Abstract
The immunomodulatory capacity of mental stress is one of the basic concepts of psychoneuroimmunology. The current prospective longitudinal study was designed to evaluate the effect of acute mental stress on neurotransmitter precursor amino acid levels in individuals with depression at 2 time points. Ten physically healthy patients with a diagnosis of major depressive episode and Montgomery–Åsberg Depression Rating Scale scores (MADRAS) ⩾20 points at inclusion were assessed on 2 study days (once with higher MADRAS scores, once with lower MADRAS scores; median 34.5 days apart) and subjected to a standardized acute mental stress test on each study day. Blood was collected at 4 time points: once prior to and at 3 time points (0, 30 minutes, 60 minutes) following mental stress. Neurotransmitter precursor amino acid levels, that is kynurenine/tryptophan (KYN/TRP) and phenylalanine/tyrosine (PHE/TYR), as well as neopterin and nitrite were analyzed in a total of 80 individual blood samples. Regression and correlation analyses were performed. Regression analyses of PHE/TYR (R2 = .547) and KYN/TRP (R2 = .440) in relation to MADRAS depression severity showed a quadratic curve fit. This was reflected by a negative linear correlation between MADRAS scores and PHE/TYR as well as KYN/TRP in the lower score range (r = −.805, P < .001 and r = −.586, P < .001 respectively) and a positive correlation in the higher MADRAS score range (r = .713, P < .001 and r = .379, P = .016 respectively). No effect of acute mental stress was found. This analysis exemplifies the implications of sampling as well as data distributions on results. The crosstalk of biological mechanisms that orchestrate metabolic and immunological signaling may vary depending on depression severity resulting in non-linear associations that may explain the heterogeneity of results found in the literature.
Keywords: Depression severity, quadratic curve fit, kynurenine, tryptophan, phenylalanine, tyrosine, neurotransmitter
Introduction
The precise pathophysiological mechanisms leading to the clinical phenotype of depressive symptoms still remain unclear.1,2 Biomarkers of depression could help to account for inter-individual variance in aetiology and phenotype of depressive symptoms, thereby classifying the heterogeneous group of patients with respect to their expected response to treatment. This is of major importance since at least one third of depressed patients fail to respond to conventional medications.3 The attempt to identify biomarker profiles of psychiatric disorders is not a phenomenon of modern psychiatry. In the 19th century, Emil Kraepelin (1856–1926) designed a writing scale which was used to measure writing pressure curves of patients suffering from psychiatric disorders.4 Since then, the search for biomarkers of depression has included “wet” biomarkers such as the monocyte signature,5 cytokines6 and neurotransmitter precursor amino acids7 or the dexamethasone/corticotropin releasing hormone test,8 and also “dry” biomarkers derived from neuroimaging studies, sleep analysis, and clinical data.4 Stratifying individuals with depression using biomarker profiles would improve personalized therapeutic strategies and outcome.
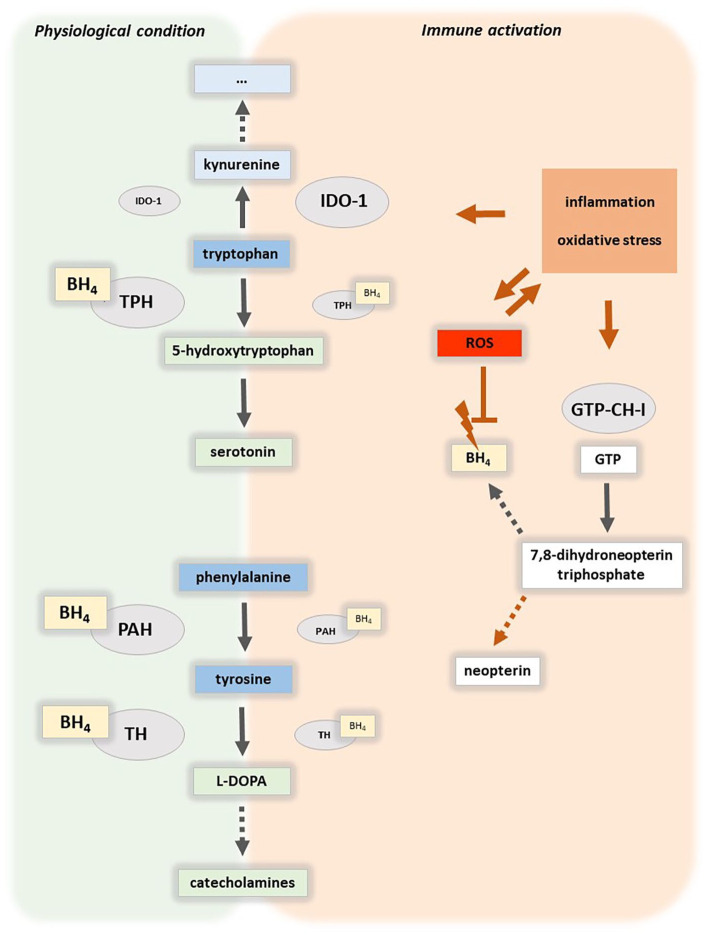
When neurotransmitter precursor amino acid levels are used as markers of depression, the most relevant pathways are the serotonin and catecholamine pathway.9 Important enzymes related to the serotonin pathway are indoleamine 2,3-dioxygenase 1 (IDO-1) or tryptophan 2,3-dioxygenase (TDO; Figure 1). Both enzymes are involved in the breakdown of tryptophan (TRP) to kynurenine (KYN).10 IDO-2 can also catalyze this reaction but has a lower affinity to TRP than IDO-1 and its specific role in the here discussed processes is still unclear.11 IDO and TDO catalyze the first and rate limiting reaction in the kynurenine pathway KYN/TRP (Figure 1).12 Inflammatory cytokines can strongly enhance the activity