Abstract
Background:
Partial supraspinatus tendon tears have frequently been treated using a subacromial corticosteroid injection or surgery. The clinical use of a platelet-rich plasma (PRP) injection is an alternative treatment method for the condition, despite the paucity of evidence of its efficacy.
Purpose:
To compare pain relief, functional improvement, and complications after an intratendinous PRP injection versus a subacromial corticosteroid injection for partial supraspinatus tears.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
A total of 32 patients with partial supraspinatus tears were randomly assigned to receive a leukocyte-poor PRP (LP-PRP) injection or a corticosteroid injection. One patient withdrew from the PRP group, leaving 15 patients in the PRP group and 16 patients in the corticosteroid group. The ultrasound-guided procedures were performed by a single experienced pain physician. Pain relief and functional improvement were evaluated using the visual analog scale (VAS) and the Oxford Shoulder Score (OSS), respectively. Treatment efficacy and complications were documented, and the 2 groups were compared at 1- and 6-month follow-up.
Results:
There were no differences in VAS and OSS scores between the PRP and corticosteroid groups at 1-month follow-up. However, the PRP group had better scores than the corticosteroid group had on both the VAS and OSS at 6-month follow-up (VAS: 14.5 ± 15.4 vs 37.5 ± 24.9, respectively; OSS: 16.2 ± 3.9 vs 25.0 ± 10.2, respectively; P < .01 for both). Both groups showed significant improvement in VAS and OSS scores from before treatment to 1-month follow-up (mean difference, 35.67 and 11.47 points, respectively, for the PRP group; mean difference, 29.69 and 11.13 points, respectively, for the corticosteroid group; P < .01 for all). The VAS and OSS scores did not change significantly at 6-month follow-up in the corticosteroid group; however, the PRP group showed continued improvement in both VAS and OSS scores between 1- and 6-month follow-up (mean difference, 15.87 and 7.40 points, respectively; P < .01 for both). There were no complications in either group.
Conclusion:
An injection using either a corticosteroid or LP-PRP resulted in a similar reduction in pain and improvement in function at 1 month in patients with a partial supraspinatus tear. However, PRP showed superior benefits over the corticosteroid at 6-month follow-up.
Keywords: platelet-rich plasma, supraspinatus tear, rotator cuff tear, pain intervention
With its wide range of motion, the shoulder is one of the most complex joints in the body, and pain from trauma or degeneration is common.6 Rotator cuff tendinopathy is a frequent cause of shoulder pain, and the progression to a partial- or full-thickness supraspinatus tendon tear can occur.8,31 Because of poor vascularization, the tendons have a limited ability to regenerate. This failure to heal is considered a principal cause of chronic shoulder pain and hinders successful outcomes from both nonoperative and surgical treatment.8,31 A subacromial corticosteroid injection is an option used widely for patients with inadequate responses to nonoperative procedures, although clinical evidence of its efficacy is conflicting.9,14 Most studies have shown pain relief and functional improvement from a corticosteroid injection in the short term but no clear benefit in the long term.1,9,14
Recently, the use of orthobiologics, such as platelet-rich plasma (PRP), has been proposed to promote regeneration of the tendon.6,19 PRP is an autologous concentration of human platelets in a small volume of plasma produced by centrifuging a patient’s own blood. Platelets contain a milieu of growth factors and mediators in their alpha granules, including transforming growth factor–β1, platelet-derived growth factor, basic fibroblast growth factor, vascular endothelial growth factor, epidermal growth factor, and insulin-like growth factor–1,6,19 which are concentrated using the centrifugation process and can then be delivered to an injured site to augment the body’s natural healing process.6 In contrast to leukocyte-rich PRP, leukocyte-poor PRP (LP-PRP) eliminates some problems caused by leukocytes such as oxygen free radical release during inflammation, which causes endothelial and subendothelial damage and leads to fibrosis that will disrupt the expected healing process while providing a sustained release of growth factors from endogenously activated platelets.
There is convincing evidence of the efficacy of PRP in the treatment of lateral epicondylitis, patellar tendinopathy, and knee osteoarthritis.30 However, there is little evidence, mostly in the form of small clinical trials, that shows the efficacy of PRP for rotator cuff injuries.2,4,5,18,23 The purpose of this study was to compare pain relief, functional improvement, and complications after an intratendinous PRP injection versus a subacromial corticosteroid injection for partial supraspinatus tears.
