Abstract
Background:
There are many clinical practice guidelines (CPGs) for the prevention, diagnosis, and treatment of knee osteoarthritis (OA). They differ by region, considering local health care systems, along with cultural and economic factors. Currently, there are conflicting CPG recommendations across the various publications, which makes it difficult for clinicians to fully understand the optimal treatment decisions for knee OA management.
Purpose:
To summarize the current published CPG recommendations for the role of injections in the nonoperative management of knee OA, specifically with the use of intra-articular hyaluronic acid (IA-HA), intra-articular corticosteroids (IA-CS), and platelet-rich plasma (PRP).
Study Design:
Systematic review.
Methods:
A comprehensive search identified all nonoperative knee OA CPGs within the ECRI (formerly Emergency Care Research Institute) Guidelines Trust database, the Guidelines International Network database, Google Scholar, and the Trip (formerly Turning Research Into Practice) database. Guideline recommendations were categorized into strong, conditional, or uncertain recommendations for or against the use of IA-HA, IA-CS, or PRP. Guideline recommendations were summarized and depicted graphically to identify trends in recommendations over time.
Results:
The search strategy identified 27 CPGs that provided recommendations. There were 20 recommendations in favor of IA-HA use, 21 recommendations in favor of IA-CS use, and 9 recommendations that were uncertain or unable to make a formal recommendation for or against PRP use based on current evidence. Most recommendations considered IA-HA and IA-CS use for symptom relief when other nonoperative options are ineffective. IA-CS were noted to provide fast and short-acting symptom relief for acute episodes of disease exacerbation, while IA-HA may demonstrate a relatively delayed but prolonged effect in comparison. The CPGs concluded that PRP recommendations currently lack evidence to definitively recommend for or against use.
Conclusion:
Available CPGs provide recommendations on injectables for knee OA treatment. General guidance from a global perspective concluded that IA-CS and IA-HA are favored for different needed responses and can be utilized within the knee OA treatment paradigm, while PRP currently has insufficient evidence to make a conclusive recommendation for or against its use.
Keywords: osteoarthritis, knee, injectables, corticosteroids, hyaluronic acid, platelet-rich plasma
Osteoarthritis (OA) can be defined as a progressive degeneration of synovial joints, with characteristic features such as articular cartilage degeneration, osteophyte formation, meniscal degeneration, and formation of bone marrow lesions.4,22 Knee OA may lead to significant disability, as progressive pain and stiffness will lead to difficulties in performing activities of daily living.4,5,29 Consequently, knee OA is a functional burden placed on the individual patient, as well as a significant socioeconomic burden on the health care system and society.23,29
Nonpharmacological therapeutic options for patients with knee OA include weight reduction, exercise, and orthotic devices, while pharmacological therapies consist of oral medications for pain management or intra-articular injection therapies.4,18 A range of surgical options are also available, including lower extremity realignment osteotomy, arthroscopy, and partial or total knee arthroplasty.30 The myriad of potential treatment options provide clinicians with many choices to make with regard to the management of their patients’ knee OA. In order to aid in this decision-making process, various national and international health care groups have published clinical practice guidelines (CPGs) that provide treatment recommendations for the management of knee OA.1
There are many CPGs for the prevention, diagnosis, and treatment of OA that differ by geographic location, taking into account local health care systems and cultural and economic factors.1 Currently, there are conflicting guideline recommendations available across these published CPGs, which makes it difficult for clinicians to fully understand available options to make the optimal treatment decisions for knee OA management. The use of intra-articular hyaluronic acid (IA-HA) in these patients has particularly been in question for knee OA management because of the seemingly conflicting recommendations in current CPGs.1,6,29 Intra-articular corticosteroid (IA-CS) injections and the more recently introduced use of platelet-rich plasma (PRP) injections for knee OA are other injectable treatments commonly assessed within the scientific literature.
