Abstract
The mitogen-activated protein (MAP) kinases, extracellular signal-related kinase 1 (ERK1) and ERK2, regulate cellular responses by mediating extracellular growth signals toward cytoplasmic and nuclear targets. A potential target for ERK is topoisomerase IIα, which becomes highly phosphorylated during mitosis and is required for several aspects of nucleic acid metabolism, including chromosome condensation and daughter chromosome separation. In this study, we demonstrated interactions between ERK2 and topoisomerase IIα proteins by coimmunoprecipitation from mixtures of purified enzymes and from nuclear extracts. In vitro, diphosphorylated active ERK2 phosphorylated topoisomerase IIα and enhanced its specific activity by sevenfold, as measured by DNA relaxation assays, whereas unphosphorylated ERK2 had no effect. However, activation of topoisomerase II was also observed with diphosphorylated inactive mutant ERK2, suggesting a mechanism of activation that depends on the phosphorylation state of ERK2 but not on its kinase activity. Nevertheless, activation of ERK by transient transfection of constitutively active mutant MAP kinase kinase 1 (MKK1) enhanced endogenous topoisomerase II activity by fourfold. Our findings indicate that ERK regulates topoisomerase IIα in vitro and in vivo, suggesting a potential target for the MKK/ERK pathway in the modulation of chromatin reorganization events during mitosis and in other phases of the cell cycle.
Growth and differentiation factors regulate the mitogen-activated protein kinases (MAPKs), extracellular signal-related kinase 1 (ERK1) and ERK2, through pathways utilizing receptor tyrosine kinases, cytokine receptors, and heterotrimeric G protein-coupled receptors (for a review, see reference 33). Activation occurs by coupling of receptors to Ras, Raf-1, and MAPK kinase 1 (MKK1) or MKK2, the latter of which activates ERK directly through phosphorylation at regulatory threonine and tyrosine residues. In response to phosphorylation, ERK translocates to nuclei, an event which has been shown to involve phosphorylation and dimerization of this kinase, although enhancement of its specific activity is not required (23). Nuclear uptake of ERK is strongly correlated with proliferation of fibroblasts and neuronal differentiation of PC12 cells (52, 54); thus, the identification of nuclear substrates for this enzyme is an important goal in elucidating mechanisms for biological control.
The MKK/ERK pathway has an essential role in promoting S phase entry, through the phosphorylation of nuclear transcription factors such as Elk/p62TCF, induction of immediate-early genes such as Fos and Egr-1, and transcriptional upregulation of cyclin D1 (3, 30, 31). In contrast, targets for MKK or ERK in somatic cell mitosis are less well defined. MKK and ERK are activated within nuclei during prophase (48, 60), indicating that this pathway may also promote early mitotic events. This is consistent with results suggesting that ERK inactivates the chromatin remodeling activity of the hSWI-SNF complex, an event necessary for mitotic chromosome condensation (50).
DNA topoisomerase II is an important constituent of chromatin, functioning primarily in chromosome condensation and sister chromatid separation during mitosis, with possible secondary functions in transcription and DNA replication (39, 55). It is the segregation of newly replicated daughter chromosomes that renders topoisomerase II essential to the survival of eukaryotic cells (14). Two mammalian forms, topoisomerases IIα (170 kDa) and IIβ (180 kDa), catalyze similar reactions involving double-stranded DNA breakage, double-strand passage, and religation (55). The protein level of the α isoform is upregulated in proliferating cells, whereas β-isoform expression does not significantly change during the cell cycle (25, 46).
The ability of topoisomerase II to generate double-stranded breaks within the genome has been exploited in the treatment of human cancers (7, 18). Antineoplastic drugs, such as the epipodophyllotoxin and etoposide, dramatically increase levels of topoisomerase II cleaved-DNA complexes, resulting in permanent DNA damage followed by apoptotic cell death. Cells containing high levels of topoisomerase II, such as cancer cells, which replicate more rapidly, are thus more susceptible to the cytotoxic effects of these drugs. Thus, signaling pathways that regulate topoisomerase II activity are highly relevant to the understanding and treatment of human disease.