Methods
This prospective randomized controlled trial was conducted at King Chulalongkorn Memorial Hospital, Bangkok, Thailand, between April 2019 and May 2020. The study protocol was approved by an institutional review board. Before enrollment, all patients provided written informed consent. Patients with shoulder pain who visited the outpatient orthopaedic clinic were examined to ascertain their eligibility. After clinical and radiological assessments, those who met the following criteria were included in the study.
The inclusion criteria for study patients were as follows:
Age between 18 and 80 years and no serious systemic diseases such as uncontrolled diabetes, hyperthyroidism, or end-stage renal failure.
Partial supraspinatus tendon tears confirmed using magnetic resonance imaging (MRI). A partial supra-spinatus tear was defined as tendon disruption that did not involve the entire thickness of the tendon, including bursal, articular, and intrasubstance tears,27 by an appointed radiologist.
Natural history of tears from repetitive trauma or overuse only.
Failed nonoperative treatment, including physical therapy and oral medication, for at least 3 months.
Ability to participate for a minimum follow-up period of 6 months.
The exclusion criteria for study patients were as follows:
Severe arthritis or other complications related to supraspinatus tears, such as generalized inflammatory arthritis and infections.
History of previous shoulder surgery.
Other concurrent shoulder conditions such as impingement from an MRI diagnosis.
Malignancy.
Current treatment using anticoagulant or antiplatelet medication.
Immunocompromised status.
Patient Allocation
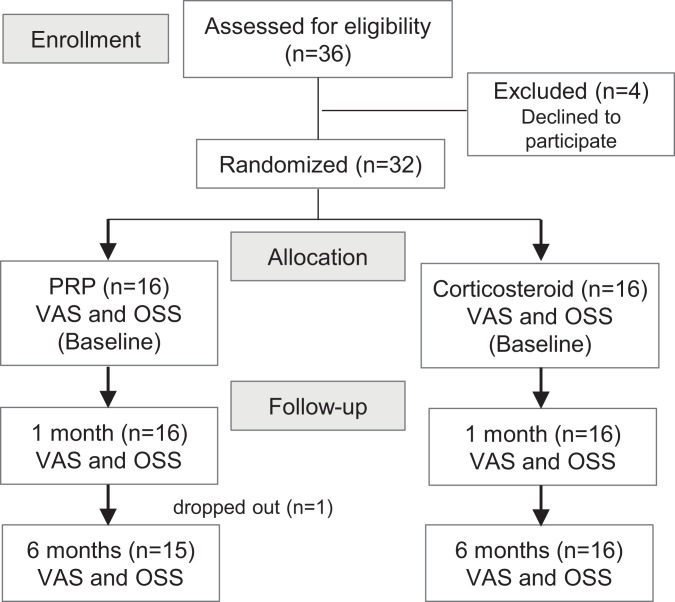
Patients were randomly assigned to receive a PRP injection (PRP group; n = 16) or a corticosteroid injection (corticosteroid group; n = 16) using a computer-generated randomization schedule (Figure 1). Group assignments were only accessible by a research assistant and were concealed from the patients throughout the study. The physician who performed the interventions was not blinded because the study compared 2 different interventional methods, and because of the blood draw required for the PRP group, patients could not be truly blinded. Characteristic information including underlying diseases was recorded. In the PRP group, 1 patient withdrew because of the inconvenience of traveling from another province, leaving 15 patients for analysis in this group. No patients were lost to follow-up in the corticosteroid group (Figure 1).
Figure 1.
Flow diagram of patient allocation process. OSS, Oxford Shoulder Score; PRP, platelet-rich plasma; VAS, visual analog scale.
