Abstract
Autoimmune gastritis (AIG) is a chronic immune-mediated, inflammatory condition that involves the destruction of the gastric oxyntic mucosa through the autoimmune-mediated loss of parietal cells, with replacement by atrophic and metaplastic tissue. Diagnosing AIG is important, given the need for ongoing clinical management and vigilance with respect to downstream complications, the most serious of which is gastric adenocarcinoma. Other clinical consequences include gastric neuroendocrine tumors, consequences related to decreased gastric acid and decreased intrinsic factor due to parietal cell destruction and antibodies against intrinsic factor (e.g. micronutrient deficiencies), as well as concomitant autoimmune disorders. Considering the prevalence of AIG and the potential for severe clinical outcomes, it is important to engage in efforts to reduce practice pattern variability related to diagnosis and management. Accordingly, herein, we review of the epidemiology, pathogenesis, clinical presentation of AIG, including both gastric and extragastric manifestations, and provide an overview of clinical management.
Keywords: atrophic gastritis, carcinoid, gastric neoplasm, Helicobacter pylori, intestinal metaplasia, iron deficiency, neuroendocrine tumors, vitamin B12 deficiency
Introduction
Autoimmune gastritis (AIG) is a chronic immune-mediated, inflammatory condition that involves the destruction of the gastric oxyntic mucosa through the autoimmune-mediated loss of parietal cells, with replacement by atrophic and metaplastic tissue. Histologically, AIG is characterized by atrophy of the oxyntic mucosa that is, corpus-predominant atrophic gastritis, with or without replacement by metaplastic mucosa. Classically, antral mucosa architecture is preserved, but this might not be the case in more advanced stages or if there is concomitant infection with Helicobacter pylori (H. pylori).1
Histologically, AIG falls under the umbrella “atrophic gastritis,” which comprises several conditions where the downstream histologic consequence of chronic environmental (e.g. H. pylori) or nonenvironmental triggers is gastric atrophy—that is, disappearance of the native gastric glands and thinning of the mucosa.2 There is abundant literature on chronic H. pylori gastritis leading to progression to multifocal atrophic gastritis (MAG) and, in a small minority, neoplastic transformation and progression to adenocarcinoma; however, the literature on pathogenesis, epidemiology, and management of AIG is sparse in comparison. Moreover, the heterogeneity across the literature with respect to criteria used to diagnose AIG further contributes to our incomplete understanding of this condition, as well as practice pattern variability. For example, pernicious anemia (PA), which is a late-stage manifestation of AIG characterized by megaloblastic anemia due to vitamin B12 deficiency, is sometimes defined in studies solely based on laboratory evidence of anemia or vitamin B12 deficiency, without endoscopic/histologic confirmation of AIG.
Notwithstanding challenges related to terminology in the literature, the prevalence of AIG is estimated to be approximately 1–2% and occurs more often in women compared to men, with increasing prevalence with age.3,4 Diagnosis of AIG is important, given the need for ongoing clinical management and vigilance with respect to downstream complications, the most serious of which is gastric adenocarcinoma. Other clinical consequences include gastric neuroendocrine tumors, consequences related to decreased gastric acid and decreased intrinsic factor due to parietal cell destruction and antibodies against intrinsic factor (e.g. micronutrient deficiencies), as well as concomitant autoimmune disorders. Considering the prevalence of AIG and the potential for severe clinical outcomes, it is important to engage in efforts to reduce practice pattern variability related to diagnosis and management. Accordingly, herein, we review of the epidemiology, pathogenesis, clinical presentation of AIG, including both gastric and extragastric manifestations, and provide an overview of clinical management for the target audience of clinical providers.
Literature search
We identified relevant literature by searching PubMed using the following MESH search terms (“Autoimmune Gastritis” OR (“Gastritis, Atrophic”(Mesh) AND “Autoimmune Diseases”(Mesh) (date of search July 27, 2020). All articles which included “autoimmune gastritis” were considered, and there were no restrictions based on study design. For epidemiological studies and clinical outcomes data, we favored studies with histological confirmation of AIG where appropriate.
Epidemiology
Estimates of AIG prevalence vary depending on the study and rigor of the diagnostic criteria—that is, whether the diagnosis was based on serology, medical coding, or endoscopy with biopsy confirmation. Indeed, some studies have classified patients as having AIG based solely on the presence of low serum vitamin B12, which has a broad differential diagnosis, without confirming the diagnosis on gastric histopathology. Other studies base the diagnosis on the presence of parietal cell antibodies (PCAs) or anti-intrinsic factor (IF) antibodies, which is also incorrect, given that these lack specificity (PCA) and sensitivity (IF) and patients may have seronegative AIG. With these considerations in mind, the prevalence of AIG is estimated to range from 0.1% to 1–2% in the general population, but higher in certain populations including women and those >60 years old (estimated 2–3%).5,6 There is at least a 2:1 female versus male predominance of AIG,6 and some data suggest that non-white individuals might have an earlier onset of AIG compared to white individuals.7,8
