation was the same as for RRS (root length in the formula is replaced by fine root length).
Statistical analyses
Three-way analyses of variance (ANOVAs) considering P levels, nutrient supply methods and genotypes were performed for plant shoot biomass, root biomass, root shoot ratio, shoot P concentration, shoot P content and root length in the nutrient-rich patch (Table 1) using SPSS statistical software (SPSS 20.0; IBM SPSS Inc., Chicago, IL, USA). One-way ANOVA was performed for all treatments with the same nutrient supply method. A t-test was performed for uniform and local nutrient supply under the same genotype and P level. Significant differences among means were separated by Tukey’s test [honest significant difference (HSD)] at the 0.05, 0.01 and 0.001 probability levels (0.01 < P ≤ 0.05, 0.001 < P ≤ 0.01 and P ≤ 0.001).
Table 1.
Three-way analysis of variance (ANOVA) of the effects of phosphorus (P), nutrient supply method (N), genotype (G) and their interaction on maize growth. Significant P values (P ≤ 0.05) are in bold
| Parameter | Shoot biomass | Root biomass | Root/shoot ratio | Shoot P concentration | Shoot P content | Root length in nutrient patch | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |
| P | 110.645 | 0 | 134.296 | 0 | 0.305 | 0.586 | 13.507 | 0.001 | 65.403 | 0 | 39.536 | 0 |
| N | 36.794 | 0 | 2.203 | 0.151 | 20.319 | 0 | 2.615 | 0.119 | 0.397 | 0.535 | 195.346 | 0 |
| G | 45.926 | 0 | 51.681 | 0 | 1.219 | 0.281 | 13.078 | 0.001 | 42.261 | 0 | 45.763 | 0 |
| P × N | 87.116 | 0 | 31.71 | 0 | 14.928 | 0.001 | 22.63 | 0 | 66.933 | 0 | 23.287 | 0 |
| P × G | 2.309 | 0.142 | 7.432 | 0.012 | 0.685 | 0.416 | 0.768 | 0.39 | 3.861 | 0.061 | 1.758 | 0.197 |
| N × G | 0.476 | 0.497 | 6.015 | 0.022 | 8.666 | 0.007 | 0.353 | 0.558 | 0.627 | 0.436 | 3.188 | 0.087 |
| P × N × G | 7.089 | 0.014 | 4.747 | 0.039 | 0.008 | 0.927 | 1.453 | 0.24 | 0.098 | 0.757 | 0.482 | 0.494 |
RESULTS
Shoot and whole root growth
The interaction among P levels, nutrient supply methods and genotypes influenced maize shoot (P = 0.014) and root biomass (P = 0.039, Table 1). At low P level, the shoot and root biomass of the rth3 mutant were significantly lower than the WT in the uniform nutrient supply treatment, but there was no significant difference under the condition of localized nutrient supply (Fig. 2A, B). Compared with the uniform nutrient treatment, localized nutrient application significantly increased the shoot biomass of both the WT and rth3 mutant, as well as the root biomass of the rth3 mutant. At high P level, there was no significant difference in the shoot biomass between the WT and rth3 mutant under the uniform nutrient supply, but the root biomass of the rth3 mutant was significantly lower than that of the WT (Fig. 2A, B). In contrast to the uniform nutrient supply, both shoot and root biomass of the rth3 mutant were significantly lower than the WT in the localized nutrient treatment. Additionally, localized nutrient application significantly reduced the shoot biomass of the rth3 mutant in comparison with uniform nutrient supply, but did not affect the shoot biomass of the WT as well as root biomass of both the WT and rth3 mutant (Fig. 2A, B).
Fig. 2.
(A) Shoot biomass, (B) root biomass (B) and (C) root/shoot ratio of two maize genotypes grown in different nutrient supply treatments. Each value is the mean (+s.e.) of four replicates. One-way ANOVA was performed for all treatments under uniform or local nutrient supply; different letters denote significant differences (P ≤ 0.05) by the Tukey test (HSD). For a given genotype, asterisks indicate significant differences between the uniform or local nutrient supply: n.s., non-significant; *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01.
For the root/shoot ratio, there was no significant difference between the two genotypes at the same P level and nutrient supply method. Overall, the root/shoot ratio of the two maize genotypes under localized nutrient supply was lower than that under uniform nutrient supply, although it was only statistically significant in the WT at low P level (Fig. 2C).