of IDO-1, therefore raising KYN/TRP in parallel to increasing concentrations of immune activation markers such as neopterin. Of note, also hepatic TDO, which is mainly involved in the regulation of physiological TRP concentrations in the bloodstream, is influenced by stress hormones.13 When TRP metabolism along the KYN pathway is intensified this leads to TRP depletion resulting in lower serotonin levels (5-HT) associated with depressive symptoms14 (Figure 1). Central nervous system (CNS) concentrations of TRP and downstream metabolites including kynurenine and 3-hydroxykynurenine are linked to peripheral levels via the leucine-preferring system L1 large amino acid transporter (LAT1).15
Figure 1.
Aromatic amino acid catabolism and related pathways. Immune activation is characterized by an oxidative milieu and release of inflammatory stimuli that for example during the cellular immune response activate indoleamine 2,3-dioxygenase (IDO-1), which catalyzes the rate-limiting reaction in the tryptophan catabolic route along the kynurenine axis. Notably, also tryptophan 2,3-dioxygenase and IDO-2 can catalyze tryptophan breakdown (not shown). GTP-cyclohydrolase 1 (GTP-CH-I) forms 7,8 trihydroneopterin triphosphate, the precursor of tetrahydrobiopterin (BH4), and neopterin. In immune activation, this biosynthetic route is shifted towards neopterin formation in human macrophages and dendritic cells, moreover, the oxidative milieu destabilizes BH4. In consequence, the activity of BH4-dependent monooxygenases such as of tryptophan 5-hydroxylases (TPH), phenylalanine 4-hydroxylase (PAH), and tyrosine 3-monooxygenase (TH) decreases, indicated by smaller symbols. Both, aromatic amino acid availability and the activity of biosynthetic enzymes are determinants of serotonergic and dopaminergic neurotransmitter levels.
Abbreviation: ROS: reactive oxygen species.
Along the catecholamine pathway the conversion of phenylalanine (PHE) to tyrosine (TYR) by phenylalanine hydroxylase (PAH) is approximated by the PHE/TYR ratio16 (Figure 1). Inflammatory stimuli can affect the catecholamine synthesis via the enzyme co-factor, tetrahydrobiopterin (BH4).17 Notably, also tryptophan hydroxylases, which convert TRP to 5-HT are dependent on BH4. Reactive oxygen species (ROS) reduce the availability of oxidation-labile BH4, thereby influencing catecholamine and serotonin synthesis, while on the other hand the pro-inflammatory cytokine interferon-γ stimulates the biosynthesis of BH4.18 However, in human and primate macrophages and dendritic cells neopterin, a cellular marker of immune activation, is formed at the expense of BH4.19 Nitrite is a frequently used biomarker for the less stable nitric oxide, which is produced from arginine by different nitric oxide synthases (NOS) and is an IDO-1 inhibitor. Inflammatory conditions can enhance its formation via inducible NOS. Also this enzyme is BH4-dependent and the pathway is less activated in human macrophages compared to rodents.20
Acute mental stress is an promising tool for the stratification of patients with depressive symptoms since there is evidence that chronic mental stress can lead to a sensitization toward acute stress, possibly representing an adaptive process to continuing challenges.21 This is further supported by the finding that acute mental stress induces changes in the hypothalamic pituitary adrenocortical axis, which have been shown to be useful in the prediction of the treatment response in patients with depressive symptoms.22
Many studies of biomarkers in depression are cross-sectional in nature, and in these studies the results and values of individuals with depression are compared to those of a group of healthy controls. In the present study we used a longitudinal design to evaluate the effect of acute mental stress and severity of depressive symptoms on neurotransmitter precursor amino acids concentrations, namely PHE/TYR and KYN/TRP ratios as well as neopterin and nitrite levels.