Population-based studies of patients undergoing esophagogastroduodenoscopy (EGD) with biopsy are available from several areas around the world. Prevalence varies from 0.3% to 2.7%. For example, a single-center retrospective study from the United States (1988–2008) reported an overall prevalence of AIG of 1.1% based on gastric biopsies from 41,245 patients with varying indications for endoscopy. The median age of diagnosis was 67.0 (range 18–94) years old with 2:1 female to male predominance. The prevalence was highest in non-white Hispanics (2.7%) but still approximately 1% in self-identified whites, Asians, and African Americans.4 In Japan, a study of 6,739 asymptomatic individuals who underwent a routine medical examination that included upper endoscopy with biopsies reported AIG prevalence to be 0.49% (0.65% in females and 0.40% in males); none of the patients diagnosed with AIG had anemia, nor did likelihood of AIG vary by H. pylori infection status.9 A large prospective study from China of 97,341 patients undergoing endoscopies with biopsies diagnosed AIG in 320 (based on histopathologic evidence. While the prevalence of AIG was lower compared to other studies, the same female: male predominance was demonstrated, albeit with a median age of diagnosis of 61 years (range: 26–86 years).3 Among the 56.9% of patients enrolled in the multi-center histoGERD trial found to have gastritis on endoscopy, approximately 2% had AIG diagnosed based on histology.10 Patients more commonly had alternative etiologies for the diagnosis of gastritis/gastropathy including H. pylori (18.7%) and reactive gastropathy (20.8%). One cross-sectional study of surgical specimens from 248 patients, who had undergone sleeve gastrectomy in which 12% (n = 30), were found to have gastritis of any type, reported that 0.8% (n = 2) had AIG with microcarcinoids (0% had AIG without microcarcinoids) versus 5.2% (n = 10) had H. pylori associated gastritis.11
Compared to the general population, AIG is more prevalent among patients with concomitant autoimmune diseases, especially autoimmune thyroid disease. Two recent retrospective cohort studies of AIG patients demonstrate 36–44% of these patients had autoimmune thyroiditis.12,13 AIG is also significantly more common in patients with type-I diabetes mellitus, with several studies estimating the risk of AIG to be approximately 3–5 times that of the general population.14–16
Pathogenesis
The earliest case series of AIG dates back to 1921 where Samuel Levine and William Ladd describe hypochlorhydria in 104 of 107 patients with PA, based primarily on a diagnosis of vitamin B12 deficiency and lack of gastric acid.17 Although the underlying etiology including the early pathways and trigger(s) of autoimmunity are not fully defined, the interaction between genetic and environmental factors is implicated. Familial clustering and concordance of AIG in twin studies support genetic predisposition to AIG, similar to other autoimmune diseases,18,19 but shared environmental triggers are also relevant in these studies. Candidate genes include certain HLA haplotypes, which may also associate with other autoimmune diseases including thyroid disease, type-1 diabetes mellitus, rheumatoid arthritis, and systemic lupus erythematosus.20
Parietal cells, which are located in the oxyntic glands, contain the gastric proton pump H + /K + ATPase, which is the predominant source of gastric acid production and secretion; parietal cells also secrete IF. Anti-PCA are often (but not always) present in patients with AIG, but do not appear to have a strong direct role in AIG pathogenesis and the characteristic loss of parietal cell mass. Instead, autoreactive T-cells recognize the H + /K + ATPase on parietal cells, which leads to T-cell-dependent activation of B-cells and subsequent PCA production; to this end, T-helper 1 cells and cytotoxic T-cells appear to be the main players in AIG pathogenesis.21–23
There is some controversy regarding whether H. pylori infection might be a trigger for AIG, and it is possible that there may be genetic predisposition underlying the association.24–26 The observation that H. pylori lipopolysaccharides and the gastric mucosa share some protein structures underlies one hypothesis that there is molecular mimicry between certain H. pylori antigens and H + /K + ATPase of parietal cells, which may in turn stimulate PCA production.27,28 Interestingly, one recent proteomic study identified overlap in proteomic signatures of gastric body biopsies between patients with AIG versus gastric cancer, both of which were significantly different compared to healthy controls with a family history of gastric cancer.6 Some of these changes in proteomic expression were also found in those with antral H. pylori only, suggesting potential shared pathways. Individuals with certain toll-like receptors (TLRs) polymorphisms may have a greater predisposition to AIG following H. pylori infection compared to individuals without these polymorphisms, suggesting also the role of host genetics.29
Diagnosis
Diagnostic delay is not uncommon for AIG, particularly because the condition is often asymptomatic or associated with mild nonspecific symptoms. Often, it is incidental laboratory findings such as anemia, which may occur in later stages that prompt diagnostic workup. One study of consecutive patients with AIG at an Italian center found that the median diagnostic delay was 14 months.19 Factors associated with diagnostic delay included female sex, previous misdiagnosis, history of infertility or miscarriages, as well as initial evaluation by a gastroenterologist (vs hematologist or internist).19 One study of patients who had been referred to a center in Japan for H. pylori eradication found that a significant proportion (19.5%, n = 43/220) of patients met the criteria for AIG. A majority of these patients (76.7%) had been previously treated twice for H. pylori, even though they were colonized with non-H. pylori urease-producing bacteria.30 In a study of 99 consecutive patients with AIG based on histology and positive PCA serology, the features which were associated with the diagnosis and may have prompted further evaluation were concomitant autoimmune disorders, neurologic symptoms, family history, vitamin B12 deficiency, and abnormal findings on gastric biopsies.31
Endoscopy
Upper endoscopy with biopsies obtained from the gastric body and antrum/incisura (at minimum) provides the gold standard for diagnosis. It is important that proceduralists recognize endoscopic features which might be consistent with AIG, although the endoscopic findings might be subtle early in the disease course. As atrophy progresses, the mucosa thins and rugae flatten which appears endoscopically as loss of gastric folds, pale mucosa, and prominent vessels visible through the thinned mucosa (Figure 1). Small lesions such as hyperplastic and pseudopolyps, neuroendocrine tumors (NETs), and even adenomas or adenocarcinoma may also be present (Figure 1). Concomitant gastric intestinal metaplasia is often easier to appreciate endoscopically, particularly with high-definition white light endoscopy and image enhancement, such as narrow band imaging. Features of gastric intestinal metaplasia include the classic tubulovillous pattern and light blue crest sign, which are more readily appreciated with near-focus or magnified endoscopy.32,33
Figure 1.