Shoot P concentration and P accumulation
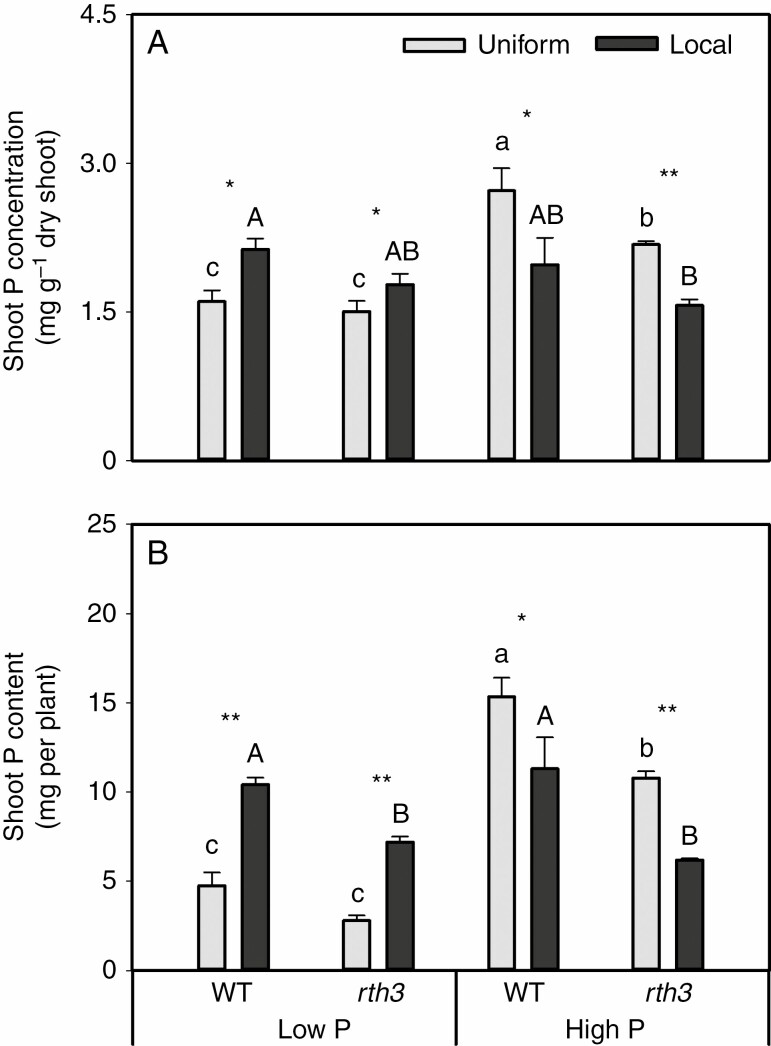
The shoot P concentration was influenced by the interaction of P levels × nutrient supply methods (P < 0.001, Table 1). At the same P level and supply method, the shoot P concentration of the rth3 mutant was significantly lower than that of the WT only at high P level and uniform treatment. The localized nutrient supply significantly increased the shoot P concentration of both the WT and rth3 mutant at low P level in comparison with the uniform treatment. By contrast, the shoot P concentration of both the WT and the rth3 mutant at high P level was significantly reduced under localized nutrient supply compared with uniform treatment (Fig. 3A).
Fig. 3.
(A) Shoot P concentration and (B) shoot P content of two maize genotypes grown in different nutrient supply treatments. Each value is the mean (+s.e.) of four replicates. One-way ANOVA was performed for all treatments under uniform or local nutrient supply; different letters denote significant differences (P ≤ 0.05) by the Tukey test (HSD). For a given genotype, asterisks indicate significant differences between the uniform or local nutrient supply: n.s., non-significant; *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01.
The shoot P content was also influenced by the interaction of P levels × nutrient supply methods (P < 0.001, Table 1). Shoot P content of the rth3 mutant tended to be always lower than in the WT under the same treatment (except for uniformly applied low P). For both WT and rth3 grown at low P, localized nutrient supply significantly increased the shoot P content of maize in comparison with uniform treatment. However, an opposite trend was observed at high P level: localized nutrient supply significantly reduced the shoot P content in both genotypes of maize (Fig. 3B).