This study aimed to systematically review the currently published CPG recommendations for knee OA management particular to the use of IA-HA, IA-CS, or PRP. Guidelines published from various international groups were summarized to assess treatment recommendations globally. We hypothesized that a large body of CPGs across the globe would be generally consistent with their recommendations of injectables, with some notable outliers.
Methods
Search Strategy
A comprehensive search to identify all guidelines relevant to the nonoperative management of knee OA was conducted within the ECRI (formerly Emergency Care Research Institute) Guidelines Trust database, the Guidelines International Network database, Google Scholar, and the Trip (formerly Turning Research Into Practice) database. All article hits within the searches were reviewed for potential eligibility. These databases were searched using varied combinations of the following terms: “knee,” “osteoarthritis,” “gonarthritis,” and “arthritis,” as systematic search strategies using Boolean terminology are not possible in these databases. Searches within Google Scholar were limited to guidelines using the term “recommendation,” “guideline,” or “CPG.” Additionally, a detailed hand search of reference lists was conducted by 2 research associates (Global Research Solutions Inc) in order to identify any additional guidelines that were relevant to the management of knee OA. Although many of these databases do not allow for a systematic search strategy to be created and utilized, this hand search of many guideline-specific databases was comprehensive in identifying all of the relevant CPGs on the topic. All retrieved articles were then screened for potential duplicates, and any duplicate articles were removed. Articles were included if they provided a treatment recommendation statement regarding the use of IA-HA, IA-CS, or PRP for knee OA. Articles of all languages were included and translated into English by using Google translate (https://translate.google.com).
Guidelines Overview
The CPG characteristics, including publication year and geographic location of the professional group publishing the guideline, were recorded. The recommendations regarding the use of IA-HA, IA-CS, and PRP for knee OA from each guideline were summarized according to the strength of the recommendation and certainty of the available evidence, if reported. Guideline recommendations were generalized into the following categories, which were adapted from the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to recommendation development: strong/unconditional recommendation against treatment use, weak recommendation against treatment use, uncertain recommendation regarding treatment use, weak/conditional recommendation for the use of treatment, and a strong/unconditional recommendation for the use of treatment.17 If recommendations were contingent on specific treatments having failed first, or specific clinical parameters being met, the recommendation was considered conditional. Based on these categories, the recommendations were graphically depicted in infographics to illustrate the general recommendation trends that are published on the use of these treatments over time. When available, summary statements about the CPG reasons for the recommendations, such as perceived treatment effect, safety, duration of treatment effect, and evidence base in the available literature, were reported.
Results
Included Guidelines
The search strategy identified 27 guidelines that provided a recommendation on the use of injectables for knee OA.** The United States was the most frequent country represented within the included guidelines (7 articles; 25.9%).4,8,13,18,21,34,37 Guidelines were published between 2003 and 2020. All 27 (100%) guidelines provided a statement regarding IA-HA use, 24†† (88.9%) provided a statement on IA-CS use, and 11‡‡ (40.7%) provided a statement on PRP use. Appendix Table A1 provides a summary of the included guidelines and recommendations.
IA-HA Recommendations
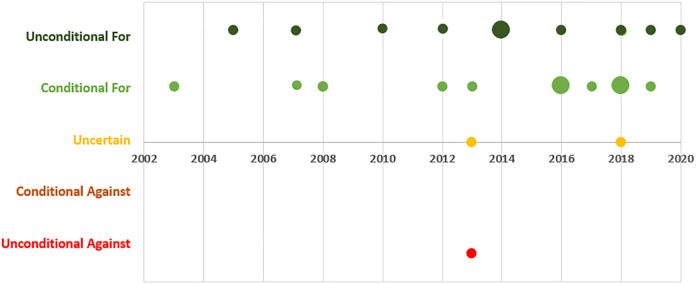
All 27 guidelines provided a statement on the use of IA-HA for treating knee OA. There were 102,3,7,10-12,15,32,35,37 (37.0%) strong recommendations for the use of IA-HA, 10§§ (37.0%) conditional recommendations for its use, 28,13 (7.4%) uncertain recommendations, 221,36 (7.4%) weak recommendations against, and 318,26,27 (11.1%) strong recommendations against the use of IA-HA for knee OA. Figure 1 provides an overview of IA-HA recommendation trends over time, demonstrating a general trend toward positive recommendations for IA-HA between 2003 and 2020.