Study Procedures
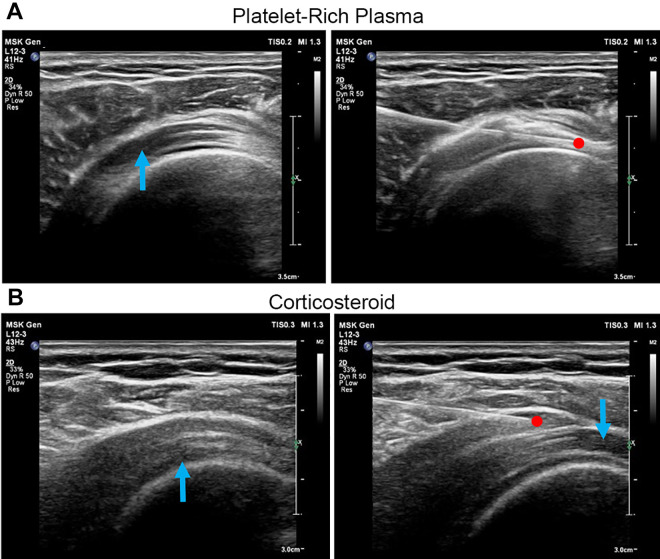
All procedures were performed in the operating theater. A study nurse who was not involved in the study evaluation prepared the injectates for all patients in both groups. All injections were administered by a single experienced pain physician (M.T.). Patients sat in the modified Crass position, and the area to be injected was disinfected using strict aseptic precautions. The posterolateral approach was used for all the patients in both groups. Real-time ultrasound guidance (Yokogawa Medical Systems) was provided during the injections using a 3- to 12-MHz linear array transducer covered with a sterile camera sleeve utilizing the plane technique. The ultrasound probe was placed on the affected shoulder. The supraspinatus tendon and the subacromial-subdeltoid bursa were identified (Figure 2).
Figure 2.
(A) Platelet-rich plasma (PRP) was injected into the supraspinatus tendon tear site. The blue arrow indicates a partial tear of the supraspinatus tendon; the red dot indicates the needle tip. (B) A corticosteroid was injected into the subacromial bursa. The blue arrows indicate partial tears of the supraspinatus tendon; the red dot indicates the needle tip.
In the PRP group, PRP was prepared using the ACP Double Syringe System (Arthrex); 15 mL of blood was aspirated in the double syringe and centrifuged at 1500 rpm for 5 minutes. This yielded around 5 mL of LP-PRP ready for an injection.17 Using a 25-gauge needle, 5 mL of PRP was infiltrated into the supraspinatus tendon tear site ≤5 seconds after being centrifuged (Figure 2A).
In the corticosteroid group, 1 mL of triamcinolone acetonide (40 mg/mL Kenacort-A suspension), together with 4 mL of 1% lidocaine, was prepared using a 5-mL syringe with a 25-gauge needle. The mixture was injected into the subacromial bursa (Figure 2B).
After the injection, the patients rested without moving the shoulder for 30 minutes in the recovery room. Arm slings were used to immobilize the injected shoulders of all patients for 3 days. After that, the patients were allowed to move their shoulders and instructed to follow a light exercise program at home as advised by our rehabilitation team. Exercise included passive range of motion, such as wall climbing in the scapular plane, abduction, and forward flexion and towel suspension in internal rotation and adduction, as well as active range of motion, such as forward flexion, scapular-plane abduction, and external rotation. Additionally, exercise included periscapular strengthening, scapular retraction, and wall planking with scapular protraction and shoulder depression. Physical therapy was not prescribed. Patients were advised to avoid sports activities for 6 weeks. Use of nonsteroidal anti-inflammatory drugs was not allowed for 6 months, and patients were prescribed acetaminophen or acetaminophen/tramadol (325/37.5 mg) for pain control. Visual analog scale (VAS) for pain scores, Oxford Shoulder Score (OSS) results, and complications were assessed before the injection and at 1 and 6 months after the injection in the outpatient clinic.
Outcome Measures
The primary outcome measure was the 100-mm VAS, which is the most common tool for validating pain, including shoulder pain.2,11,26–28,30,31 The participants were told to choose a number between 0 and 10 marked on a 100-mm line, with 0 indicating no pain and 10 indicating the worst pain possible. Because our patients were neither athletes nor heavy physical workers, the secondary outcome measure was the OSS10,24 to assess function, with 12 indicating good function and 60 indicating the worst function. Baseline characteristics were also collected from all participants. Outcome scores were obtained at baseline and at 1 and 6 months after the injection by a blinded researcher (P.T.). Patients were requested to report any adverse effects at each visit.
Data Management and Statistical Analysis
Calculation of the sample size was based on previous studies11,23 with a clinically relevant difference in the VAS score of 30% between the PRP and corticosteroid groups. This was based on a power of 80% and a 2-tailed alpha of .05. Assuming a 10% loss to follow-up, we estimated that the final sample size required was 16 patients per group.
For the descriptive data, categorical variables were described via frequency and percentage, while continuous variables were described via mean and standard deviation. The response to treatment was measured using the VAS and OSS: the VAS score ranged from 1 to 100, and the OSS ranged from 12 to 60, with lower scores indicating fewer signs and symptoms of disease. A blinded investigator (P.T.) assessed the objective portions of the scoring systems. The scores were assessed 3 times: before the injection and at 1 and 6 months after the injection.