Root distribution
The distribution of roots of the rth3 mutant and WT down the profile was influenced by the type of nutrient supply (Figs 1D, E and 4). At low P level, localized nutrient supply in the nutrient-enriched layer (11–22 cm) significantly induced root proliferation in both rth3 mutant and WT: root length in the nutrient-enriched layer (11–22 cm) was significantly greater than that in 11–22 cm layer in the uniform nutrient supply treatment (Fig. 4B). By contrast, negative effects (the root length of localized treatments were shorter than under uniform treatments) were recorded above and below the nutrient-enriched zone (0–11 and 33–44 cm; Fig. 4A, D). There was also no significant difference in root length between localized and uniform treatments in the 22–33 cm soil layer. At high P level, roots of the rth3 mutant and WT followed similar trends as in low P (Fig. 4C). The trend of variation of root surface area in different soil layers was basically similar with that of root length (Supplementary Data Fig. S1A–D).
Fig. 4.
Effects of different nutrient treatments on root length in different soil layers (A, 0–11 cm; B, 11–22 cm; C, 22–33 cm; D, 33–44 cm) of two maize genotypes. Each value is the mean (+s.e.) of four replicates. One-way ANOVA was performed for all treatments under uniform or local nutrient supply; different letters denote significant differences (P ≤ 0.05) by the Tukey test (HSD). For a given genotype, asterisks indicate significant differences between the uniform or local nutrient supply: n.s., non-significant; *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01.
A closer examination of the different root diameter classes in the 11–22 cm layer revealed that roots with smaller diameter were more affected by local nutrient treatment (Supplementary Data Fig. S2). Root lengths were significantly increased at all three diameter classification levels, but compared with the uniform treatment there was a significant increase in fine roots <0.2 mm (Fig. S2).
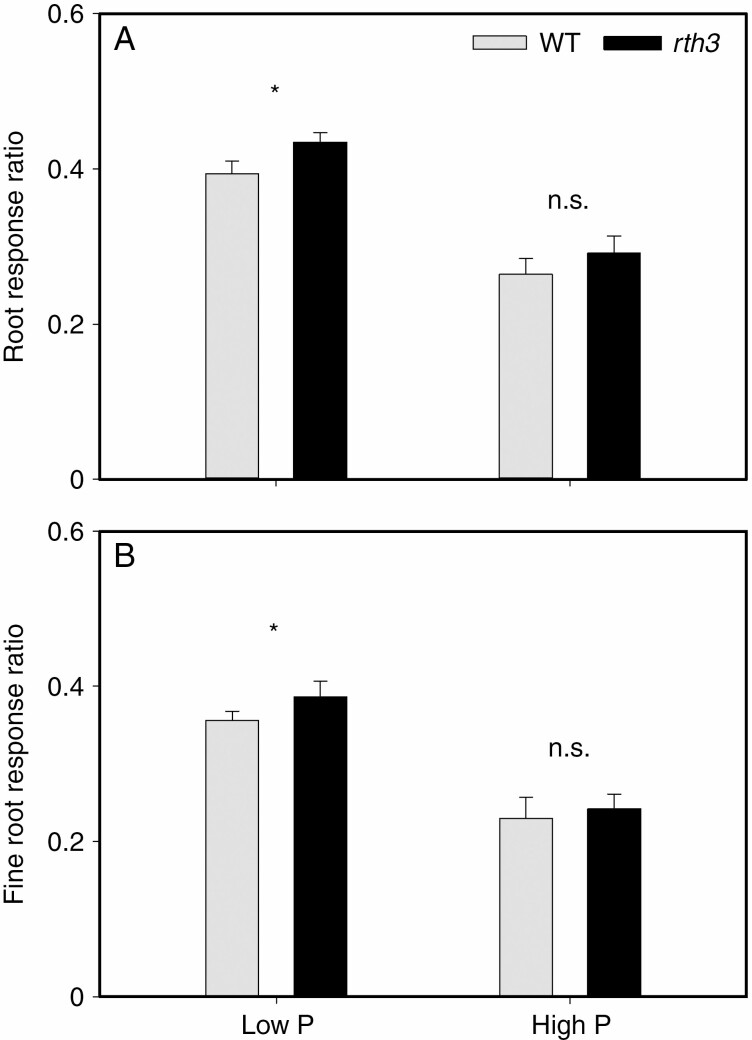
Root response ratios
The RRS of the rth3 mutant was significantly higher than that of the WT at low P level (Fig. 5A). However, there was no significant difference in RRS between the rth3 mutant and the WT at high P level. In addition, the RRS of both the WT and rth3 mutant grown in low P soil was significantly higher than in high P (Fig. 5A). The trend in fine root response ratios was the same as with RRS (Fig. 5B).