Figure 1.
Intra-articular hyaluronic acid recommendations over time—subjective strength of recommendations. Data point size is weighted by number of repeat recommendations in the same year.
IA-CS Recommendations
Twenty-four guidelines provided a statement on the use of IA-CS for treating knee OA. There were 10∥∥ (41.7%) strong recommendations for the use of IA-CS, 11¶¶ (45.8%) conditional recommendations for, 215,18 (8.3%) uncertain recommendations, 0 (0.0%) weak recommendations against, and 125 (4.2%) strong recommendation against the use of IA-CS for knee OA. Figure 2 provides an overview of IA-CS recommendation trends over time, demonstrating a general trend toward positive recommendations for the use of IA-CS between 2003 and 2020.
Figure 2.
Intra-articular corticosteroid recommendations over time—subjective strength of recommendations. Data point size is weighted by number of repeat recommendations in the same year.
PRP Recommendations
There were 11 guidelines that provided a statement on the use of PRP for treating knee OA. There were 9## (81.8%) recommendations that were either uncertain or unable to make a formal recommendation on the use of PRP for knee OA and 24,21 (18.2%) against the use of PRP for knee OA. Figure 3 provides an overview of PRP recommendation trends over time, demonstrating a general uncertainty and inconclusive recommendations regarding PRP between 2013 and 2020.
Figure 3.
Platelet-rich plasma recommendations over time—subjective strength of recommendations. Data point size is weighted by number of repeat recommendations in the same year.
Discussion
This study has provided a comprehensive overview of current guideline recommendations on knee IA-HA, IA-CS, and PRP for knee OA. While disagreement seems to exist among individual guidelines, there are general trends in the guidance provided on IA-HA, IA-CS, and PRP. Guidelines, in general, have been favorable for IA-HA use for knee OA—especially when used after conservative options have failed. Similarly, IA-CS is consistently recommended in this same scenario, with an understanding that the treatment effects have a quicker onset but shorter period of symptom relief. Currently, the guidelines that provided recommendations for PRP have noted that there is insufficient evidence to support the use of PRP for knee OA at this time.
IA-HA in most CPGs was recommended, with an equal number of recommendations for general use and secondary treatment if other conservative options fail. While some guidelines provided unfavorable recommendations against IA-HA use, the reasoning was typically for a lack of certainty and risk of bias within the available evidence.21,26,36 There are considerations and nuances within the IA-HA literature that may contribute to this lack of certainty in the evidence, despite there being a relatively large number of trials assessing this intervention.29 There is a growing body of literature demonstrating that product difference, particularly the HA molecular weight, may have a significant effect on the outcomes of this treatment.29 The distinction of IA-HA molecular weight differences has been acknowledged in some CPGs, particularly more recent recommendations. A recent network meta-analysis (NMA) has demonstrated this difference, which suggests that not all IA-HA treatments provide equal outcomes.29
IA-CS recommendations were generally favorable, with many guidelines acknowledging the fast onset of symptom relief in acute flare-up situations. Guidelines did not generally provide insights into a recommended schedule for repeated injections. More recent guidelines and the underlying evidence, however, have cited the potential harms of the repeated use of IA-CS—particularly when used in mild to moderate stages of knee OA severity.24 Similar to IA-HA, many guidelines also suggested using IA-CS only after more conservative treatment options have failed.4,35,38 Recently, physical therapy has demonstrated better pain and function improvements over a 1-year time frame when compared with IA-CS, which further highlights the short-term effectiveness of IA-CS.14
Current guidelines on PRP use have primarily provided an uncertain recommendation because of the lack of high-quality evidence. While some guidelines provided a stronger recommendation specifically against the use of PRP, their reasoning was also for a lack of available evidence.2,12,35,36,38 Currently, the lack of high-quality evidence on the use of PRP for knee OA has precluded guidelines from giving an informed recommendation based on both effectiveness and potential harms. A recent meta-analysis of injectables used for knee OA showed very wide confidence intervals for PRP effectiveness and unclear certainty for using PRP as a potential knee OA therapy.29 There is a lack of clear definition and standardization of PRP products and varying costs, which also contribute to the uncertainty of PRP products. Although there has been some recent evidence that demonstrates potential benefits of PRP, this evidence has not driven CPG recommendations of PRP in a more favorable direction.