Methods
Ethics statement
The study was approved by the ethics committee of the Medical University Innsbruck, Austria. Informed written consent was obtained from all participants prior to inclusion in the study.
Study design
The presented data represent a secondary analysis and are part of a larger study evaluating the effect of mental stress on biological markers in individuals with depression and healthy controls.23,24 In the present study we assessed 10 individuals with depression and a MADRAS (Montgomery–Åsberg Depression Rating Scale scores) score ⩾20 points at inclusion indicating at least moderate depression.25 Two time points were assessed: study day 1, with the participants displaying higher MADRAS scores and study day 2 with the participants displaying lower MADRS scores. In between study days individuals underwent treatment as usual at our department consisting of multimodal therapeutic interventions. At each study day patients were subjected to a standardized acute mental stress test. Blood was collected at 4 time points: at baseline after 30 minutes of rest after arrival to the study location (T0), immediately after the acute mental stress test (T1), 30 minutes post-acute mental stress (T2), and 60 minutes post-acute mental stress (T3) (Figure 2a).
Figure 2.
Flowchart of study design (a) and patient recruitment (b).
Abbreviations: MADRAS, Montgomery–Åsberg Depression Rating Scale.
Participants
Inclusion criteria: depressive episode (ICD-10: F32) or recurrent depressive disorder (ICD-10: F33) and depression as the primary psychiatric diagnosis, MADRAS26 score ⩾20 at inclusion, no somatic disorder requiring medical attention especially no allergy or active hay fever, no adherence to any specific diet, no caffeinated or alcoholic beverages prior to the assessment, no excessive exercise or sleep deprivation 24 hours before the assessment, no immunomodulatory medication or medication affecting coagulation in the 14 days prior to the assessment. Exclusion: manic episode (ICD-10: F30), bipolar disorder (ICD-10: F31), persistent mood disorder (ICD-10: F34), alcohol dependency, electroconvulsive therapy in the 14 days prior to study inclusion, acute infection (even if not requiring medical attention). Mental health was screened for using the German Version of the Mini International Neuropsychiatric Interview 5.0 M.I.N.I.27 Somatic health was assessed by questions about current and previous health conditions. Lifestyle data such as smoking, and BMI were assessed. The following general, participant-related parameters were collected: sociodemographic data, physical activity (self-administrable German version of the International Physical Activity Questionnaire (IPAQ)28), and sleep quality (German version of the Pittsburgh Sleep Quality Index (PSQI)29). Patient recruitment is shown in Figure 2b.
Acute stress protocol
The acute stress test was administered as described previously.23 In brief it consisted of the STROOP color and word test and a standardized arithmetic test of 2 × 2.5 minutes.30,31 This paradigm has been shown to be associated with increased cortisol levels when previously applied.24
Assessment of stress-related questionnaires and scales
MADRAS was used to evaluate depression severity prior to study inclusion.26 The following cut-off points were used: 0 to 6—symptom absent, 7 to 19—mild, 20 to 34—moderate, 35 to 60—severe depression.25 The “Perceived Stress Scale 14” (PSS-14)32 was used for stress-related assessment. The “State Trait Anxiety Inventory” (STAI) was used to assess anxiety symptoms on the day of study.33 The participants rated their subjective amount of mental stress from each acute stress task on a 5-point Likert Scale following the acute mental stress test.
Blood sampling
A peripheral venous catheter was inserted into the antecubital vein of the non-dominant hand in all participants. Participants rested for 30 minutes following the insertion of the catheter before the first (resting) blood sample was drawn. The first 2 ml were discarded during each draw. Blood was drawn without stasis. The samples were collected in commercially available tubes (Sarstedt, Vienna, Austria). Routine blood parameters were determined at the hospital standard laboratory parameters. For analysis of neurotransmitter precursor amino acids aliquots of serum were processed immediately and shock frozen in liquid nitrogen and stored at −80°C until use. The first blood draw (T0) was done between 8:00 and 8:30 am in all participants. Care was taken to schedule study day 1 and 2 in the same season of the year.