Fig. 5.
(A) Root response ratios and (B) fine root (<0.2 mm) response ratios to localized nutrient supply treatments in comparison to the uniform nutrient supply. Each value is the mean (+s.e.) of four replicates. For a given genotype, asterisks indicate significant differences between the uniform or local nutrient supply: n.s., non-significant; *0.01 < P ≤ 0.05.
Root hair length and density
For root hair parameters in different soil layers, significant changes in root hair length were observed in nutrient-rich patches, while root hair density was insensitive to P levels and heterogeneous nutrients (Fig. 6; Supplementary Data Fig. S3). Roots growing in a localized nutrient patch had about twice the length of root hairs in comparison with those under uniform nutrient supply, and this increase was independent of the P levels (Fig. 6A). However, no significant increase in root hair density was observed under local nutrient treatment. Roots formed about 20 hairs mm–1, whether they were uniformly and locally supplied with low or high P (Fig. 6B).
Fig. 6.
(A) Root hair length and (B) root hair density of the WT grown in the 11–22 cm layer under uniform and local nutrient supply. Asterisks indicate significant differences between the uniform or local nutrient supply: n.s., non-significant; ***P < 0.001.
Discussion
The present study confirms the importance of root hairs for P acquisition in maize, especially in soil with limited resources. Localized supply of P plus NH4+-N could promote shoot growth and P accumulation of maize regardless of root hairs (Hypothesis 1), but it also depended on P supply intensity (Figs 1C, 2A and 3). For the WT, root proliferation in the nutrient-rich patch was accompanied by increasing root hair elongation (Fig. 6); for the rth3 mutant, RRS was significantly greater than that of the WT in the nutrient-rich patch (Fig. 5), indicating that lateral root proliferation partially compensated for lack of root hairs (Hypothesis 2), but this compensation mechanism was affected by soil P supply intensity.
Positive effects of local nutrient supply on maize growth
Genotypes with short root hairs or without root hairs are restricted in growth when P resources were limited, as demonstrated in the present study and previous reports (Bates and Lynch, 2001; Dodd and Diatloff, 2016; Klamer et al., 2019). The rth3 mutants performed (including shoot growth and P accumulation) worse than WT under uniform low P supply conditions, while rth3 showed similar performance to WT under uniform high P supply (Figs 1C, 2A and 3), indicating the importance of root hairs for P acquisition in low P soil. The uniform supply of low P strongly reduced internal shoot P concentration (below the critical level of 2.5 mg g−1; Wen et al., 2017) of both the WT and the rth3 mutant as well as induced P deficiency symptoms such as leaf purpling and senescence of old leaves (Figs 1C and 3A). However, shoot biomass and P accumulation were greatly improved when the same amount of low P was locally supplied with NH4+-N (Figs 1C, 2A and 3), indicating that localized nutrient supply improved maize growth. Possible explanations include: (1) concentrated supply of nutrients increases P concentration in soil solutions, which is very important for P because the diffusion coefficient of P in soil is only 10–12–10–15 m2 s−1 (Schachtman et al., 1998); (2) root proliferation in a nutrient-rich patch and higher ratios of root length or root surface area to volume more effectively explore a given soil volume (Lynch, 1995), compensating for the uneven supply of nutrients to the whole root system (Robinson, 2001); and (3) ammonium-induced rhizosphere acidification improves the bioavailability of soil P (Jing et al., 2010; Shen et al., 2013). However, the mechanism of positive effects of local nutrient supply on plant growth is complex and may be a combination of multiple causes.
Many previous reports have investigated plant responses to local nutrients, and most of which did not observe an increase in the biomass and nutrient accumulation of individual plants (Robinson, 1994; He et al., 2012; Li et al., 2014). However, these studies are usually carried out in soil with sufficient nutrients, and the nutrient concentration was not lower than the critical level. Therefore, regardless of localized or uniform nutrient supply, the plant does not experience nutrient deficiency, so the superiority of local nutrients is not reflected in the biomass or nutrient accumulation. In the present study, both the WT and rth3 mutant supplied with low P had internal P concentrations lower than the critical level (2.5 mg g−1), and plants were in P starvation status (Fig. 3A). With locally restricted nutrient supply, the P concentration in both WT and rth3 mutant was increased (Fig. 3A), which significantly improved the internal P nutrition and possibly increased uptake of N, iron and zinc in maize as previously noted (Ma et al., 2014). Consistent with previous reports, the positive effects of local nutrients on shoot growth and nutrient accumulation were significantly reduced when externally applied P was high, and even showed negative effects on rth3 mutants (Figs 1C, 2A and 3). Therefore, a comprehensive consideration of nutrient patch attributes, including size and concentration, patch distribution and even nutrient composition and specific environment, is needed to stimulate the biological potential of plants to efficiently obtain soil nutrients (Hodge, 2004; Li et al., 2014; Zhang et al., 2019).