Both topoisomerases IIα and IIβ are phosphorylated throughout the cell cycle but become more highly phosphorylated during the G2 and M phases of the cell cycle (6, 8, 20, 21, 25, 46, 51). Casein kinase II (CKII) and protein kinase C (PKC) have been shown to phosphorylate and activate topoisomerase II in vitro (1, 8, 9, 13, 45, 56). In addition, cyclin B/cdc2 and sea star MAP kinase have been shown to phosphorylate topoisomerase IIα in vitro (57), although a corresponding effect on topoisomerase activity or function has not been reported. Importantly, several residues phosphorylated in vitro have been shown to correspond to in vivo phosphorylation sites. In topoisomerase IIα, two of the four known sites targeted by proline-directed kinases have been shown to be enhanced during mitosis (57).
In this article, we present evidence for direct interactions between topoisomerase IIα and ERK and show that topoisomerase IIα can be phosphorylated and activated by ERK2 in vitro. However, we found that topoisomerase II activation depends on the phosphorylation state, rather than the activity state, of ERK2, suggesting that binding interactions between phospho-ERK and topoisomerase IIα are more relevant to the activation mechanism than is topoisomerase II phosphorylation. These studies suggest a novel function for the MKK/ERK pathway in the control of topoisomerase IIα activity and chromatin structure.
MATERIALS AND METHODS
Enzyme purification. (i) Topoisomerase II.
Topoisomerase II was purified from the nuclei of Drosophila melanogaster embryonic cells (Kc) by the procedure of Shelton et al. (49), yielding a preparation that was ≥95% homogeneous and had no contaminating kinase activity (1). Human topoisomerase IIα was expressed and purified from the yeast Saccharomyces cerevisiae (28), yielding a preparation that was 95% homogeneous and had contaminating kinase activity ascribed to CKII (4).
(ii) (His)6-ERK2.
Wild-type or K52R mutant (His)6-tagged rat ERK2 (a gift of Melanie Cobb) was expressed in bacteria, purified by Ni2+-nitrilotriacetic acid (NTA) metal affinity chromatography (Qiagen), and activated with MKK1-G7B (ΔN4/S218D/M219D/N221D/S222D) (35), which was expressed in bacteria and subjected to proteolysis with enterokinase to remove the (His)6 tag. Reaction mixtures contained 170 μg of ERK2, 15 μg of MKK1-G7B, 4 mM ATP, 15 mM MgCl2, 20 mM HEPES (pH 7.4), and 0.2% (vol/vol) β-mercaptoethanol, in a final volume of 1 ml, and were incubated for 3 h at 30°C. ERK2 was purified away from MKK and ATP by adsorption to Ni2+-NTA resin for 20 min at room temperature; washed three times, each with 1 ml of 10 mM HEPES (pH 7.4)–0.2% β-mercaptoethanol; and eluted with a solution containing 10 mM Tris (pH 8.0), 0.3 M imidazole, and 0.2% β-mercaptoethanol. Aliquots of the activated ERK2 were snapfrozen in liquid nitrogen and stored at −80°C. The specific activity of this preparation was 1.5 μmol/min/mg of protein, as measured with 0.3 mg of myelin basic protein (Sigma)/ml as the substrate (37). A (His)6-tagged ERK2 mutant deficient in dimerization (ERK2-H176E/L4A [H176E/L333,336,341,344A] [see reference 23]) was coexpressed in bacteria with untagged, constitutively active MKK1 (MKK1-R4F [ΔN3/S218E/S222D] [36]), yielding partially phosphorylated ERK2-H176E/L4A, which was further phosphorylated as described above for wild-type ERK. ERK proteins were desalted by using 50- to 150-μl macrospin columns (G-10; Amika Co.) equilibrated in 20 mM Tris-HCl (pH 7.9) containing 50 mM NaCl and 1 mM dithiothreitol (DTT).
Cell culture and transfection.
Mouse NIH 3T3, human A431, human 293, and rat kangaroo PtK1 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (Gibco-BRL). Human CC19 cells were grown in McCoy’s medium with penicillin, streptomycin, and 15% fetal bovine serum.
NIH 3T3 cells were seeded at a density of 4 × 105 cells per 6-cm-diameter plate and at 50 to 80% confluence were transfected with 1 μg of cDNA, using 5 μl of Lipofectamine (Gibco-BRL), according to the manufacturer’s instructions; they were harvested 40 h posttransfection. The transfection efficiency was estimated to be 30 to 40%, based on cell fluorescence in parallel transfections using a construct expressing green fluorescent protein (pK7-GFP; a gift of Ian Macara). The cDNA constructs for expression of wild-type MKK1 and constitutively active MKK1 (MKK1-G1C [ΔN4/S218E/S222D]) were previously described (35, 58).
Preparation of nuclear extracts.