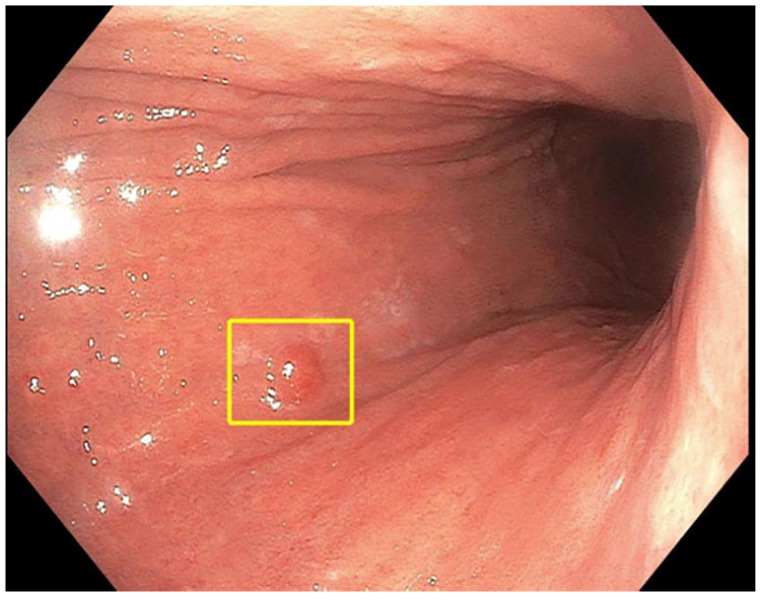
Endoscopic view of type-1 gastric neuroendocrine tumor on background of atrophic gastritis. The typical endoscopic features of atrophic gastritis are apparent, including loss of rugal folds and pallorous-appearing gastric mucosa; not as appreciated here is the increased prominence of submucosal blood vessels in the gastric body due to the thinned gastric mucosa. Endoscopic image is courtesy of Michelle K Kim, MD PhD.
In addition to a thorough endoscopic assessment in order to evaluate the extent of pre-neoplastic changes and identify/characterize any concerning lesions, obtaining biopsies according to the Sydney protocol is recommended and allows for the greatest diagnostic yield, as well as the opportunity to classify disease severity and extent using the Operative Link for Gastritis Assessment (OLGA)34 and Operative Link for Gastric Intestinal Metaplasia (OLGIM)35 staging systems. The updated Sydney protocol includes two sets of biopsies obtained from the following locations placed in separately labeled jars: greater and lesser curvature of the gastric corpus, greater and lesser curvatures of the gastric antrum, and the incisura. These anatomic biopsies should be placed in separately labeled jars, as should any biopsies obtained from visualized abnormalities.36
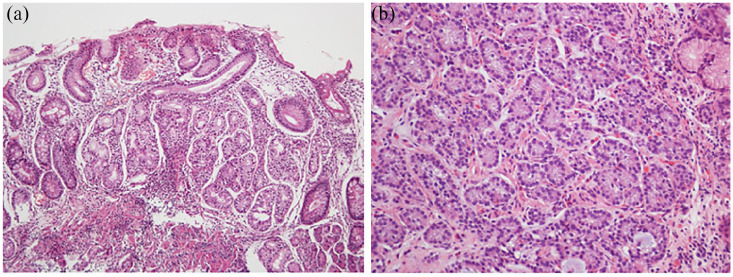
Histology (Figure 2(a) and (b))
Figure 2.
(a) Histopathological features of autoimmune gastritis (H&E stain, 100×, oxyntic mucosa). As characteristic of autoimmune gastritis, there is absence of parietal and chief cells. These are replaced by intestinal metaplasia and pseudopyloric metaplasia. There is background chronic inflammation. (b) Histopathologic image of type-I gastric neuroendocrine tumor. The tumor cells are well differentiated as evidenced by monomorphic round nuclei. These appear as “nests” infiltrating the lamina propria. Chromogranin A stain could be used to confirm neuroendocrine differentiation (stain not shown). Images are courtesy of Dr M Blanca Piazuelo.
On histopathology, the early phase of AIG is characterized by inflammatory cell invasion of the parietal cells (the non-atrophic phase); over time, there is progressive destruction of oxyntic glands (atrophic phase). The presence of relatively normal antral mucosa with inflammatory infiltrates in the oxyntic mucosa, including plasma cells, lymphocytes, eosinophils, neutrophils, and mast cells, with patchy oxyntic gland destruction is characteristic of AIG.37 With ongoing inflammation, the mucosa atrophies, and glands are replaced by fibrous tissue.38 The hypochlorhydric environment also leads to increased gastrin secretion, which in turn exerts a trophic effect on both parietal cell glands and enterochromaffin-like cells (ECLs), evidence of which may also be seen on histopathology in the early stages.21–23 A prospective longitudinal study of 270 patients with AIG showed that the histopathology of all patients progressed over time, from mild (mild atrophy of oxyntic mucosa with lymphocytic and plasma cell infiltration) to florid (moderate atrophy of oxyntic mucosa with lymphocytic and plasma cell infiltration) to end stage (severe atrophy of oxyntic mucosa), generally within 3 years.6 No patients had regression to milder stages. In this study, patients with ECL dysplasia, neuroendocrine dysplasia or neoplasia, or adenocarcinoma were defined as “complicated stage.”6
The severity and extent of gastric atrophy based on histopathology are important risk determinants for subsequent gastric neoplasia. Both the OLGA and OLGIM systems incorporate extent and severity of gastric atrophy, with OLGIM having the advantage of improved interobserver agreement compared to OLGA.39 Compared to OLGA/OLGIM stages 0–II, stage III, and stage IV are consistently associated with a significantly higher risk of gastric cancer.40,41 Of note, despite the utility of OLGA/OLGIM staging in risk prognostication and despite promotion by international society guidelines for guiding subsequent endoscopic surveillance (see below), the OLGA/OLGIM systems have not been widely used in the United States, but a change in practice has been advocated.33
Serologic markers
Serologic autoimmune markers, namely PCA and IF, are also relevant for AIG diagnosis, although they are neither necessary nor sufficient for the diagnosis, given their imperfect test characteristics. The test characteristics of IFA and PCA vary depending on the study. PCA has a high sensitivity and is generally present in 85–90% of patients with AIG but is not specific for the diagnosis because positive PCA is seen in other conditions, including H. pylori infection and other autoimmune diseases.42–45 Furthermore, PCA declines with age, and may be a less-reliable marker for those age 50 years or older.46 IFA by contrast, has a low sensitivity and often appears later in the course of AIG when the atrophy has progressed; indeed, while highly specific for AIG (and PA), the sensitivity of IFA for the diagnosis is ~50% for PA, and even lower for AIG.47,48
Pepsinogens (PG) are useful in diagnosing corpus atrophy. PG I is produced only in the corpus, whereas PG II may also be produced in the antrum. Therefore, in AIG, with progressive atrophy of the corpus both PG-I and PG-II levels decrease; however, because there is antral sparing, PG-II decline is less than that of PG I. As a result, the ratio of PG I:II is useful in the diagnosis of corpus atrophy. The cutoffs of PG I is less than 70, and PG I:II is less than or equal to 3 are most often used, with good discrimination of moderate–severe corpus atrophy.49 The caveat is these cutoffs are from studies almost universally performed in regions where H. pylori prevalence, and by extension, incidence of H. pylori-associated gastric (pre)cancer, is high. This test may not have the same performance in non-endemic countries, although further data and well-designed studies are needed. One cross-sectional single-center study from the United States reported that PG had poor discriminatory ability, but this study did not remark specifically on atrophic gastritis nor on the location (corpus vs antrum) or severity of mucosal changes.50