Positive effects of local nutrient supply on root hair growth
An elongation and increased density of root hairs is generally observed in homogeneous low P conditions (Jungk, 2001; Lambers et al., 2006). The effect of local nutrients on root hair growth was first quantified in the present study. Obviously, root hair length increased significantly in the nutrient-rich patch in comparison with uniform treatment (Fig. 6A), which may be another important phenotype of the root response to heterogeneous nutrients. Root hairs expand the radial rhizosphere by increasing root surface area and the root–soil contact area to explore a larger soil volume and thus increase soil exploration and nutrient acquisition (Jungk, 2001; Brown et al., 2013; Ma et al., 2021). In a study of Arabidopsis, some genotypes reduced root hair length or density when local P was deficient in agar plates (Stetter et al., 2015), again implying a positive response of root hair to P-rich patches. However, great care must be taken to predict the behaviour of root hairs in real soils based on these results from the controlled experiments, and further testing of more species and genotypes will be needed in future studies.
Plants can sense and respond to variations in nutrient availability and distribution in the soil (Hodge, 2004; Cahill et al., 2010; Li et al., 2014). Some plants invest resources into proliferating roots, in particular increasing the proportion of fine roots when root encounter nutrient-rich patch (Fransen and De Kroon, 2001; Hodge, 2004; Zhang et al., 2019). Studies on root foraging in nutrient-rich soil patches tend to focus on the fine root level, ignoring root hairs – a cheap, ‘cost’-effective strategy to acquire soil P. Compared with roots or root exudates, the carbon cost allocated directly to root hairs is generally considered to be lowest (Bates and Lynch, 2000). Root hairs are efficient at foraging for P resources in smaller cracks and/or pores where roots are unable to penetrate (Misra et al., 1988; Rongsawat et al., 2021). Additionally, the benefits of root hairs in P acquisition reach a peak when the P consumption zones around each root hair first begin to overlap (Ma et al., 2001b). This seems to imply that increasing the length and longevity of root hairs, rather than increasing their density, may maximize the efficiency of P acquisition, as shown in the root hair development model by Brown et al. (2013).
In the present study, root hair elongation in a nutrient-rich patch was observed under both localized low P and high P supply (Fig. 6A), indicating that the response of root hairs to a soil nutrient-rich patch was regulated by the local nutrient signal, rather than the systematic signal of the shoot nutritional status. This is consistent with local signal regulation of lateral root proliferation (Wang et al., 2020). It has been reported that although increasing root hair length could improve P acquisition and shoot growth, considering longer root hairs alone without other root traits is not enough to benefit P nutrition (Zhang et al., 2018). Similarly, the importance of root hairs has been questioned in rice because root hairs improved P efficiency only in some genotypes (Nestler and Wissuwa, 2016). Additionally, studies have suggested that maize is more dependent on mycorrhiza than on root hairs under P-deficient conditions (Ma et al., 2021). Therefore, future attention needs to be given to the selection of genotypes with strong plasticity to the soil environment and the comprehensive consideration of the response of various root traits to improve the current situation of severe P resource shortage.
Trade-offs between root hairs and lateral roots in nutrient-rich patches
Previous reports have suggested that root responses to nutrient-rich patches vary from species to species (Robinson, 1994; Fransen and De Kroon, 2001; Li et al., 2014), but genotypic variations in different traits have rarely been reported. In the present study, both the WT and rth3 mutant had a positive response to the nutrient-rich patch (Fig. 4B), and the RRS of the rth3 mutant was significantly higher than the WT at low P, but this response difference disappeared with the increase in soil P supply intensity (Fig. 5). The increase of RRS in rth3 at low P indicated that the mutant maize compensated for the absence of root hairs by increased proliferation of lateral roots in the nutrient-rich patch. However, these plants did not reach the same biomass or shoot P content, showing that this compensation was only partial. In barley, Dodd and Diatloff (2016) reported increased root growth in brb mutants in dry soil, making the shoot physiological response to soil water deficit similar with that of WT plants. Likewise, rth2, a very short root hair mutant of maize, had more fine roots and larger SRL than the WT under low P and drought conditions, resulting in a similar shoot growth and P nutrition as for the WT (Weber et al., 2018; Klamer et al., 2019). To sum up, the stronger fine root proliferation in the rth3 mutant indicated that the lack of one functional root trait (e.g. root hairs) caused shifts to other traits (e.g. fine roots, mycorrhiza; Ma et al., 2021) with complementary functions (Fig. 7), which may be a general adaption mechanism of plants to their surroundings formed during long-term evolution.