NIH 3T3 cells were grown to 80% confluence in three 6-cm-diameter culture dishes, washed once with cold phosphate-buffered saline (PBS), and scraped, in 1 ml of cold PBS, into 1.5-ml microcentrifuge tubes. The cells were pelleted by centrifugation at 2,500 × g for 30 s. This was followed by removal of the PBS; addition of 400 μl of a cold solution containing 10 mM HEPES (pH 7.5), 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, and 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and incubation on ice for 15 min. The cells were lysed by slowly drawing the cell suspension through a 26-gauge hypodermic needle and then rapidly ejecting it; this was repeated 10 times. Homogenates were then centrifuged at 23,000 × g for 30 s, producing a crude nuclear pellet and a postnuclear or cytoplasmic fraction. The crude nuclear pellet was incubated on ice with frequent vortexing for 30 min in a solution containing 20 mM HEPES (pH 7.5), 1.5 mM MgCl2, 25% (vol/vol) glycerol, 420 mM NaCl, 0.2 mM EDTA, 1 mM DTT, and 0.5 mM PMSF; then it was centrifuged at 23,000 × g for 5 min. The resulting supernatant, containing the nuclear fraction, was dialyzed for 2 h against a cold solution consisting of 20 mM HEPES (pH 7.5), 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 1 mM DTT, and 0.5 mM PMSF.
Binding assays and coimmunoprecipitations.
Glutathione S-transferase (GST)–ERK2 (a gift of Melanie Cobb) was expressed in bacteria, purified on glutathione-Sepharose resin (Pharmacia), and stored in a solution containing 20 mM HEPES (pH 7.5), 120 mM NaCl, 10% glycerol, 2 mM EDTA, 0.5% Triton X-100, 1 mM benzamidine, 0.5 mM PMSF, and 10 μg of aprotinin/ml. In some cases, GST-ERK2 bound to glutathione-Sepharose was activated with constitutively active MKK1-G7B in kinase buffer (KB; 25 mM HEPES [pH 7.4], 15 mM MgCl2, 1 mM ATP, 1 mM sodium orthovanadate, and 1 mM DTT). Following reaction with MKK1, active immobilized GST-ERK2 was washed extensively with KB to remove the MKK1 protein. Inactive immobilized GST-ERK2 was also washed with KB. Activation of GST-ERK2 was confirmed by gel mobility retardation and by reactivity on immunoblots with anti-active MAPK antibody (Promega), which recognizes active, diphosphorylated ERK1 and ERK2. Bacterially expressed GST-p38 MAPK (a gift of Roger Davis) and GST–p21-activated kinase (PAK) (a gift of Melanie Cobb) were used in controls. Concentrations of GST fusion proteins were normalized based on Coomassie staining following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
In binding assays using cell extracts, GST-ERK2 or GST-p38 MAPK (5 μg) bound to 5 μl of glutathione-Sepharose resin, or GST-PAK (5 μg) bound to 5 μl of resin, was added to nuclear extract (50 to 100 μg of protein) and incubated with end-over-end mixing for 1 h at 4°C. Resin-bound protein complexes were washed once with wash buffer (25 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 2 mM DTT, and 1 mM sodium orthovanadate) containing 100 mM KCl and three times with unsupplemented wash buffer.
Binding assays with purified topoisomerase II were carried out by incubating purified Drosophila topoisomerase II or human topoisomerase IIα (0.8 μg) in 30 μl of KB plus 1 mM cold ATP in the presence of GST-ERK2 or GST-p38 MAPK (5 μg, prepared and immobilized as described above) for 1 h at 30°C with occasional mixing. Kinase-topoisomerase IIα complexes were washed as described above and separated by SDS-PAGE, and topoisomerase IIα was visualized by immunoblotting. For immunoblotting, 40 μl of Laemmli sample buffer was added to washed protein complexes; proteins were resolved by SDS-PAGE and electrophoretically transferred (Ellard Instruments) onto Immobilon P membranes (Millipore). The membranes were blocked for 1 h in Tris-buffered saline (TBS; 50 mM Tris [pH 7.6], 0.15 M NaCl) supplemented with 0.1% (vol/vol) Tween 20 and 5% (wt/vol) nonfat dry milk. The blots were then incubated with anti-topoisomerase IIα antibody (0.5 μg/ml; mouse monoclonal antibody OM-11-930A or rabbit polyclonal antibody OA-11-752; Genosys) in TBS plus 0.1% (vol/vol) Tween 20 (TBS-Tween 20) supplemented with 1% (wt/vol) bovine serum albumin (BSA) for 1 h. The blots were washed four times with TBS-Tween 20 and then incubated for 1 h with horseradish peroxidase-coupled anti-rabbit or anti-mouse secondary antibody (0.8 μg/ml; Jackson ImmunoResearch Laboratories) in TBS-Tween 20 containing 1% BSA. Following further washes in TBS-Tween 20, proteins were visualized by enhanced chemiluminescence (Amersham).