One study tested a variety of potential biomarkers (vitamin B12, chromogranin A, mean corpuscular volume, hemoglobin, and gastrin) and built a prediction model for AIG; the model notably did not include H. pylori or PG. The authors performed a single-center validation study and reported that a simple score that combined hemoglobin, mean corpuscular volume, and gastrin had a sensitivity of 85.7% and specificity of 83.7% for histologically confirmed AIG.51
While these biomarkers may be useful for the diagnosis of AIG, there are currently no reliable algorithms or risk stratification systems to predict which patients with AIG will develop complications, including malignancy (prognosis/natural history). One recent prospective study of 282 AIG patients found that over 18 years of follow-up time, the presence of PCA and the quantitative value was not associated with histological progression (defined in the study as the degree (mild, moderate, or severe) of atrophy of the oxyntic mucosa as well as the presence of inflammatory cells and complications were defined as the presence of dysplasia or neoplasia).6
Clinical manifestations
Symptoms
AIG is most often asymptomatic. When present, symptoms are usually nonspecific with patients most commonly reporting dyspepsia, especially post-prandial distress.52–54 One retrospective cohort analysis of 379 consecutive patients with AIG whose symptoms were scored using the Rome IV criteria found that 56.7% had GI symptoms, with dyspepsia being the most common.52 On multivariable analysis, younger age, and non-smoking status were more commonly associated with dyspepsia. Other cohort studies have demonstrated similar associations between younger age and dyspepsia.53 Another study of 41 patients with AIG who underwent pH impedance suggested that acid reflux rarely occurred and is thus likely not a common mechanism underlying symptoms in patients with AIG.54 Notably, though, patients with AIG are frequently treated with anti-acid therapy, which is likely to only worsen hypochlorhydria/achlorhydria.54
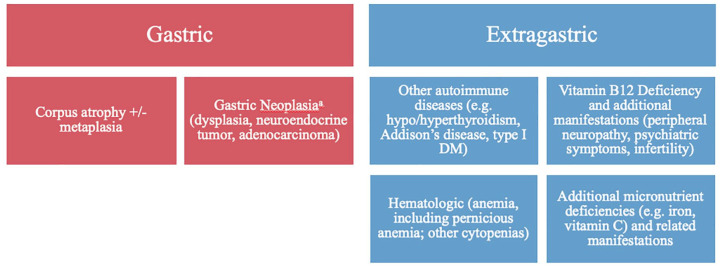
Extra-gastrointestinal symptoms include a number of other organ systems (Figure 3). Vitamin B12 deficiency can lead to subacute combined degeneration, a peripheral neuropathy and myelopathy of the posterior and lateral columns that can be irreversible if treatment is delayed.55 In severe cases, this can also cause optic neuropathy and encephalopathy. One retrospective study of 65 patients with both neuropathy and vitamin B12 deficiency, reported that low serum PG-1 levels (PG 1 < 70 ng/mL) and serum PG 1:2 ratios (<3.0) correlated both with more severe anemia and more severe neurologic symptoms.55 Lower PG-1 levels (22.4 ng/mL or less) was a more specific measure. AIG may also be associated with miscarriage and infertility related to vitamin B12 deficiency.56 Infertility among patients with AIG may also be a manifestation of hypothyroidism or other autoimmune diseases commonly associated with AIG; practitioners should take care to replete B12 and investigate other potential etiologies.57
Figure 3.
Clinical and histological manifestations of autoimmune gastritis.
Anemia
The anemia of AIG (with or without PA) may be microcytic, normocytic, or macrocytic, although macrocytic is the classic association (Figure 3).58 Anemia is common in AIG, with a prevalence around 50% depending on the study, with the most often etiologies iron and vitamin B12 deficiency.59 Gastric acid is important for enzyme activation and freeing micronutrients from dietary sources. Gastric acid is also important for the reduction and absorption of iron. As previously described, vitamin B12 deficiency in patients with AIG may be attributed to parietal cell loss as well as autoantibodies targeting IF. IF is responsible for binding vitamin B12 and facilitating uptake in the terminal ileum. Iron stores are depleted more quickly than vitamin B12 stores and thus iron deficiency anemia is often an early manifestation of AIG.53,60,61 While the presence of vitamin B12 deficiency and anemia often prompts an AIG workup,5,62 it should be equally appreciated that vitamin B12 deficiency has a broad differential, and more common causes should be evaluated in patients.33 The diagnosis of anemia may also be confounded by other underlying diseases, such as hypothyroidism. To this end, one multi-center study of 654 consecutive AIG patients, nearly 50% of whom had anemia, demonstrated that certain patient characteristics were associated with patterns of anemia.59 PA was more common in males and older patients, whereas iron deficiency anemia was more common in females and younger patients. Iron deficiency was present in both those with and without anemia. These findings were similar to another cohort study of 160 patients with AIG based on serologies.63 In this study, 83 of the 160 patients with AIG were also diagnosed with iron deficiency anemia. Again, those with iron deficiency were more often women, younger (median age 41 vs 62 with macrocytic anemia), and had a higher prevalence of H. pylori (41.3% vs 28.6%).