Primary and secondary outcomes in the analysis were described using mean, standard deviation, mean difference, and 95% confidence interval. The Pearson chi-square test was then used to analyze the data and calculate the P value of the outcomes, comparing within the group, between the groups, and among time points (ie, before and after the injection at 1 and 6 months). The chi-square test was used to analyze multiple sets of categorical data to determine if they were homogeneous. Continuous variables of the PRP and corticosteroid groups were compared using the Student t test. Assumption was checked before using the Student t test via Q-Q plots for normality and the F test for variance. A P value of <.05 was considered statistically significant. The 95% confidence interval was also reported. Data analysis was performed using STATA version 10 (StataCorp).
Results
Table 1 shows the characteristics of the study groups. The PRP group was significantly younger than was the corticosteroid group (P = .006), but the other characteristics were similar between the groups.
Table 1.
Descriptive Dataa
| PRP Group (n = 15) | Corticosteroid Group (n = 16) | P | |
|---|---|---|---|
| Age, y | 51.3 ± 10.3 | 62.4 ± 10.5 | .006 |
| Male sex, n (%) | 3 (20.0) | 3 (18.8) | ≥.999 |
| Body mass index | 25.1 ± 4.1 | 24.6 ± 3.6 | .719 |
| Pain duration, mo | 8.3 ± 11.6 | 13.5 ± 12.5 | .901 |
| Some strenuous work (lifting in daily routine), n (%) | 9 (60.0) | 10 (62.5) | .88 |
| Underlying diseases, n (%)b | 4 (26.7) | 3 (18.8) | .613 |
| Hypertension | 3 (20.0) | 3 (18.8) | .933 |
| Dyslipidemia | 1 (6.7) | 2 (12.5) | .598 |
| Migraine | 1 (6.7) | 0 (0.0) | .310 |
| Benign prostate hyperplasia | 1 (6.7) | 0 (0.0) | .310 |
| Nondominant arm, n (%) | 3 (20.0) | 2 (12.5) | .585 |
a Data are reported as mean ± SD unless otherwise indicated. Bolded P value indicates a statistically significant difference between groups. PRP, platelet-rich plasma.
bTwo patients had 2 underlying diseases.
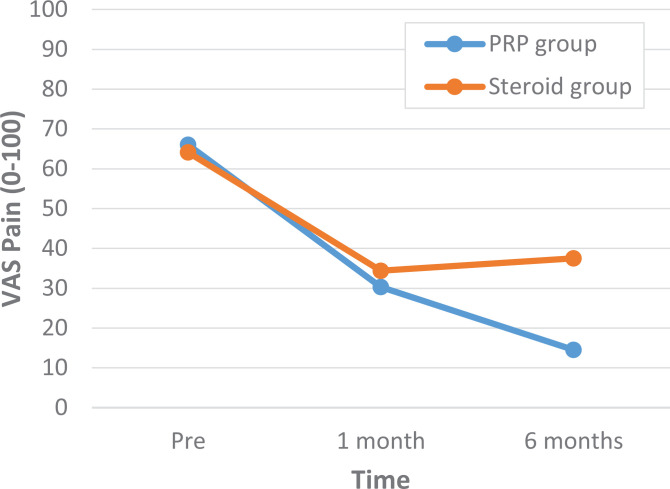
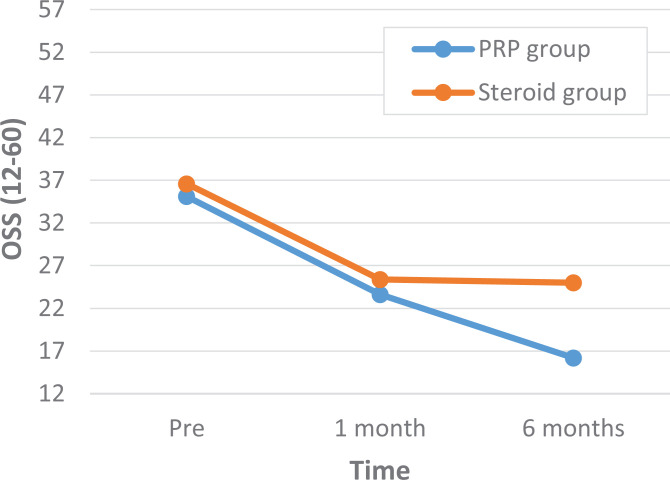
A comparison of outcome scores between the PRP and corticosteroid groups showed that there were no statistically significant differences in the VAS and OSS scores at baseline or at 1-month follow-up (Tables 2 and 3). At 6-month follow-up, both the VAS and OSS scores were significantly different: the PRP group had a VAS score of 14.5 compared to a VAS score of 37.5 in the corticosteroid group (P < .01) and an OSS score of 16.2 versus 25.0, respectively (P < .01) (Figures 3 and 4 and Tables 2 and 3).