Fig. 7.
Illustration showing the response of roots to nutrient-rich patches in the presence (WT) and absence (rth3) of root hairs. In the nutrient-rich patch, root hair elongation replaced partial root proliferation when root hairs were present (left). Conversely, root proliferation was enhanced in the root hairless mutant to compensate for the absence of root hairs (right).
The compensating effect among root traits was regulated by plant internal P status and/or external nutrient availability. Generally, modifications in root traits associated with P mobilization were enhanced by shoot P starvation or low soil P availability, and were inhibited by high shoot P status or high soil P availability (Wen et al., 2017; Klamer et al., 2019). In the present study, although the shoot growth of the rth3 mutuant grown in localized high P supply soil was obviously inferior to that under uniform treatment, root proliferation in the nutrient-rich zone was not affected (Figs 1C, 2A and 4). However, the RRS of rth3 under localized high P supply decreased significantly in comparison to under low P, indicating the weakening of root proliferation in an adequate external nutrient environment. All these highlight the importance of root hairs and fine roots for plant P acquisition. Furthermore, when plants grow in nutrient-rich soil, the greater trade-off for retaining both root hairs and a large amount of fine root exceeds their benefits to the plant, which might have resulted in weakened root proliferation (lower RRS at high P; Fig. 6) with high P application. Combined with localized supply under low P, the significantly enhanced RRS of rth3 indicates that plants are able to sense the external nutrient environment and regulate the combination of their root traits towards a balance between costs and benefits (Fig. 7).
Conclusions
Root hair traits are critical for maize growth and P acquisition, especially under P-limited conditions. In terms of nutrient management, localized application of nutrients could greatly improve P uptake and maize growth (both in WT and rth3), but this also depends on external nutrient supply intensity. This study also confirmed that root hairs could respond to heterogeneous nutrients in the soil, manifested as significant increases in root hair length, which were largely regulated by local nutrient signals. In addition, plants can regulate the combination of root traits, and enhanced root proliferation in nutrient-rich patch compensates for the negative effects of the absence of root hairs on nutrient acquisition. This study provides basic insights for a better understanding of the interaction between roots and soil nutrients.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: Effects of different nutrient treatments on root surface area in different soil layers of two maize genotypes. Fig. S2: Root length of different root diameter classes in the 11–22 cm layer. Fig. S3: Root hair length and root hair density of the WT in different soil layers.
ACKNOWLEDGEMENTS
We thank Professor Dr Frank Hochholdinger (University of Bonn) for providing maize seeds. L.W., J.S. and U.L. conceived and designed the study; L.W., X.L. and M.M. conducted the experiment; L.W. analysed the data and wrote the manuscript; U.L., J.S., X.L. and M.M. revised the manuscript. The authors declare that they have no conflicts of interest.
FUNDING
This work was supported financially by the National Natural Science Foundation of China (31772402, 31330070), the National Key Research and Development Program of China (2017YFD0200200/2017YFD0200202), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)-328017493/GRK 2366 (Sino-German International Research Training Group ‘Adaptation of maize-based food-feed-energy systems to limited phosphate resources’), and The 2115 Talent Development Program of China Agricultural University.