Neurotransmitter precursor amino acid analysis
Neopterin concentrations were measured by enzyme-linked immunosorbent assay (BRAHMS Diagnostics, Berlin, Germany). TRP and KYN serum concentrations, as well as concentrations of PHE and TYR, were determined by high-performance liquid chromatography, as described elsewhere.18,34 The ratios of KYN/TRP and PHE/TYR were calculated as indices of IDO-1 and PAH activity respectively.35 Serum nitrite concentrations, which are considered surrogate markers for nitric oxide, were measured via the Griess reaction36 Absolute values of neopterin, nitrite, KYN/TRP, and PHE/TYR were compared to normal values (95th percentile) from the literature.37
Statistical analysis
Sample characteristics and results are given as means, standard deviations, and absolute frequencies. Due to data distributions, time between study days is given as median (range). Group comparisons between inclusion and follow-up regarding questionnaires and scales were performed using non-parametric analyses (Wilcoxon Test). For neurotransmitter precursor amino acid, neopterin, and nitrite analysis, associations were analyzed with regression models, separately with linear and quadratic curve fit.
To demonstrate the effect of analyzing data covering only a part of the depression continuum, we also conducted separate correlation analyses with Pearson correlation coefficients for the upper and lower range of the MADRAS score. Additionally, we also calculated Spearmen rank correlations to investigate the robustness of the results. P < .05 was considered significant in these analyses. All analyses were done in SPSS 20.0.
Results
Study population
Ten individuals with a MADRAS (Montgomery–Åsberg Depression Rating Scale) score ⩾20 and a primary diagnosis of major depressive episode, but without relevant somatic comorbidities were included in this study (Figure 2b). Routine laboratory values were determined and without clinically significant pathology in all participants. All patients were assessed on 2 different study days (Figure 2a). Patient characteristics are shown in Table 1. The results of collected questionnaires and scales can be found in Table 2. Severity of depression differed significantly between the 2 study time points (Wilcoxon test, P = .005), while other parameters (Table 2) as well as BMI (Wilcoxon test, P = .08), number of cigarettes smoked (Wilcoxon test, P = .357), or acute mental stress induced by the mental stress task (Wilcoxon test for Stroop task, P = .159 and for mental arithmetics task, P = .698) were comparable across study days.
Table 1.
Participant characteristics.
| Patient variable | Value |
|---|---|
| Age in years (mean ± SD) | 39.7 ± 14.1 |
| Sex (absolute numbers) | 9 female (1 male) |
| Smoking yes (absolute numbers) | 7/10 |
| BMI, kg/m2 (mean ± SD) | 25.3 ± 5.6 |
| Medication (absolute numbers) | |
| Antidepressants | 10/10 |
| Atypical antipsychotics | 4/10 |
| Other mood stabilizers | 2/10 |
| BZD | 0/10 |
| Additional diagnosis (M.I.N.I., absolute numbers) | |
| Anxiety disorder | 4/10 |
| Panic disorder | 2/10 |
| Social phobia | 2/10 |
| Agoraphobia | 2/10 |
| Personality disorder | 1/10 |
| PTSD | 1/10 |
Abbreviations: BMI, body mass index; BZD, benzodiazepines; M.I.N.I., Mini International Neuropsychiatric Interview; PTSD, posttraumatic stress disorder; SD, standard deviation.
Table 2.
Results of analyzed questionnaires and scales.
| Parameter (mean _+ SD) | Study day 1 | Study day 2 | P-Value |
|---|---|---|---|
| Depression (MADRAS, range 0-60 points) | 29.5 ± 5.4 | 16.7 ± 8.7 | .005* |
| Stress (PSS, range 0-56 points) | 46.0 ± 8.8 | 42.1 ± 5.6a | .144 |
| State anxiety scale (STAI, range 20-80 points) | 55.6 ± 8.5 | 45.9 ± 7.3 | .036* |
| Sleep (PSQI, range 0-21) | 13.5 ± 5.0 | 10.8 ± 5.0 | .116 |
| Physical activity (IPAQ) moderate in metabolic equivalents | 3642.0 ± 4367.5 | 4308.0 ± 4532.1 | .477 |
| Physical activity (IPAQ) intensive in metabolic equivalents | 4248.0 ± 3424.2 | 1728.0 ± 2284.7 | .097 |
Abbreviations: IPAQ, International Physical Activity Questionnaire; MADRAS, Montgomery–Åsberg Depression Rating Scale; PSQI, Pittsburgh Sleep Quality Index; PSS, Perceived Stress Scale; STAI, State-Trait Anxiety Scale.