Table 2.
Between-Group Comparison of VAS Scoresa
| PRP Group | Corticosteroid Group | Mean Difference (95% CI) | P | |
|---|---|---|---|---|
| Before treatment | 66.0 ± 14.6 | 64.1 ± 21.1 | 1.94 (–11.45 to 15.32) | .76 |
| 1-mo follow-up | 30.3 ± 20.6 | 34.4 ± 27.6 | –4.04 (–22.00 to 13.92) | .64 |
| 6-mo follow-up | 14.5 ± 15.4 | 37.5 ± 24.9 | –23.03 (–38.37 to –7.69) | <.01 |
a Data are reported as mean ± SD unless otherwise indicated. Bolded P value indicates statistical significance. PRP, platelet-rich plasma; VAS, visual analog scale.
Table 3.
Between-Group Comparison of OSS Scoresa
| PRP Group | Corticosteroid Group | Mean Difference (95% CI) | P | |
|---|---|---|---|---|
| Before treatment | 35.1 ± 7.6 | 36.6 ± 7.2 | –1.50 (–6.94 to 3.95) | .58 |
| 1-mo follow-up | 23.6 ± 6.5 | 25.4 ± 10.5 | –1.84 (–8.31 to 4.63) | .57 |
| 6-mo follow-up | 16.2 ± 3.9 | 25.0 ± 10.2 | –8.84 (–14.57 to –3.03) | <.01 |
a Data are reported as mean ± SD unless otherwise indicated. Bolded P value indicates statistical significance. OSS, Oxford Shoulder Score; PRP, platelet-rich plasma.
Figure 3.
Visual analog scale (VAS) scores between the platelet-rich plasma (PRP) and corticosteroid groups before treatment, at 1-month follow-up, and at 6-month follow-up.
Figure 4.
Oxford Shoulder Score (OSS) results between the platelet-rich plasma (PRP) and corticosteroid groups before treatment, at 1-month follow-up, and at 6-month follow-up.
Both groups showed significant improvements in the VAS and OSS scores at all time points compared to baseline (before treatment), with a large mean reduction or mean difference (P < .01). The corticosteroid group had no significant change in VAS or OSS scores between the 1- and 6-month time points; however, the PRP group showed continued improvement in both VAS and OSS scores at the 6-month time point (Tables 4 and 5). There were no complications in either group.
Table 4.
Within-Group Comparison of VAS Scoresa
| Mean Difference (95% CI) | P | |
|---|---|---|
| PRP group | ||
| Before treatment vs 1-mo follow-up | 35.67 (25.69 to 45.64) | <.01 |
| Before treatment vs 6-mo follow-up | 51.53 (39.56 to 63.51) | <.01 |
| 1- vs 6-mo follow-up | 15.87 (4.31 to 27.42) | <.01 |
| Corticosteroid group | ||
| Before treatment vs 1-mo follow-up | 29.69 (12.22 to 47.16) | <.01 |
| Before treatment vs 6-mo follow-up | 26.56 (12.64 to 40.48) | <.01 |
| 1- vs 6-mo follow-up | –3.13 (–20.28 to 14.03) | .70 |
a Bolded P values indicate statistical significance. PRP, platelet-rich plasma; VAS, visual analog scale.
Table 5.
Within-Group Comparison of OSS Scoresa
| Mean Difference (95% CI) | P | |
|---|---|---|
| PRP group | ||
| Before treatment vs 1-mo follow-up | 11.47 (6.65 to 16.28) | <.01 |
| Before treatment vs 6-mo follow-up | 18.86 (14.51 to 23.22) | <.01 |
| 1- vs 6-mo follow-up | 7.40 (4.00 to 10.80) | <.01 |
| Corticosteroid group | ||
| Before treatment vs 1-mo follow-up | 11.13 (6.40 to 15.85) | <.01 |
| Before treatment vs 6-mo follow-up | 11.56 (6.63 to 16.49) | <.01 |
| 1- vs 6-mo follow-up | 0.44 (–5.13 to 6.00) | .87 |
a Bolded P values indicate statistical significance. OSS, Oxford Shoulder Score; PRP, platelet-rich plasma.