LITERATURE CITED
- Bates TR, Lynch JP. 1996. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell and Environment 19: 529–538. [Google Scholar]
- Bates TR, Lynch JP. 2000. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany 87: 964–970. [PubMed] [Google Scholar]
- Bates TR, Lynch JP. 2001. Root hairs confer a competitive advantage under low phosphorus availability. Plant and Soil 236: 243–250. [Google Scholar]
- Brown LK, George TS, Dupuy LX, White PJ. 2013. A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Annals of Botany 112: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill JF, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, Clair CCS. 2010. Plants integrate information about nutrients and neighbors. Science 328: 1657. [DOI] [PubMed] [Google Scholar]
- Dittmer HJ. 1937. A quantitative study of the roots and root hairs of a winter rye plant (Secale cereale). American Journal of Botany 24: 417–420. [Google Scholar]
- Dodd IC, Diatloff E. 2016. Enhanced root growth of the brb (bald root barley) mutant in drying soil allows similar shoot physiological responses to soil water deficit as wild-type plants. Functional Plant Biology 43: 199–206. [DOI] [PubMed] [Google Scholar]
- Drew MC. 1975. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist 75: 479–490. [Google Scholar]
- Fransen B, De Kroon H. 2001. Long-term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. Journal of Ecology 89: 711–722. [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE. 1997. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant and Soil 191: 181–188. [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. 2001. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant and Soil 235: 211–219. [Google Scholar]
- He W, Shen Y, Cornelissen JHC. 2012. Soil nutrient patchiness and plant genotypes interact on the production potential and decomposition of root and shoot litter: evidence from short-term laboratory experiments with Triticum aestivum. Plant and Soil 353: 145–154. [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237: 173–195. [Google Scholar]
- Hinsinger P, Brauman A, Devau N, et al. 2011. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant and Soil 348: 29–61. [Google Scholar]
- Hochholdinger F, Wen TJ, Zimmermann R, et al. 2008. The maize (Zea mays L.) roothairless3 gene encodes a putative GPI-anchored, monocot-specific, COBRA-like protein that significantly affects grain yield. The Plant Journal 54: 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist 162: 9–24. [Google Scholar]
- Hodge A. 2006. Plastic plants and patchy soils. Journal of Experimental Botany 57: 401–411. [DOI] [PubMed] [Google Scholar]
- Holz M, Zarebanadkouki M, Kuzyakov Y, Pausch J, Carminati A. 2018. Root hairs increase rhizosphere extension and carbon input to soil. Annals of Botany 121: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Rui Y, Zhang F, Rengel Z, Shen J. 2010. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Research 119: 355–364. [Google Scholar]
- Johnson CM, Ulrich A. 1959. Analytical methods for use in plant analysis. California Agricultural Experiment Station Bulletin 767: 25–78. [Google Scholar]
- Jungk A. 2001. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science 164: 121–129. [Google Scholar]
- Klamer F, Vogel F, Li XL, et al. 2019. Estimating the importance of maize root hairs in low phosphorus conditions and under drought. Annals of Botany 124: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Shahbaz M, Koirala M, et al. 2019. Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. Journal of Plant Nutrition and Soil Science 182: 945–952. [Google Scholar]
- Lambers H, Plaxton WC. 2015. Phosphorus: back to the roots. Annual Plant Reviews 48: 3–22. [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ma Q, Li H, Zhang F, Rengel Z, Shen J. 2014. Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant and Soil 376: 151–163. [Google Scholar]
- Lynch JP. 1995. Root architecture and plant productivity. Plant Physiology 109: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Ho MD. 2005. Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil 269: 45–56. [Google Scholar]
- Lyu Y, Tang H, Li H, et al. 2016. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Frontiers in Plant Science 7: 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Wang X, Li H, et al. 2014. Localized application of NH4+-N plus P enhances zinc and iron accumulation in maize via modifying root traits and rhizosphere processes. Field Crops Research 164: 107–116. [Google Scholar]
- Ma X, Li X, Ludewig U. 2021. Arbuscular mycorrhizal colonisation outcompetes root hairs in maize under low phosphorus availability. Annals of Botany 127: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Bielenberg D, Brown KM, Lynch JP. 2001a. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell and Environment 24: 459–467. [Google Scholar]
- Ma Z, Walk TC, Marcus A, Lynch JP. 2001b. Morphological synergism in root hair length, density, initiation and geometry for phosphorus acquisition in Arabidopsis thaliana: a modeling approach. Plant and Soil 236: 221–235. [Google Scholar]