Results are given as means ± standard deviation. Wilcoxon test was used to compare the results of baseline and follow-up condition; significant P-values are indicated by an asterisk. Ranges of the respective tests or subtests are given to aid with interpretation.
Two values missing.
Results of PHE/TYR analysis in relation to depression severity
Using a linear modeling approach for the PHE/TYR values in relation to the MADRAS sum score R2 was calculated at .008 (P = .437). In comparison a quadratic modeling approach resulted in R2 of .547 with (P < .001). This translates to 54.7% of the PHE/TYR variance being explained by MADRAS sum in the quadratic model, while it is only 0.8% in the linear model. An effect of acute mental stress could not be detected as indicated by the small differences between the lines (Figure 3). PHE/TYR absolute values were above the cut-off for healthy individuals37 in all but 5 individual measurements.
Figure 3.
Scatter plots with quadratic regression curves showing the association between the MADRAS (Montgomery–Åsberg Depression Rating Scale) score (x-axis) and the phenylalanine to tyrosine ratio (PHE/TYR) (y-axis, left figure a) and the kynurenine to tryptophan ratio (KYN/TRP) (y-axis, right figure b). Data points and quadratic regression curves are shown separately for the 4 study time points, that is for baseline after 30 minutes of rest (T0, blue), immediately after the acute mental stress test (T1, red), 30 minutes post-acute mental stress (T2, green), and 60 minutes post-acute mental stress (T3, orange).
Results of KYN/TRP analysis with respect to depression severity
Using a linear modeling approach for the KYN/TRP values in relation to the MADRAS sum score R2 was calculated at .251 with a P < .001. In comparison, a quadratic modeling approach resulted in a R2 of .440 with a P < .001. This translates to 44% of the KYN/TRP variance being explained by MADRAS sum in the quadratic model, while it is only 25.1% in the linear model. An effect of acute mental stress could not be detected as indicated by the small distance between the lines (Figure 3). KYN/TRP absolute values were above the cut-off for healthy individuals in 9 values attributed to 4 participants.37
Neopterin and nitrite
Using a linear modeling approach for the neopterin values in relation to the MADRAS sum score R2 was calculated at .01 (P = .366). In comparison a quadratic modeling approach resulted in a R2 of .05 (P = .163). This translates to 5% of the neopterin variance being explained by MADRAS sum in the quadratic model and 1% in the linear model. An effect of acute mental stress could not be detected. Neopterin absolute values were above the predefined cut off score in 7 measurements attributed all to a single participant. For nitrite R2 was .001 (P = .741) for the linear model and R2 was .070 (P < .061) for the quadratic model. This translates to 7% of the nitrite variance being explained by MADRAS sum in the quadratic model and 0.1% in the linear model. An effect of acute mental stress could not be detected. All values were within pre-defined normal range.37
Correlation analyses
Results of correlation analysis confirmed the observed results and exemplify what would happen when all data points would be used in a single correlation analysis ignoring the non-linear association. When the full data are used, correlations between PHE/TYR and MADRAS scores were not significant, but when data were analyzed separately for high and low scores separated by the vertex of the quadratic curve (Figure 3, Table 3), it become evident that lower MADRAS scores are negatively correlated with PHE/TYR values, while higher scores correlate positively. Comparable results can be found for KYN/TRP (Figure 3, Table 3). Non-parametric analyses relying on Spearmen rank correlation showed comparable results.
Table 3.
Results of correlation analysis.
| Nitrite | Neopterin | PHE/TYR | KYN/TRP | ||
|---|---|---|---|---|---|
| MADRAS score (all cases) | r | .038 | −.102 | .088 | −.501 |
| P | .741 | .366 | .437 | <.001 | |
| MADRAS score (cases MADRAS < 25) | r | .410 | .034 | −.805 | −.586 |
| P | .008 | .833 | <.001 | <.001 | |
| MADRAS score (cases MADRAS >25) | r | .340 | −.130 | .713 | .379 |
| P | .032 | .425 | <.001 | .016 |
Abbreviation: MADRAS, Montgomery–Åsberg Depression Rating Scale scores; P, significance level; r, Pearson correlation coefficient.
Discussion
The main finding of this study evaluating neurotransmitter precursor amino acid levels in individuals with depression is, that PHE/TYR and KYN/TRP values correlated negatively with depression severity in no to mild depression, while a positive correlation was found for moderate to severe scores. The current data show a quadratic curve fit for PHE/TYR and KYN/TRP values, which indicates that 2 or more distinct biochemical mechanisms are responsible for this U shaped distribution. This finding could help to explain some of the contradictory results of PHE/TYR and KYN/TRP in depression. Somewhat unexpectedly, no effect of acute mental stress was found on PHE/TYR and KYN/TRP as well as on neopterin and nitrite in this study. Likewise there was no interaction between depression and acute mental stress.