Discussion
The results from the current study showed that both PRP and a corticosteroid had beneficial effects for the treatment of partial supraspinatus tears. However, the corticosteroid effects plateaued after 1 month, with no significant change between the 1- and 6-month assessments in this group.
A comparison of the 2 groups at the 1-month time point indicated that there was no significant difference between them in terms of pain and function. However, at the 6-month time point, the differences were statistically significant, reflecting the extended effects of PRP compared to the corticosteroid. Many studies have shown similar results on the relative efficacy of PRP,2,4,5,18,23 but others have found no overall significant effects of PRP on functional outcomes and repair integrity.17,26–29 A review by Chen et al7 reported that LP-PRP appeared to significantly reduce the retear rate compared with the control and may improve tendon-to-bone healing based on significant differences in the failure-to-heal rate along with improving patient pain scores. It has been hypothesized that the primary cause of chronic tendinopathy is not related to the inflammatory process but to an insufficient body healing process.3 From these concepts, PRP as a regenerative substance has its role in promoting tissue healing because platelets are known to release growth factors, cytokines, and chemokines to modulate inflammation and tissue regeneration. In our study, as we used pain as a primary outcome, our evidence on PRP injections supports a beneficial effect on long pain reduction for rotator cuff injuries.
In contrast to other studies, which have suggested a return to baseline over time using corticosteroid injections,9,21,25 our results showed a sustained improvement in pain and OSS scores in the corticosteroid group. Partial supraspinatus tears are considered a chronic overuse disease in which inflammation is not characterized pathologically; hence, a corticosteroid as an anti-inflammatory drug might not be able to exhibit long-term effects.21,25 Previous studies reported a significantly better functional outcome, sustained for 6 months, with exercise therapy compared with placebo in rotator cuff diseases.15,22 Part of the prolonged pain control without returning to baseline for both groups in our study might be a consequence of patients following an early, long-term light shoulder exercise program at home.12,20,22
The pathogenetic mechanisms of partial rotator cuff tears should be considered, although these are not fully understood. In the initial phase, inflammation is prevalent, whereas in the later phase, degeneration varying according to the intensity and duration of overuse and individual predisposing factors is commonly seen. During relapses, inflammation is frequently superimposed over degeneration.1,11,18 Because there are several aspects in the pathogenesis of tears,19,27 we selectively included patients with a natural history of tears from repetitive trauma due to daily work/activities, excluding sports injuries and accidents. All participants had unsuccessful nonoperative treatment for at least 3 months, so improvement should be from the effects of PRP or the corticosteroid. The nonoperative management of partial rotator cuff tears for at least 6 weeks to 3 months is recommended; prolonged nonoperative management in symptomatic patients can have negative consequences. These include an increase in tear size, tear retraction, difficulty in repair, and muscle atrophy with fatty infiltration, all of which can result in a diminished outcome.11
A local corticosteroid injection counteracts the inflammatory and immune cascades, so it is beneficial in the early phase of the disease, corresponding to our current results. However, its efficacy is limited or absent in the late phase when degeneration is prevalent,9,11,13 which corresponded to our results. In the corticosteroid group, results showed significant improvement from baseline at 1 and 6 months but no improvement from 1 to 6 months (Tables 4 and 5). The rationale for sustained improvement at 6 months from baseline might be the anti-inflammatory effects of the corticosteroid at the first time of treatment. However, definite verification should be performed in a future study.
Platelets are known for their importance in clotting. Furthermore, platelet products represent an enriched autologous source of platelets containing growth factors at higher concentrations than normal physiological levels. These factors augment revascularization of the injury areas and promote tendon healing, resulting in the improvement of pain and function.4,5,18 Many animal models have proven the beneficial effects of growth factors in PRP on tendon healing.14,16,24 Clinical reports have been inconclusive on the details of different PRP formulations, injection techniques, and tear sizes, and many have uncontrolled biases.4,5,7,17 The concentrations and activities of the platelet components may vary according to the volume and timing of application.19 Both LP-PRP and leukocyte-rich PRP promote the regeneration of many kinds of injured tissue, including tendons. Owing to these regenerative benefits of PRP and the detrimental effects of repetitive steroid injections to tendons, PRP seemed to be an interesting option for injured tendons. We chose LP-PRP from Arthrex (ACP Double Syringe System), as this system was the most convenient and cost-effective commercial PRP kit available at our institution.