A previous study that assessed knee OA guideline quality provided insights into the evidence cited by major knee OA treatment guidelines. This study demonstrated that these guidelines generally included only a very small subset of the available evidence.1 With knee OA guidelines citing a selective subset of the available IA-HA literature, it is understandable that some may come to different conclusions depending on the evidence subset they have reviewed. Two key publications have been the NMAs of Jevsevar et al19 and Bannuru et al5 that had somewhat conflicting conclusions. Jevsevar et al19 demonstrated a limited effect of IA-HA and a larger effect with IA-CS use, comparing only studies with 30 or more participants and a time frame of 4 weeks postinjection. In comparison, Bannuru et al5 found that IA-HA had the largest treatment effect of knee OA treatments assessed, with IA-CS having a smaller effect when not limiting study size and using a 3-month time point for comparison. These results initially seem conflicting; however, important methodology considerations can explain these differences. The NMA of Jevsevar et al assessed an earlier follow-up period—around 1 month after treatment, while the NMA of Bannuru et al assessed the treatment effects 3 months after the injection. Depending on the needs of specific patients, it is important to consider whether 1-month benefits or 3-month benefits are more favorable to the patient.
When the evidence and current CPGs are assessed as a whole within this review, the clinical pathway for the care of patients with knee OA becomes more apparent. After initial attempts with conservative treatment options, knee OA injectables are generally recommended. For faster onset but shorter-term symptom relief, IA-CS injection is generally recommended. For a delayed but longer-term symptom relief period, IA-HA should be considered. Currently, there is not enough evidence to confidently identify a place for PRP within the knee OA care pathway.
This review was strengthened by its thorough analysis of available guideline recommendations on knee OA injectables. The inclusion of guidelines in all available languages provided the most comprehensive overview of global guidance, as previous investigations have focused only on English guidelines. While this broad inclusion provided strength, it also created limitations in the ability to systematically search the literature. Many of the included guidelines were not retrieved within traditional databases (eg, PubMed) that are typically searched during systematic reviews. Owing to this, a much broader hand search was required to find many of the included guidelines. Although they were not retrieved using systematic database searches, the large number of included guidelines globally represents a far more comprehensive assessment of knee OA guideline literature than does any previously published assessment of the topic.
Conclusion
Available CPGs provide recommendations on injectables for knee OA treatment. General guidance from a global perspective concludes that IA-CS and IA-HA are both favored for different needed responses and can be utilized within the knee OA treatment paradigm, while PRP currently has insufficient evidence to make a conclusive recommendation for or against its use.
Appendix
Table A1.