In vitamin B12 deficiency, patients may be asymptomatic while other patients can have sequalae of symptoms as the disease progresses. This includes subtle findings including glossitis or more catastrophic symptoms of infertility, early recurrent miscarriages, or even thrombosis as well as the neurologic symptoms described above.56
Other nutrient deficiencies
Achlorhydria/hypochlorhydria may also lead to other micronutrient deficiencies, in addition to iron and vitamin B12 described above. One single-center study of 122 patients with AIG on endoscopic biopsy found that 76 had at least one nutritional deficiency (vitamin B12, iron, vitamin D, and folic acid) and 52 had more than one.64 The progression of vitamin deficiencies occurs over a long period of time due to natural reserves in the body and can present with vague, non-localizing symptoms. Thus, providers should be attuned to other nutritional deficiencies in patients with AIG and manage these appropriately.
Concomitant autoimmune diseases
As noted, AIG not uncommonly coexists with other autoimmune conditions, some of which have GI manifestations. Concomitant thyroid disease is most common, with two recent retrospective cohort studies of AIG patients showing 36–44% of these patients had autoimmune thyroiditis.12,13 Conversely, among patients with autoimmune thyroid disease, approximately 40% had atrophic body gastritis and 16% had PA.12,65 Among patients with type-1 diabetes mellitus (T1DM), the risk of AIG is elevated 3–5 times that of the general population.14–16 Similarly, another study of other autoimmune disease include Addison’s disease (4.8% of Addison’s patients also had PA),66 T1DM (5–10% with AIG, 2–4% with PA),67 and some other diseases such as vitiligo, chronic spontaneous urticarial, myasthenia gravis, and perioral cutaneous autoimmune conditions.68 Indeed, in patients with an autoimmune disease who present with anemia, a diagnosis of AIG should be considered.
Neoplasms
AIG/PA is considered a preneoplastic condition, given the established increased risk of gastric adenocarcinoma or gastric NETs associated with these conditions.69 A case–control study in the Surveillance, Epidemiology, and End Results (SEER) database found that PA was associated with increased odds of gastric adenocarcinoma (odds ratio (OR): 2.18, 95% confidence interval [CI]: 1.94–2.45) and gastric NETs (OR: 11.43; 95% CI: 8.90–14.69). Several single-center studies suggest an elevated risk of both gastric adenocarcinoma and type-1 NETs, with an incidence of approximately 1.4% per person-year in patients enrolled in surveillance.70–72 A recent single-center prospective study from Italy of patients with biopsy-proven AIG demonstrated little disease regression over time.6 More advanced disease, as defined based on the extent of atrophic/metaplastic changes and histologic severity, is associated with up to 7-fold higher risk of gastric neoplasia compared to less-advanced disease (for example, as defined based on OLGA/OLGIM systems detailed above).73
There have been several case–control and cohort studies confirming this elevated risk; that being said, it is possible that the risk might even be underestimated, given the heterogeneity with respect to terminology and rigor in confirming a diagnosis of AIG/PA.4,74,75 For example, the US population-based study by Murphy and colleagues described above defined AIG solely on having an ICD-9 code for PA without endoscopic and histologic confirmation (or concordant serologic antibodies). It should also be noted that since this was a Medicare-linked population, only patients >66 years old were included and thus might not reflect the risk among younger patients.74 Another large study from Sweden among 21,265 patients hospitalized with PA (unverified diagnosis based on ICD coding) followed for a mean of 7.1 years reported a standardized incidence rate of gastric adenocarcinoma of 2.4 (95% CI: 2.1–2.7) and NETs of 26.4 (95% CI: 14.8–43.5).75 A retrospective study of stomach biopsy specimens over 20 years at one institution, reported that 143 of 461 (31%) patients had lesions including polyps, neuroendocrine neoplasms, lymphoma, GI stromal tumor, and adenocarcinoma that were identified on endoscopy, 17 of whom had polyps requiring close follow-up, and 11 (2%) had gastric adenocarcinoma.4 A recent meta-analysis that included 14 studies and 2688 patients found that PA (as defined based on the individual study) was associated with a 2.84-fold (95% CI 2.30–3.50) higher risk of gastric cancer compared to patients without PA.76 The histology was not specified. A different meta-analysis that included 27 studies and 22,417 patients reported that PA was associated with 6.8-fold (95% CI: 2.6–18.1) higher relative risk of gastric cancer versus patients without PA.77 This meta-analysis also did not specify the cancer histology, as many included studies did not provide that information.
Clinical management
There are no randomized controlled studies evaluating the benefit of endoscopic surveillance, nor of ideal surveillance intervals/methods in AIG for the purpose of earlier detection of neoplasia (dysplasia, cancer). Instead, observational data demonstrating a significantly increased risk of gastric neoplasia associated with AIG and PA provide the clinical rationale for performing endoscopic surveillance. Several international societies provide clinical guidance on screening, diagnosis, and management of chronic atrophic gastritis, many times embedded within guidelines for other gastric (pre)neoplasia.