According to the basic biological knowledge of PRP and corticosteroids, injection techniques had to be performed differently. Treatment modalities and injection sites may have influenced the response outcomes. However, we could not inject the corticosteroid intratendinously as with PRP, and we intended to compare these 2 methods, not to duplicate other studies. Corticosteroids may have detrimental effects on tendons, including an impairment of fibroblast viability, arrest of cell proliferation, and depletion of the tenocyte stem cell pool, leading to decreased collagen synthesis.1,9,11,13 The release of metalloproteinases after a corticosteroid injection has been associated with tendon degeneration and ruptures.1 Therefore, it is preferable to perform a peritendinous corticosteroid injection into the subacromial bursa rather than directly into the supraspinatus tendon.1,9,11,13 On the other hand, PRP should be injected directly into the injured tendon, where released growth factors can promote the healing process.1,32 PRP injections are generally safe and cost-effective. The platelets are harvested from the patient’s own blood; thus, the risk of any incompatible blood reactions or disease transmission is extremely low. Reactions from additives are also rarely reported. PRP therapy is quick with no downtime from daily activities.4,5,18,31
There are some limitations of this study. First, we had a relatively short follow-up period of only 1 and 6 months. With a longer period, the results might be more meaningful. VAS and OSS scores are subjective and self-reported. Inadequate blinding of the physician who performed the injections and the participants could be a potential source of bias. The OSS is a good measure for our patients, as our patients had normal activity levels and were not physical laborers or athletes. The OSS also had validation in our local language. Second, despite computer-generated randomization of patients, there was a significant difference in the age range of both groups, with younger patients in the PRP group. The mean age difference of 11.1 years could be a significant factor that contributed to our results. The healing process was studied by Balasubramaniam et al,5 who suggested that a lower rate of healing occurred in patients older than 65 years. We did not differentiate the site of tears (bursal vs articular vs intrasubstance). Because of limited resources, the characterization of PRP growth factors was not tested. Blinding of the physician who performed the injections and participants could not be done.
However, to the best of our knowledge, this is the first study to compare these 2 methods that are most often used for the treatment of partial supraspinatus tears. The current study may be a preliminary trial for a large-scale future study with a longer follow-up period. We realized that MRI would be an ideal method to assess tendon healing, but our primary aim was to measure pain and function. MRI should be another tool to include in future studies.
Conclusion
An injection of either a corticosteroid or LP-PRP resulted in a similar reduction of pain and improvement of function at 1 month in patients with partial supraspinatus tears. At 6 months after the injection, continued improvement was seen in the PRP group, whereas no further improvement was seen in the corticosteroid group. A PRP injection showed superior benefits over a corticosteroid injection for partial supraspinatus tears at 6-month follow-up.
Footnotes
Final revision submitted January 29, 2021; accepted February 25, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study received funding support from the Pain Management Research Unit, Department of Anesthesiology, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Faculty of Medicine, Chulalongkorn University (No. 752/61).
References
- 1.Abate M, Salini V, Schiavone C, Andia I. Clinical benefits and drawbacks of local corticosteroids injections in tendinopathies. Expert Opin Drug Saf. 2017;16(3):341–349. [DOI] [PubMed] [Google Scholar]
- 2.Akan O, Mete B, Koçyiğit H, Bayram KB, Yilmaz HE, Tosun A. Efficacy of ultrasound guided platelet-rich plasma in the repair of partial and full-thickness supraspinatus tears. Int J Clin Exp Med. 2019;12(9):11. [Google Scholar]
- 3.Alfredson H. Chronic midportion Achilles tendinopathy: an update on research and treatment. Clin Sports Med. 2003;22(4):727–741. [DOI] [PubMed] [Google Scholar]
- 4.Andia I, Latorre PM, Gomez MC, Burgos-Alonso N, Abate M, Maffulli N. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110(1):99–115. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramaniam U, Dissanayake R, Annabell L. Efficacy of platelet-rich plasma injections in pain associated with chronic tendinopathy: a systematic review. Phys Sportsmed. 2015;43(3):253–261. [DOI] [PubMed] [Google Scholar]