Mechanisms underlying the low-moderate depression range
In the present study PHE/TYR and KYN/TRP correlated negatively with depression severity in the no to mild depression score range. Low-grade inflammation is probably not the main factor in this process. Rather the results fit with our recently published observation that in healthy young individuals PHE/TYR correlated negatively with chronic stress parameters and was decreased in chronic mental stress compared to no mental stress.23 These findings could be interpreted as sign of immunological suppression as has been described previously in such young and otherwise healthy cohorts.38 It is also possible that the observed negative correlation of PHE/TYR and KYN/TRP in no to mild depression is related to a direct biochemical mechanism related to BH4 availability. Reduced BH4 availability can be the result of impaired synthesis, low recovery from BH2, or destruction by ROS.17,39 We cannot make any claims as to which mechanism is the most important in our study setting. A number of feedback mechanisms regulate BH4 availability such as via nitric oxide (NO) synthase enzymes: in the case of sufficient supply with BH4 NO is produced, but when BH4 supply is insufficient, uncoupling leads to ROS formation.40 Nutrition and lifestyle have been shown to influence neurotransmitter precursor amino acid metabolism also outside of pathogenic states.41 In addition there is an influence of diet on the nitrate-nitrite-NO cycle.42
Mechanisms underlying the severe depression range
The inflammatory hypothesis of depression involves complex interactions of environmental factors as well as such of mental and physical health and has been well reviewed and discussed in much detail.9,43 Neurotransmitter precursor amino acids such as PHE/TYR and KYN/TRP have mostly been shown to correlate positively with symptom severity in depressed subjects. In patients following treatment for breast cancer, PHE/TYR correlated positively with depression severity and KYN/TRP with anxiety measures.44 In the same study PHE/TYR was increased in individuals with depression and most markedly in individuals with a diagnosis of both breast cancer and depression. Somato-psychic comorbidities seem to play an important role since it has also been shown that changes in depression severity prior to and following surgery correlate with the differences in PHE/TYR.45 Chronic low grade inflammation as a driver of depressive symptoms plays a bi-directional role: higher inflammatory markers predict the development of depressive symptoms, while the presence of depressive symptoms per se is also associated with higher inflammatory markers.46 Chronic low grade inflammation can influence both IDO and TDO activity leading to an increase in KYN/TRP ratio.13 PAH activity is influenced mostly by BH4 availability. Both increased use and loss of BH4 driven by a chronic inflammatory state may synergistically act to alter the function of BH4-dependent enzymes and then compromise the biosynthesis of monoamines, which may contribute to development of mood disorders.43 While many studies have been performed analyzing peripheral levels of neurotransmitter precursor amino acid levels, there is evidence that this might reflect the central situation.47
The effect of acute mental stress
In the current study we did not find an effect of acute mental stress on neurotransmitter precursor amino acid ratios or on neopterin and nitrite. This is surprising because in a study evaluating the effect of acute mental stress in healthy individuals with and without chronic mental stress we found an effect of acute mental stress on neurotransmitter precursor amino acids using a comparable methodology.23 Furthermore chronic mental stress (which is often used as a model of depression) has been shown to sensitize toward the effect of acute mental stress.21 In individuals with depressive symptoms, it has been shown that the acute inflammatory response induced by acute mental stress is not terminated promptly by the organism,48 hypercortisolism cannot be sufficiently experimentally suppressed, and the physiological response to synthetic glucocorticoids is lost (dexamethasone/CRH test).49
Neopterin and nitrite
Neopterin and nitrite showed no clear dependence on depression severity in this study. This might be due to low numbers of included participants or the fact that our participants were somatically healthy, not overweight, and not an elderly population. This is reflected by the fact that most neopterin and all nitrite levels were within the normal limits.37 Moreover, also dietary nitrate/nitrite sources can contribute to NO levels.42
Limitations
One limitation is the small number of individuals included in the study. This was in part due to the strict inclusion criteria, which only allowed physically healthy individuals to be included. This also limits the generalizability of the findings since many individuals with depression are also affected by physical disorders, especially metabolic or cardiovascular disease. Excluding individuals with signs of infection might have further led to exclusion of patients in whom the inflammatory markers of depression were most markedly elevated. On the other hand the very homogenous collective allows for better detangling of underlying pathophysiological processes. While we tried to control for many confounding factors using the described strict inclusion criteria and by assessing variables such as physical activity or sleep, it is of course never possible to fully exclude that there are unrecognized factors which might have had an influence on the results. Effects of gender could not be evaluated due to the low number of male participants.50
Conclusion
We aimed to evaluate the effect of acute mental stress and depression severity on neurotransmitter precursor amino acids in this study. While no effect of acute mental stress was found, the current data reveal a previously undescribed and very interesting quadratic relationship between depression severity and neurotransmitter precursor amino acid levels. The current data might help to explain some of the divergent findings in the literature on PHE/TYR and KYN/TRP in depression. With increasing data available, it becomes more and more evident that the crosstalk of the different mechanisms that contribute to the regulation of neurotransmitter precursor amino acid levels in individuals with depression is highly complex. A more differentiated view is necessary to unwire the connections. The future challenge will be to characterize the different subgroups in more detail to improve personalized and tailored treatment approaches.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Fond der Oesterreichischen Nationalbank OENB 15174.