Endoscopy
Because of the malignancy risk, endoscopic surveillance should be considered or is recommended according to international society guidelines—namely, the British Society of Gastroenterology (BSG),40 the American Gastroenterological Association (AGA),33 the European Society of Gastrointestinal Endoscopy (ESGE),41 the American Society of Gastrointestinal Endoscopy (ASGE),78 a consensus statement by multiple Italian societies for gastroenterology, endoscopy and internal medicine,79 and Kyoto Consensus Report80—but the optimal interval for surveillance is not established, with most generally recommending every 3–5 years and potentially shorter intervals depending on additional risk factors for gastric cancer, including family history. Guidance varies with respect to surveillance in patients with AG/GIM due to AIG specifically, with some suggesting surveillance every 3–5 years,41,79 and others, namely US societies, advocating determination of intervals according to individualized assessment and shared decision-making.33,78 The clinical or cost-effectiveness of these practices has not been demonstrated, making it low-quality evidence. The ASGE, AGA, BSG, Italian Societies, and ESGE acknowledge that there is a significantly increased prevalence of gastric neoplasia at the time of diagnosis of PA and that the risk for neoplasia is highest within the first year of PA diagnosis. Accordingly, the ASGE recommends endoscopy within 6 months of PA diagnosis and the BSG recommends that baseline endoscopy with biopsies be considered in individuals >50 years old with PA. Once the diagnosis of AIG is made, all patients should be evaluated for concomitant PA. However, it is unclear if and at what interval these serologies should be repeated if they are negative initially. The recent AGA Clinical Practice Update advises screening for type-1 gastric NETs with upper endoscopy among patients with AIG, along with resection, with or without ongoing surveillance, depending on the size and burden of NETs.33
Medical management
There is limited guidance on the management of non-neoplastic complications of AIG/PA, including frequency of interval surveillance and monitoring for vitamin B12 deficiency and iron deficiency. Importantly, vitamin B12 deficiency can occur in the absence of anemia and if not addressed can lead to irreversible neurologic deficits. Parenteral rather than oral supplementation is preferred, especially if neurologic symptoms are present. Appropriate supplementation of vitamin B12 and iron is important, along with workup of other etiologies for these not uncommon deficiencies based on the clinical scenario.
Conclusion
AIG is an important condition with both benign and malignant complications. Unfortunately, delays in diagnosis are common, given the indolent course and often subtle clinical presentation, if even at all, particularly in the early stages. Accordingly, clinicians should be aware of optimal practices related to diagnosis and management to reduce the likelihood of poor outcomes.
Acknowledgments
The authors would like to sincerely thank Dr Michelle Kim and Dr M Blanca Piazuelo for providing images used in this article, as well as Samantha Walsh, the biomedical librarian who assisted with the literature search.
Footnotes
Author contributions: Conceptualization—S.D.R. and S.C.S.; literature search and data curation—S.D.R., P.B., and S.C.S.; manuscript writing—original draft, review and editing: S.D.R., P.B., S.C.S.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SCS is funded by a 2019 American Gastroenterological Association Research Scholar Award and Veterans Affairs Career Development Award under award no. ICX002027A01.
ORCID iDs: Sheila D. Rustgi  https://orcid.org/0000-0002-1889-1652
https://orcid.org/0000-0002-1889-1652
Shailja C. Shah  https://orcid.org/0000-0002-2049-9959
https://orcid.org/0000-0002-2049-9959
Contributor Information
Sheila D. Rustgi, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY, USA; Division of Digestive and Liver Diseases, Columbia University Irving Medical Center, New York, NY, USA
Priyesha Bijlani, Department of Medicine, University of California, San Diego, La Jolla, CA, USA.
Shailja C. Shah, Section of Gastroenterology, VA San Diego Healthcare System, 3350 La Jolla Villa Drive, San Diego, CA 92161, USA; Division of Gastroenterology, University of California, San Diego, La Jolla, CA, USA.
References
- 1.Judd LM, Gleeson PA, Toh BH, et al. Autoimmune gastritis results in disruption of gastric epithelial cell development. Am J Physiol 1999; 277: G209–G218. [DOI] [PubMed] [Google Scholar]
- 2.Strickland RG, Mackay IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis 1973; 18: 426–440. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Jin Z, Cui R, et al. Autoimmune metaplastic atrophic gastritis in Chinese: a study of 320 patients at a large tertiary medical center. Scand J Gastroenterol 2017; 52: 150–156. [DOI] [PubMed] [Google Scholar]
- 4.Park JY, Cornish TC, Lam-Himlin D, et al. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol 2010; 34: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 5.Andres E, Serraj K. Optimal management of pernicious anemia. J Blood Med 2012; 3: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miceli E, Vanoli A, Lenti MV, et al. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther 2019; 50: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 7.Carmel R, Johnson CS. Racial patterns in pernicious anemia. Early age at onset and increased frequency of intrinsic-factor antibody in black women. N Engl J Med 1978; 298: 647–650. [DOI] [PubMed] [Google Scholar]
- 8.Carmel R, Johnson CS, Weiner JM. Pernicious anemia in Latin Americans is not a disease of the elderly. Arch Intern Med 1987; 147: 1995–1996. [PubMed] [Google Scholar]
- 9.Notsu T, Adachi K, Mishiro T, et al. Prevalence of autoimmune gastritis in individuals undergoing medical checkups in Japan. Intern Med 2019; 58: 1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf EM, Plieschnegger W, Geppert M, et al. Changing prevalence patterns in endoscopic and histological diagnosis of gastritis? Data from a cross-sectional Central European multicentre study. Dig Liver Dis 2014; 46: 412–418. [DOI] [PubMed] [Google Scholar]