Overview of Guidelines and Recommendationsa
| Association | Year | Region (Language) | IA-HA Recommendation | IA-CS Recommendation | PRP Recommendation |
|---|---|---|---|---|---|
| American College of Rheumatology (ACR)21 | 2020 | US (English) | Conditionally recommended against IA-HA because of the risk of bias within current evidence | Strong recommendation for IA-CS injection because of demonstrated short-term efficacy | Strong recommendation against PRP |
| Osteoarthritis Research Society International (OARSI)4 | 2019 | US (English) | Conditional recommendation for the use of IA-HA—may have a better long-term safety profile than IA-CS | Conditional recommendation for the use of IA-CS—may provide short-term pain relief | Strong recommendation against PRP because of extremely low-quality evidence |
| Arthroscopy Association of Canada (AAC)2 | 2019 | Canada (English) | Intra-articular injections of HMW-HA can be considered in patients with mild to moderate knee OA | IA-CS injections provide short-term pain relief in patients with early knee OA | Cannot recommend for or against the use of PRP until further, high-quality clinical studies become available |
| Royal Australian College of General Practitioners (RACGP)36 | 2018 | Australia (English) | Conditional recommendation against the use of IA-HA because of an uncertainty in the evidence | Conditional recommendation for the use of IA-CS for short-term pain; potential harms of repeated use | Uncertain recommendation because of the very low quality of current evidence, cost, and variation in IA PRP treatments |
| German Society for Orthopedics and Orthopedic Surgery (DGOOC)16 | 2018 | Germany (German) | Recommended if NSAIDs are not effective or contraindicated | IA-CS recommended for short-term therapy, but the effective dose should be as low as possible | No recommendation can be given because of the uncertainty in the mechanism of action, evidence, and differences in IA PRP products |
| Mexican Consensus Meeting (MCM)15 | 2018 | Mexico (Spanish) | Recommended as a safe and effective treatment that can reduce the direct and indirect costs associated with the disease | No recommendation, as short-term efficacy is well established, but uncertainty on long-term efficacy | No recommendation because of the currently limited evidence |
| EUROpean VIScosupplementation COnsensus Group (EUROVISCO)31 | 2018 | Europe (English) | Recommended when NSAIDs are not effective | Not reported | Not reported |
| Turkish League Against Rheumatism (TLAR)38 | 2018 | Turkey (English) | IA-HA may be recommended in patients with moderate to severe symptoms | IA-CS may be recommended if other treatment options have failed but not more than 3 times per year | No recommendation because of currently insufficient scientific evidence |
| Columbia Experts in OA (CEOA)12 | 2017 | Columbia (Spanish) | HMW IA-HA is useful in the management of knee OA | The use of IA-CS is recommended in acute processes or articulate effusion | No recommendation because of the current low level of evidence |
| Chinese Medical Doctor Association (CMDA)11 | 2017 | China (Mandarin) | Recommended for knee OA, especially in patients for whom NSAIDs and analgesics did not provide efficacy | Not reported | Not reported |
| Pan-American League of Associations for Rheumatology (PANLAR)32 | 2016 | South America (English) | Recommended for knee OA, as IA-HA has proven to be beneficial | Recommended for knee OA, as it may be beneficial to provide fast pain relief | Uncertain recommendation, as PRP may help to relieve pain associated with knee OA, but higher-quality studies required |
| Spanish Society of Sports Medicine (SSSM)35 | 2016 | Spain (Spanish) | Recommended, particularly in patients who do not respond to nondrug therapy, analgesics, or NSAIDs | Recommended only when conservative treatment has failed; no more than 3 injections several weeks apart | No recommendation because of the current uncertainty in the clinical evidence |
| American Medical Society for Sports Medicine (AMSSM)37 | 2016 | US (English) | Recommended in patients who meet OMERACT-OARSI criteria | Not reported | Not reported |