Declaration Of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceptualization, KH, JMGo, JE, PK-T and BS-U; Data curation, KH, JE. and PK-T; Formal analysis, KH, and JMGi.; Funding acquisition, KH and BS-U; Investigation, KH, JMGo, JE, PK-T, TV and DF; Methodology, KH, JMGi, DF and BS-U; Project administration, BS-U; Resources: JMGo, PK-T, TV and DF; Supervision, DF and BS-U; Validation, KH, JMGi, JMGo, JE, DF, BS-U; Visualisation: KH, JMGi; Writing—original draft, KH; Writing—review & editing, all authors.
ORCID iDs: Katharina Hüfner  https://orcid.org/0000-0002-5453-8792
https://orcid.org/0000-0002-5453-8792
Barbara Sperner-Unterweger  https://orcid.org/0000-0001-8936-676X
https://orcid.org/0000-0001-8936-676X
Data availability statement: Original data are available from the corresponding author upon request.
References
- 1.Aan Het Rot M, Mathew SJ, Charney DS.Neurobiological mechanisms in major depressive disorder. Can Med Assoc J. 2009;180:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan V, Nestler EJ.Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917. [DOI] [PubMed] [Google Scholar]
- 4.Turck CW.Biomarkers for Psychiatric Disorders. 1st ed. Springer; 2009. [Google Scholar]
- 5.Padmos RC, Hillegers MH, Knijff EM, et al. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65:395-407. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer R, Wollman E, Vitkovic L, Yirmiya R.Cytokines and depression: fortuitous or causative association? Mol Psychiatry. 1999;4:328-332. [DOI] [PubMed] [Google Scholar]
- 7.Strasser B, Sperner-Unterweger B, Fuchs D, Gostner JM.Mechanisms of inflammation-associated depression: immune influences on tryptophan and phenylalanine metabolisms. Curr Top Behav Neurosci. 2017;31:95-115. [DOI] [PubMed] [Google Scholar]
- 8.Bardeleben U, Holsboer F.Cortisol response to a combined dexamethasone-human corticotrophin-releasing hormone challenge in patients with depression. J Neuroendocrinol. 1989;1:485-488. [DOI] [PubMed] [Google Scholar]
- 9.Haroon E, Raison CL, Miller AH.Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906-914. [DOI] [PubMed] [Google Scholar]
- 11.Pantouris G, Serys M, Yuasa HJ, Ball HJ, Mowat CG.Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids. 2014;46:2155-2163. [DOI] [PubMed] [Google Scholar]
- 12.Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D.Neopterin production, tryptophan degradation, and mental depression – what is the link? Brain Behav Immun. 2002;16:590-595. [DOI] [PubMed] [Google Scholar]
- 13.Oxenkrug GF.Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. Isr J Psychiatry Relat Sci. 2010;47:56-63. [PMC free article] [PubMed] [Google Scholar]
- 14.Myint AM.Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375-1385. [DOI] [PubMed] [Google Scholar]
- 15.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR.Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007-2017. [DOI] [PubMed] [Google Scholar]
- 16.Sperner-Unterweger B, Kohl C, Fuchs D.Immune changes and neurotransmitters: possible interactions in depression? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:268-276. [DOI] [PubMed] [Google Scholar]
- 17.Werner-Felmayer G, Golderer G, Werner ER.Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab. 2002;3:159-173. [DOI] [PubMed] [Google Scholar]
- 18.Neurauter G, Schrocksnadel K, Scholl-Burgi S, et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9:622-627. [DOI] [PubMed] [Google Scholar]
- 19.Sucher R, Schroecksnadel K, Weiss G, Margreiter R, Fuchs D, Brandacher G.Neopterin, a prognostic marker in human malignancies. Cancer Lett. 2010;287:13-22. [DOI] [PubMed] [Google Scholar]
- 20.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A.Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358-1363. [DOI] [PubMed] [Google Scholar]
- 21.Pike JL, Smith TL, Hauger RL, et al. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 1997;59:447-457. [DOI] [PubMed] [Google Scholar]
- 22.Ising M, Horstmann S, Kloiber S, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression – a potential biomarker? Biol Psychiatry. 2007;62:47-54. [DOI] [PubMed] [Google Scholar]