- 11.Raess PW, Baird-Howell M, Aggarwal R, et al. Vertical sleeve gastrectomy specimens have a high prevalence of unexpected histopathologic findings requiring additional clinical management. Surg Obes Relat Dis 2015; 11: 1020–1023. [DOI] [PubMed] [Google Scholar]
- 12.Chan JC, Liu HS, Kho BC, et al. Pattern of thyroid autoimmunity in Chinese patients with pernicious anemia. Am J Med Sci 2009; 337: 432–437. [DOI] [PubMed] [Google Scholar]
- 13.Kalkan C, Soykan I. Polyautoimmunity in autoimmune gastritis. Eur J Intern Med 2016; 31: 79–83. [DOI] [PubMed] [Google Scholar]
- 14.De Block CE, De Leeuw IH, Bogers JJ, et al. Helicobacter pylori, parietal cell antibodies and autoimmune gastropathy in type 1 diabetes mellitus. Aliment Pharmacol Ther 2002; 16: 281–289. [DOI] [PubMed] [Google Scholar]
- 15.De Block CE, De Leeuw IH, Van Gaal LF. High prevalence of manifestations of gastric autoimmunity in parietal cell antibody-positive type 1 (insulin-dependent) diabetic patients. The Belgian Diabetes Registry. J Clin Endocrinol Metab 1999; 84: 4062–4067. [DOI] [PubMed] [Google Scholar]
- 16.De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab 2008; 93: 363–371. [DOI] [PubMed] [Google Scholar]
- 17.Levine SA, Ladd WS. Pernicious anemia: a clinical study of one-hundred and fifty consecutive cases with special reference to gastric anacidity. Bull Johns Hopkins Hosp 1921; 32: 254–266. [Google Scholar]
- 18.Delva PL, Macdonell JE, Macintosh OC. MEGALOBLASTIC ANEMIA OCCURRING SIMULTANEOUSLY IN WHITE FEMALE MONOZYGOTIC TWINS. Can Med Assoc J 1965; 92: 1129–1131. [PMC free article] [PubMed] [Google Scholar]
- 19.Lenti MV, Miceli E, Cococcia S, et al. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment Pharmacol Ther 2019; 50: 167–175. [DOI] [PubMed] [Google Scholar]
- 20.Arango MT, Perricone C, Kivity S, et al. HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol Res 2017; 65: 82–98. [DOI] [PubMed] [Google Scholar]
- 21.Chlumská A, Boudová L, Benes Z, et al. Autoimmune gastritis. A clinicopathologic study of 25 cases. Cesk Patol 2005; 41: 137–142. [PubMed] [Google Scholar]
- 22.Solcia E, Fiocca R, Villani L, et al. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am J Surg Pathol 1995; 19(Suppl. 1): S1–S7. [PubMed] [Google Scholar]
- 23.Stolte M, Bethke B, Rühl G, et al. Omeprazole-induced pseudohypertrophy of gastric parietal cells. Z Gastroenterol 1992; 30: 134–138. [PubMed] [Google Scholar]
- 24.Claeys D, Faller G, Appelmelk BJ, et al. The gastric H+, K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 1998; 115: 340–347. [DOI] [PubMed] [Google Scholar]
- 25.Eidt S, Oberhuber G, Schneider A, et al. The histopathological spectrum of type A gastritis. Pathol Res Pract 1996; 192: 101–106. [DOI] [PubMed] [Google Scholar]
- 26.Fong T-L, Dooley CP, Dehesa M, et al. Helicobacter pylori infection in pernicious anemia: a prospective controlled study. Gastroenterology 1991; 100: 328–332. [DOI] [PubMed] [Google Scholar]
- 27.Amedei A, Bergman MP, Appelmelk BJ, et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+–adenosine triphosphatase in human gastric autoimmunity. J Exp Med 2003; 198: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol 2009; 15: 5121–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Re V, Repetto O, De Zorzi M, et al. Polymorphism in toll-like receptors and Helicobacter pylori motility in autoimmune atrophic gastritis and gastric cancer. Cancers 2019; 11: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuta T, Baba S, Yamade M, et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. Pylori eradication. Aliment Pharmacol Ther 2018; 48: 370–377. [DOI] [PubMed] [Google Scholar]
- 31.Miceli E, Lenti MV, Padula D, et al. Common features of patients with autoimmune atrophic gastritis. Clin Gastroenterol Hepatol 2012; 10: 812–814. [DOI] [PubMed] [Google Scholar]
- 32.Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, et al. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc 2017; 86: 857–865. [DOI] [PubMed] [Google Scholar]
- 33.Shah SC, Kuipers EJ, Li D.American Gastroenterological Association clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology 2021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007; 56: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010; 71: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 36.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 37.Coati I, Fassan M, Farinati F, et al. Autoimmune gastritis: pathologist’s viewpoint. World J Gastroenterol 2015; 21: 12179–12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenti MV, Rugge M, Lahner E, et al. Autoimmune gastritis. Nat Rev Dis Primers 2020; 6: 56. [DOI] [PubMed] [Google Scholar]
- 39.Isajevs S, Liepniece-Karele I, Janciauskas D, et al. Gastritis staging: interobserver agreement by applying OLGA and OLGIM systems. Virchows Arch 2014; 464: 403–407. [DOI] [PubMed] [Google Scholar]
- 40.Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019; 68: 1545–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019; 51: 365–388. [DOI] [PubMed] [Google Scholar]
- 42.Rusak E, Chobot A, Krzywicka A, et al. Anti—parietal cell antibodies—diagnostic significance. Adv Med Sci 2016; 61: 175–179. [DOI] [PubMed] [Google Scholar]
- 43.Bizzaro N, Antico A. Diagnosis and classification of pernicious anemia. Autoimmun Rev 2014; 13: 565–568. [DOI] [PubMed] [Google Scholar]
- 44.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med 1999; 159: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 45.Toh BH. Diagnosis and classification of autoimmune gastritis. Autoimmun Rev 2014; 13: 459–462. [DOI] [PubMed] [Google Scholar]
- 46.Conti L, Lenti MV, Di Sabatino A, et al. Seronegative autoimmune atrophic gastritis is more common in elderly patients. Dig Liver Dis 2020; 52: 1310–1314. [DOI] [PubMed] [Google Scholar]