| European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO)9 | 2016 | Europe (English) | Recommend the use of IA-HA in mild to moderate knee OA or in patients wishing to delay surgery | Recommended after using other treatments (ie, NSAIDs) | Not reported |
| National Institute for Health and Care Excellence (NICE)26 | 2014 | UK (English) | Do not recommend because of the uncertainty in the current evidence | IA-CS should be considered as an adjunct to core treatments for the relief of moderate to severe pain in people with OA, generally for short-term relief | Not reported |
| Veterans Affairs/Department of Defense (VA/DoD)13 | 2014 | US (English) | Uncertain recommendation, but suggested use if other treatments are not effective | IA-CS recommended for patients with symptomatic OA of the knee | Not reported |
| American Academy of Orthopaedic Surgeons (AAOS)18 | 2013 | US (English) | Do not recommend because of a lack of efficacy in the published literature | Uncertain recommendation for or against the use of IA-CS for symptomatic knee OA because of a lack of current evidence | Uncertain recommendation for or against the use of PRP for symptomatic knee OA because of a lack of current evidence |
| Ministry of Health of the Russian Federation (MHRF)25 | 2013 | Russia (Russian) | Positive effects of IA-HA variable depending on severity of OA and characteristics of the IA-HA product | IA-CS is not recommended for use in patients with OA because of the short lasting effect and possible destruction of articular cartilage | Not reported |
| Association of Rheumatologists of Russia (ARR)3 | 2013 | Russia (Russian) | IA-HA may be used to reduce OA pain within a recommended treatment algorithm | IA-CS recommended for knee OA for short-term relief of pain and inflammation but no more than 2-3 injections per year | Not reported |
| American Academy of Family Physicians (AAFP)34 | 2012 | US (English) | Recommended in severe OA when other treatment options are not effective | Recommended in severe OA to provide short-term pain relief; limited to 4 injections annually when other treatment options are not effective | Not reported |
| Czech Rheumatology Society (CSR)28 | 2012 | Czech Republic (Czech) | Recommended in patients with painful knee OA who have unsuccessful or are contraindicated for NSAID treatment | IA-CS may be recommended for painful OA cases but should be repeated 4 times a year at most | Not reported |
| Chinese Medical Association (CMA)10 | 2010 | China (Mandarin) | Recommended as a safe and effective treatment for mild to moderate knee OA | Recommended application of IA-CS no more than 3 times per year for short-term pain relief | Not reported |
| National Collaborating Centre for Chronic Conditions (NCC-CC)27 | 2008 | UK (English) | Do not recommend IA-HA because of the uncertainty of the evidence | IA-CS recommended as an addition to core treatments for short-term pain relief of moderate to severe OA | Not reported |
| Agency for Healthcare Research and Quality (AHRQ)8 | 2007 | US (English) | Recommendation of IA-HA is uncertain because of variability in the evidence | IA-CS may be considered when NSAIDs are not effective | Not reported |
| Singapore Ministry of Health (MOH)33 | 2007 | Singapore (English) | Recommended where general measures or systemic therapies have failed or are contraindicated | Consider IA-CS (especially if joint effusion present) | Not reported |
| Spanish Society of Rheumatology (SER)7 | 2005 | Spain (Spanish) | Recommended as an effective treatment for knee OA | Recommended as an effective treatment for short-term pain control in knee OA | Not reported |
| European League Against Rheumatism (EULAR)20 | 2003 | Europe (English) | Acknowledgment of potential benefits of IA-HA use | IA-CS recommended for flare of knee pain, especially if accompanied by effusion | Not reported |
aHA, hyaluronic acid; HMW, high molecular weight; IA, intra-articular; IA-CS, intra-articular corticosteroid; IA-HA, intra-articular hyaluronic acid; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis; OMERACT-OARSI, Standing Committee for Clinical Trials Response Criteria Initiative and the Outcome Measures in Rheumatology–Osteoarthritis Research Society International; PRP, platelet-rich plasma; UK, United Kingdom; US, United States.