- 23.Hufner K, Galffy M, Egeter J, et al. Acute and chronic mental stress both influence levels of neurotransmitter precursor amino acids and derived biogenic amines. Brain Sci. 2020;10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koudouovoh-Tripp P, Hufner K, Egeter J, et al. Stress enhances proinflammatory platelet activity: the impact of acute and chronic mental stress. J Neuroimmune Pharmacol. 2021;16:500-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snaith RP, Harrop FM, Newby DA, Teale C.Grade scores of the montgomery—Åsberg depression and the clinical anxiety scales. Br J Psychiatry. 1986;148:599-601. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA, Asberg M.A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22-33;quiz 34-57. [PubMed] [Google Scholar]
- 28.Hagströmer M, Oja P, Sjöström M.The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755-762. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ.The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 30.Vella EJ, Friedman BH.Hostility and anger in: cardiovascular reactivity and recovery to mental arithmetic stress. Int J Psychophysiol. 2009;72:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garde AH, Laursen B, Jorgensen AH, Jensen BR.Effects of mental and physical demands on heart rate variability during computer work. Eur J Appl Physiol. 2002;87:456-461. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Kamarck T, Mermelstein R.A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396. [PubMed] [Google Scholar]
- 33.Laux L, Glanzmann P, Schaffner P, Spielberger CD.Das State-Trait-Angstinventar (STAI), Manual. Beltz Test; 1981. [Google Scholar]
- 34.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D.Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424-2426. [PubMed] [Google Scholar]
- 35.Capuron L, Schroecksnadel S, Feart C, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175-182. [DOI] [PubMed] [Google Scholar]
- 36.Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R.Adaptation of the Griess reaction for detection of nitrite in human plasma. Free Radic Res. 2004;38:1235-1240. [DOI] [PubMed] [Google Scholar]
- 37.Geisler S, Mayersbach P, Becker K, Schennach H, Fuchs D, Gostner JM.Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines. 2015;26:31-36. [Google Scholar]
- 38.Dhabhar FS.The short-term stress response – mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front Neuroendocrinol. 2018;49:175-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thony B, Auerbach G, Blau N.Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1-16. [PMC free article] [PubMed] [Google Scholar]
- 40.Crabtree MJ, Channon KM.Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D.Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology. 2020;79:89-99. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Weitzberg E, Gladwin MT.The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156-167. [DOI] [PubMed] [Google Scholar]
- 43.Vancassel S, Capuron L, Castanon N.Brain kynurenine and BH4 pathways: relevance to the pathophysiology and treatment of inflammation-driven depressive symptoms. Front Neurosci. 2018;12:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hufner K, Oberguggenberger A, Kohl C, et al. Levels in neurotransmitter precursor amino acids correlate with mental health in patients with breast cancer. Psychoneuroendocrinology. 2015;60:28-38. [DOI] [PubMed] [Google Scholar]
- 45.Hüfner K, Fuchs D, Blauth M, Sperner-Unterweger B.How acute and chronic physical disease may influence mental health – an analysis of neurotransmitter precursor amino acid levels. Psychoneuroendocrinology. 2019;106:95-101. [DOI] [PubMed] [Google Scholar]
- 46.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB.The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. Published online August 17, 2020. doi: 10.1038/s41380-020-00867-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kofler M, Schiefecker AJ, Gaasch M, et al. A reduced concentration of brain interstitial amino acids is associated with depression in subarachnoid hemorrhage patients. Sci Rep. 2019;9:2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller GE, Rohleder N, Stetler C, Kirschbaum C.Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679-687. [DOI] [PubMed] [Google Scholar]
- 49.Holsboer F, Von Bardeleben U, Gerken A, Stalla GK, Müller OA.Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984;311:1127. [DOI] [PubMed] [Google Scholar]
- 50.Reininghaus EZ, Dalkner N, Riedrich K, Fuchs D, Gostner JM, Reininghaus B.Sex specific changes in tryptophan breakdown over a 6 week treatment period. Front Psychiatry. 2019;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]