- 6.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429–439. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Jones IA, Togashi R, Park C, Vangsness CT. Use of platelet-rich plasma for the improvement of pain and function in rotator cuff tears: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2020;48(8):2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement ND, Nie YX, McBirnie JM. Management of degenerative rotator cuff tears: a review and treatment strategy. Sports Med Arthrosc Rehabil Ther Technol. 2012;4(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751–1767. [DOI] [PubMed] [Google Scholar]
- 10.Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. J Bone Joint Surg Br. 1996;78(4):593–600. [PubMed] [Google Scholar]
- 11.Gialanella B, Prometti P. Effects of corticosteroids injection in rotator cuff tears. Pain Med. 2011;12(10):1559–1565. [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez-Espinoza H, Araya-Quintanilla F, Pinto-Concha S, et al. Effectiveness of supervised early exercise program in patients with arthroscopic rotator cuff repair: study protocol clinical trial. Medicine (Baltimore). 2020;99(4):e18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart L. Corticosteroid and other injections in the management of tendinopathies: a review. Clin J Sport Med. 2011;21(6):540–541. [DOI] [PubMed] [Google Scholar]
- 14.Ho LK, Baltzer WI, Nemanic S, Stieger-Vanegas SM. Single ultrasound-guided platelet-rich plasma injection for treatment of supraspinatus tendinopathy in dogs. Can Vet J. 2015;56(8):845–849. [PMC free article] [PubMed] [Google Scholar]
- 15.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 16.Jinming W, Caiyue L, Baojin W, et al. Effects of platelet-rich plasma on tissue expansion in rabbits. Aesthetic Plast Surg. 2017;41(2):454–460. [DOI] [PubMed] [Google Scholar]
- 17.Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41(11):2609–2616. [DOI] [PubMed] [Google Scholar]
- 18.Lange S. Are Platelet-Rich Plasma Injections a Better Choice Compared to Glucocorticoid Injections for the Treatment of Rotator Cuff Tendinopathy in Adult Patients? Thesis. Augsburg University; 2018. Accessed August 24, 2021. https://idun.augsburg.edu/cgi/viewcontent.cgi?article=1347&context=etd [Google Scholar]
- 19.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Platelet-rich plasma. Clin Sports Med. 2019;38(1):17–44. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J. Rotator cuff related shoulder pain: assessment, management and uncertainties. Man Ther. 2016;23:57–68. [DOI] [PubMed] [Google Scholar]
- 21.Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative effectiveness of injection therapies in rotator cuff tendinopathy: a systematic review, pairwise and network meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(2):336–349.e15. [DOI] [PubMed] [Google Scholar]
- 22.Littlewood C, Ashton J, Chance-Larsen K, May S, Sturrock B. Exercise for rotator cuff tendinopathy: a systematic review. Physiotherapy. 2012;98(2):101–109. [DOI] [PubMed] [Google Scholar]
- 23.Mautner K, Colberg RE, Malanga G, et al. Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM R. 2013;5(3):169–175. [DOI] [PubMed] [Google Scholar]
- 24.Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244–250. [DOI] [PubMed] [Google Scholar]
- 25.Mohamadi A, Chan JJ, Claessen FM, Ring D, Chen NC. Corticosteroid injections give small and transient pain relief in rotator cuff tendinosis: a meta-analysis. Clin Orthop Relat Res. 2017;475(1):232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27(2):113–122. [DOI] [PubMed] [Google Scholar]
- 27.Schwitzguebel AJ, Kolo FC, Tirefort J, et al. Efficacy of platelet-rich plasma for the treatment of interstitial supraspinatus tears: a double-blinded, randomized controlled trial. Am J Sports Med. 2019;47(8):1885–1892. [DOI] [PubMed] [Google Scholar]
- 28.Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26(8):837–842. [DOI] [PubMed] [Google Scholar]
- 29.Tantisiriwat N, Wongmatikul V, Jaroenarpornwatana A, Janchai S. Validation and reliability of the Thai version of the Oxford Shoulder Score. J Med Assoc Thai. 2018;101(4):7. [Google Scholar]
- 30.Unlu MC, Kivrak A, Kayaalp ME, Birsel O, Akgun I. Peritendinous injection of platelet-rich plasma to treat tendinopathy: a retrospective review. Acta Orthop Traumatol Turc. 2017;51(6):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Wehren L, Blanke F, Todorov A, Heisterbach P, Sailer J, Majewski M. The effect of subacromial injections of autologous conditioned plasma versus cortisone for the treatment of symptomatic partial rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3787–3792. [DOI] [PubMed] [Google Scholar]
- 32.Waldman S.Ultrasound-guided injection techniques for supraspinatus. In: Comprehensive Atlas of Ultrasound-Guided Pain Management Injection Techniques. 1st ed. Saunders Elsevier; 2014:234–240. [Google Scholar]