- 47.Antico A, Tampoia M, Villalta D, et al. Clinical usefulness of the serological gastric biopsy for the diagnosis of chronic autoimmune gastritis. Clin Dev Immunol 2012; 2012: 520970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan S, Del-Duca C, Fenton E, et al. Limited value of testing for intrinsic factor antibodies with negative gastric parietal cell antibodies in pernicious anaemia. J Clin Pathol 2009; 62: 439–441. [DOI] [PubMed] [Google Scholar]
- 49.Zagari RM, Rabitti S, Greenwood DC, et al. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther 2017; 46: 657–667. [DOI] [PubMed] [Google Scholar]
- 50.Huang R, Park S, Shen J, et al. Pepsinogens and gastrin demonstrate low discrimination for gastric precancerous lesions in a multi-ethnic United States Cohort. Clin Gastroenterol Hepatol. Epub ahead of print 10 January 2021. DOI: 10.1016/j.cgh.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Miceli E, Padula D, Lenti MV, et al. A laboratory score in the diagnosis of autoimmune atrophic gastritis: a prospective study. J Clin Gastroenterol 2015; 49: e1–e5. [DOI] [PubMed] [Google Scholar]
- 52.Carabotti M, Lahner E, Esposito G, et al. Upper gastrointestinal symptoms in autoimmune gastritis: a cross-sectional study. Medicine 2017; 96: e5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalkan C, Soykan I. Differences between older and young patients with autoimmune gastritis. Geriatr Gerontol Int 2017; 17: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 54.Tenca A, Massironi S, Pugliese D, et al. Gastro-esophageal reflux and antisecretory drugs use among patients with chronic autoimmune atrophic gastritis: a study with pH-impedance monitoring. Neurogastroenterol Motil 2016; 28: 274–280. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Wang R, Huang X, et al. The level of serum pepsinogen in diagnosing and evaluating the severity of subacute combined degeneration due to vitamin B12 deficiency. Front Neurol 2021; 12: 604523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenti MV, Miceli E, Padula D, et al. Infertility and recurrent miscarriage in a patient with autoimmune atrophic gastritis. Intern Emerg Med 2018; 13: 815–816. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Q, Xu QH, Xie T, et al. Recent insights into the impact of immune dysfunction on reproduction in autoimmune thyroiditis. Clin Immunol 2021; 224: 108663. [DOI] [PubMed] [Google Scholar]
- 58.Stabler SP. Vitamin B12 deficiency. N Engl J Med 2013; 368: 149–160. [DOI] [PubMed] [Google Scholar]
- 59.Lenti MV, Lahner E, Bergamaschi G, et al. Cell blood count alterations and patterns of anaemia in autoimmune atrophic gastritis at diagnosis: a multicentre study. J Clin Med 2019; 8: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villanacci V, Casella G, Lanzarotto F, et al. Autoimmune gastritis: relationships with anemia and Helicobacter pylori status. Scand J Gastroenterol 2017; 52: 674–677. [DOI] [PubMed] [Google Scholar]
- 61.Elloumi H, Sabbah M, Debbiche A, et al. Systematic gastric biopsy in iron deficiency anaemia. Arab J Gastroenterol 2017; 18: 224–227. [DOI] [PubMed] [Google Scholar]
- 62.Lahner E, Norman GL, Severi C, et al. T1077 occurrence of intrinsic factor and parietal cell autoantibodies in atrophic body gastritis patients with or without pernicious anemia. Gastroenterology 2008; 134: A-478. [Google Scholar]
- 63.Hershko C, Ronson A, Souroujon M, et al. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood 2006; 107: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 64.Zilli A, Cavalcoli F, Ciafardini C, et al. Deficiency of micronutrients in patients affected by chronic atrophic autoimmune gastritis: a single-institution observational study. Dig Liver Dis 2019; 51: 505–509. [DOI] [PubMed] [Google Scholar]
- 65.Lahner E, Centanni M, Agnello G, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med 2008; 121: 136–141. [DOI] [PubMed] [Google Scholar]
- 66.Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8: 121–130. [DOI] [PubMed] [Google Scholar]
- 67.Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev 2016; 15: 644–648. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Castro KI, Franceschi M, Miraglia C, et al. Autoimmune diseases in autoimmune atrophic gastritis. Acta Biomed 2018; 89: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rustgi N, Shroff SG, Katona BW. Two types of gastric cancer caused by the same underlying condition. Gastroenterology 2018; 154: 1246–1248. [DOI] [PubMed] [Google Scholar]
- 70.Lahner E, Esposito G, Pilozzi E, et al. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol 2015; 50: 856–865. [DOI] [PubMed] [Google Scholar]
- 71.Mahmud N, Stashek K, Katona BW, et al. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: a renewed call for surveillance. Ann Gastroenterol 2019; 32: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borch K. Epidemiologic, clinicopathologic, and economic aspects of gastroscopic screening of patients with pernicious anemia. Scand J Gastroenterol 1986; 21: 21–30. [DOI] [PubMed] [Google Scholar]
- 73.Rugge M, Fassan M, Pizzi M, et al. Autoimmune gastritis: histology phenotype and OLGA staging. Aliment Pharmacol Ther 2012; 35: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 74.Murphy G, Dawsey SM, Engels EA, et al. Cancer risk after pernicious anemia in the US elderly population. Clin Gastroenterol Hepatol 2015; 13: 2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye W, Nyren O. Risk of cancers of the oesophagus and stomach by histology or subsite in patients hospitalised for pernicious anaemia. Gut 2003; 52: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song M, Latorre G, Ivanovic-Zuvic D, et al. Autoimmune diseases and gastric cancer risk: a systematic review and meta-analysis. Cancer Res Treat 2019; 51: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013; 37: 375–382. [DOI] [PubMed] [Google Scholar]
- 78.ASGE Standards of Practice Committee, Evans JA, Chandrasekhara V, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015; 82: 1–8. [DOI] [PubMed] [Google Scholar]
- 79.Lahner E, Zagari RM, Zullo A, et al. Chronic atrophic gastritis: natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis 2019; 51: 1621–1632. [DOI] [PubMed] [Google Scholar]
- 80.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64: 1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]