- Miguel MA, Postma JA, Lynch JP. 2015. Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiology 167: 1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra RK, Alston AM, Dexter AR. 1988. Role of root hairs in phosphorus depletion from a macrostructured soil. Plant and Soil 107: 11–18. [Google Scholar]
- Mommer L, van Ruijven J, Jansen C, van de Steeg HM, de Kroon H. 2012. Interactive effects of nutrient heterogeneity and competition: implications for root foraging theory? Functional Ecology 26: 66–73. [Google Scholar]
- Mpanga IK, Gomez-Genao N, Moradtalab N, et al. 2019. The role of N form supply for PGPM–host plant interactions in maize. Journal of Plant Nutrition and Soil Science 182: 908–920. [Google Scholar]
- Nestler J, Keyes SD, Wissuwa M. 2016. Root hair formation in rice (Oryza sativa L.) differs between root types and is altered in artificial growth conditions. Journal of Experimental Botany 67: 3699–3708. [DOI] [PubMed] [Google Scholar]
- Nestler J, Wissuwa M. 2016. Superior root hair formation confers root efficiency in some, but not all, rice genotypes upon P deficiency. Frontiers in Plant Science 7: 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E. 2007. Mycorrhiza technology for sustainable agriculture – results and ideas. Berlin: Mensch & Buch. [Google Scholar]
- Neumann G, Bott S, Ohler MA, et al. 2014. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Frontiers in Microbiology 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Officer S, Dunbabin V, Armstrong R, Norton R, Kearney G. 2009. Wheat roots proliferate in response to nitrogen and phosphorus fertilisers in Sodosol and Vertosol soils of south-eastern Australia. Soil Research 47: 91–102. [Google Scholar]
- Pausch J, Loeppmann S, Kuhnel A, Forbush K, Kuzyakov Y, Cheng W. 2016. Rhizosphere priming of barley with and without root hairs. Soil Biology and Biochemistry 100: 74–82. [Google Scholar]
- Postma JA, Lynch JP. 2012. Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Annals of Botany 110: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. 1994. The responses of plants to non-uniform supplies of nutrients. New Phytologist 127: 635–674. [DOI] [PubMed] [Google Scholar]
- Robinson D. 2001. Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. Plant and Soil 232: 41–50. [Google Scholar]
- Rongsawat T, Peltier JB, Boyer JC, Véry AA, Sentenac H. 2021. Looking for root hairs to overcome poor soils. Trends in Plant Science 26: 83–94. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physology 116: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Li C, Mi G, et al. 2013. Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. Journal of Experimental Botany 64: 1181–1192. [DOI] [PubMed] [Google Scholar]
- Shen J, Wang L, Jiao X, et al. 2019. Innovations of phosphorus sustainability: implications for the whole chain. Frontiers of Agricultural Science and Engineering 6: 321–331. [Google Scholar]
- Shen J, Yuan L, Zhang J, et al. 2011. Phosphorus dynamics: from soil to plant. Plant Physiology 156: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter MG, Schmid K, Ludewig U. 2015. Uncovering genes and ploidy involved in the high diversity in root hair density, length and response to local scarce phosphate in Arabidopsis thaliana. PLoS ONE 10: e0120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Sanchez-Gomez D, Zavala MA. 2006. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology 94: 1103–1116. [Google Scholar]
- Wang L, Shen J. 2019. Root/rhizosphere management for improving phosphorus use efficiency and crop productivity. Better Crops with Plant Food 103: 36–39. [Google Scholar]
- Wang X, Feng J, White PJ, Shen J, Cheng L. 2020. Heterogeneous phosphate supply influences maize lateral root proliferation by regulating auxin redistribution. Annals of Botany 125: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lambers H. 2019. Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant and Soil 447: 135–156. [Google Scholar]
- Weber N, Herrmann I, Hochholdinger F, Ludewig U, Neumann G. 2018. PGPR-induced growth stimulation and nutrient acquisition in maize: do root hairs matter? Scientia Agriculturae Bohemica 49: 164–172. [Google Scholar]
- Wen TJ, Schnable PS. 1994. Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. American Journal of Botany 81: 833–842. [Google Scholar]
- Wen Z, Li H, Shen J, Rengel Z. 2017. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant and Soil 416: 377–389. [Google Scholar]
- Wen Z, Li H, Shen Q, et al. 2019. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytologist 223: 882–895. [DOI] [PubMed] [Google Scholar]
- Zhang C, Simpson RJ, Kim CM, et al. 2018. Do longer root hairs improve phosphorus uptake? Testing the hypothesis with transgenic Brachypodium distachyon lines overexpressing endogenous RSL genes. New Phytologist 217: 1654–1666. [DOI] [PubMed] [Google Scholar]
- Zhang D, Lyu Y, Li H, et al. 2019. Neighbouring plants modify maize-root foraging for phosphorus: coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytologist 226: 244–253. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang C, Lynch JP. 2010. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology 37: 313–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.