Footnotes
Final revision submitted May 3, 2021; accepted June 1, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by a grant from the HA Viscosupplement Coalition. The authors solely hold the right to access and analyze study data and publish this paper without influence from the funder. Global Research Solutions Inc provided medical writing support. M.P. is an employee of Global Research Solutions. M.B. has received grants from Acumed, Bioventus, DJO, Flexion Therapeutics, Sanofi Aventis, Stryker, and Titan Spine and personal fees from AgNovos Healthcare and Pendopharm. J.G. has received research support from JRF Ortho, Arthrex, and Aesculap Biologics; education payments from Pinnacle; consulting fees from JRF Ortho and Ossur; speaking fees from JRF Ortho, ConMed Linvatec, and Vericel; honoraria from JRF Ortho; and hospitality payments from Smith & Nephew. A.B. has received consulting fees from Arthrex and Flexion Therapeutics; speaking fees from Arthrex and Synthes; royalties from Arthrex and Smith & Nephew; and hospitality payments from GE Healthcare. E.S. has received consulting fees from Sanofi Aventis. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1.Altman RD, Schemitsch E, Bedi A. Assessment of clinical practice guideline methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Semin Arthritis Rheum. 2015;45(2):132–139. doi:10.1016/j.semarthrit.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 2.Arthroscopy Association of Canada; Kopka M, Sheehan B, Degen R, et al. Arthroscopy Association of Canada position statement on intra-articular injections for knee osteoarthritis. Orthop J Sports Med. 2019;7(7):2325967119860110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association of Rheumatologists of Russia. Федеральные клинические рекомендации по диагностике и лечению остеоартроза. Published online 2013. Accessed August 1, 2021. http://pharm-spb.ru/docs/lit/Revmatologia_Rekomendazii%20po%20diagnostike%20i%20lecheniyu%20osteoartroza%20(ARR,%202013).pdf
- 4.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi:10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 5.Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54. doi:10.7326/M14-1231 [DOI] [PubMed] [Google Scholar]
- 6.Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis. 2017;9(9):231–246. doi:10.1177/1759720X17729641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco FJ. Primer documento de consenso de la Sociedad Española de Reumatología sobre el tratamiento farmacológico de la artrosis de rodilla. Reumatol Clin. 2005;1(1):38–48. doi:10.1016/S1699-258X(05)72711-X [DOI] [PubMed] [Google Scholar]
- 8.Blue Cross and Blue Shield Association Technology Evaluation Center Evidence-based Practice Center; Samson D, Grant M, Ratko TA, et al. Treatment of Primary and Secondary Osteoarthritis of the Knee. Evidence Report/Technology Assessment Number 157. Agency for Healthcare Research and Quality; 2007.
- 9.Bruyère O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3–S11. doi:10.1016/j.semarthrit.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 10.Chinese Medical Association Rheumatology Branch. Diagnosis and treatment guide for osteoarthritis. Chin J Rheumatol. 2010;14(6). [Google Scholar]
- 11.Chinese Medical Doctor Association Orthopedics Branch Sports Medicine Committee. Expert consensus on the application of sodium hyaluronate in orthopedics and sports medicine-related diseases (2017 revision). Chin J Med Front Electron Version. 2017;9. doi:10.12037/YXQY.2017.11-01 [Google Scholar]
- 12.Choueka MC, Pilonieta CEA, Cortés MED, et al. Recomendaciones sobre diagnóstico, prevención y tratamiento farmacológico y no farmacológico de la osteoartritis (OA) de rodilla. Rev Colomb Med Física Rehabil. 2017;27(2):160–184. doi:10.28957/rcmfr.v27n2a4 [Google Scholar]
- 13.Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the non-surgical management of hip and knee osteoarthritis. Guideline Central. Published 2014. Accessed August 23, 2020. https://www.guidelinecentral.com/summaries/vadod-clinical-practice-guideline-for-the-non-surgical-management-of-hip-and-knee-osteoarthritis/#section-society
- 14.Deyle GD, Allen CS, Allison SC, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382(15):1420–1429. doi:10.1056/NEJMoa1905877 [DOI] [PubMed] [Google Scholar]
- 15.Espinosa-Morales R, Alcántar-Ramírez J, Arce-Salinas CA, et al. Multidisciplinary meeting of experts for diagnosis and treatment of osteoarthritis: up-to-date based on evidence. Med Interna México. 2018;34(3):443–476. [Google Scholar]
- 16.German Society of Orthopedics and Orthopedic Surgery (DGOOC); Stove J. Gonarthrose S2k-Leitlinie. AWMF Online. 2018;033–004. [Google Scholar]
- 17.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 18.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571–576. doi:10.5435/JAAOS-21-09-571 [DOI] [PubMed] [Google Scholar]
- 19.Jevsevar DS, Shores PB, Mullen K, Schulte DM, Brown GA, Cummins DS. Mixed treatment comparisons for nonsurgical treatment of knee osteoarthritis: a network meta-analysis. J Am Acad Orthop Surg. 2018;26(9):325–336. doi:10.5435/JAAOS-D-17-00318 [DOI] [PubMed] [Google Scholar]
- 20.Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145–1155. doi:10.1136/ard.2003.011742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020;72(2):220–233. doi:10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lespasio MJ, Piuzzi NS, Husni ME, Muschler GF, Guarino A, Mont MA. Knee osteoarthritis: a primer. Perm J. 2017;21:16–183. doi:10.7812/TPP/16-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67(2):203–215. doi:10.1002/acr.22412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–1975. doi:10.1001/jama.2017.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health of the Russian Federation. ГОНАРТРОЗ И СХОДНЫЕ С НИМ КЛИНИЧЕСКИЕ СОСТОЯНИЯ. Published 2013. https://mzur.ru/upload/Гонартроз.pdf
- 26.National Clinical Guideline Centre (UK). Osteoarthritis: Care and Management in Adults. National Institute for Health and Care Excellence (UK); 2014. Accessed August 23, 2020. http://www.ncbi.nlm.nih.gov/books/NBK248069/ [PubMed] [Google Scholar]
- 27.National Collaborating Centre for Chronic Conditions. Osteoarthritis: National Clinical Guidelines for Care and Management in Adults. Royal College of Physicians; 2008. [PubMed] [Google Scholar]
- 28.Pavelka K. Doporučení České revmatologické společnosti pro léčbu osteoartrózy kolenních, kyčelních a ručních kloubů. Česká Revmatol. 2012;20(3):138–157. [Google Scholar]
- 29.Phillips M, Vannabouathong C, Devji T, et al. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):3031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn RH, Murray JN, Pezold R, Sevarino KS. Surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2018;26(9):e191–e193. doi:10.5435/JAAOS-D-17-00424 [DOI] [PubMed] [Google Scholar]
- 31.Raman R, Henrotin Y, Chevalier X, et al. Decision algorithms for the retreatment with viscosupplementation in patients suffering from knee osteoarthritis: recommendations from the EUROpean VIScosupplementation COnsensus Group (EUROVISCO). Cartilage. 2018;9(3):263–275. doi:10.1177/1947603517693043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rillo O, Riera H, Acosta C, et al. PANLAR Consensus recommendations for the management in osteoarthritis of hand, hip, and knee. J Clin Rheumatol. 2016;22(7):345–354. doi:10.1097/RHU.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 33.Singapore Ministry of Health. Clinical practice guidelines: osteoarthritis of the knees. Published online 2007. https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_oa-kness_booklet.pdf
- 34.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 35.Soto M del V, Díaz JFJ, Marqueta PM, Parenteau CR, Vicente JMR, Fernández LS. Consenso sobre utilización de las infiltraciones en el deporte: Documento de Consenso de la Sociedad Española de Medicina del Deporte. Arch Med Deporte. 2016;33(172):114–125. [Google Scholar]
- 36.The Royal Australian College of General Practitioners. Guideline for the Management of Knee and Hip Osteoarthritis. 2nd ed. The Royal Australian College of General Practitioners; 2018. [Google Scholar]
- 37.Trojian TH, Concoff AL, Joy SM, Hatzenbuehler JR, Saulsberry WJ, Coleman CI. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Clin J Sport Med. 2016;26(1):1–11. doi:10.1097/JSM.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 38.Tuncer T, Cay FH, Altan L, et al. 2017 update of the Turkish League Against Rheumatism (TLAR) evidence-based recommendations for the management of knee osteoarthritis. Rheumatol Int. 2018;38(8):1315–1331. doi:10.1007/s00296-018-4044-y [DOI] [PubMed] [Google Scholar]