For coimmunoprecipitations of endogenous ERK2 and topoisomerase IIα, nuclear extracts from nocodazole-treated NIH 3T3 cells were incubated with 0, 0.2, or 2 μg of anti-ERK2 antibody (C-14; Santa Cruz Biotechnology) for 2 h on ice; this was followed by addition of 20 μl of protein A-Sepharose (Pharmacia) that had been pretreated with BSA at 0.5 mg/ml. After further incubation for 2 h at 4°C with constant mixing, the protein A-Sepharose resin was washed twice with 0.5 ml of a solution consisting of 25 mM HEPES (pH 7.4), 25 mM MgCl2, 1 mM DTT, and 100 mM KCl; this was followed by two washes, each with 0.5 ml of a solution containing 25 mM HEPES (pH 7.4), 25 mM MgCl2, and 1 mM DTT. ERK2 and coprecipitating topoisomerase IIα were visualized by immunoblotting with a mixture of anti-ERK2 and anti-topoisomerase IIα antibodies.
Phosphorylation and immunoprecipitation assays.
The activity of immunoprecipitated ERK was measured by incubating washed, immobilized immune complexes in a final volume of 30 μl containing KB with 10 μCi of [γ-32P]ATP, 30 μM ATP, and 5 μg of myelin basic protein for 10 min at 30°C. Reactions were quenched with Laemmli sample buffer, products were resolved by SDS-PAGE, and 32P incorporation was quantified by phosphorimager analysis. Cyclin B/cdc2 activity was measured by phosphorylation of histone IIIS (5 μg; Sigma), under similar conditions, following immunoprecipitation with anti-p34 cdc2 antibodies (catalog no. 17; Santa Cruz).
Topoisomerase II phosphorylation by ERK2 was measured in vitro by incubating Drosophila topoisomerase II (0.6 μg) or human topoisomerase IIα (0.8 μg) with ERK2 (2 μg) in a final volume of 20 μl containing KB with 10 μCi of [γ-32P]ATP and 2 mM cold ATP at 30°C. At various times, reactions were quenched by addition of Laemmli sample buffer and products were resolved by SDS-PAGE. 32P incorporation into topoisomerase II was quantified by phosphorimager analysis.
DNA relaxation and decatenation assays.
Topoisomerase II activity was measured by relaxation of supercoiled DNA (40) or decatenation of kinetoplast DNA (34) as described previously. Unless otherwise specified, the effect of ERK2 on topoisomerase II activity in vitro was measured by preincubating Drosophila topoisomerase II (0.6 μg) or human topoisomerase IIα (1.6 μg) with purified (His)6-ERK2 (2 μg) for 1 h at 30°C in a final volume of 20 μl containing 25 mM HEPES, 15 mM MgCl2, 1 mM DTT, and 2 mM cold ATP. After preincubation, aliquots (containing 60 ng of Drosophila topoisomerase II or 160 ng of human topoisomerase IIα) were removed and added to 200 ng of supercoiled pUC119 DNA or 0.2 μg of kinetoplast DNA prepared as described by Englund (17) (kindly provided by Daniel Bogenhagen). After incubation at 30°C (15 min for relaxation assays; 30 min for decatenation assays), reactions were quenched with 10 mM EDTA–0.1% SDS, and DNA products were resolved on 1% agarose–Tris-borate-EDTA gels and visualized with ethidium bromide. Fluorescence was quantified by using a charge-coupled device camera with digital imaging software (Alpha Innotech Corp.).
The effect of ERK activation on topoisomerase II activity in vivo was measured by transfecting NIH 3T3 cells with wild-type MKK1 or constitutively active MKK1-G1C, from which nuclear extracts were prepared. Prior to assay, levels of topoisomerase IIα in nuclear extracts were determined by immunoblotting. Decatenation assays were carried out by incubating 0.2 μg of kinetoplast DNA with nuclear extract (1 to 2 μg of protein) or topoisomerase IIα immunoprecipitated from an equivalent volume of nuclear extract. After 10 to 60 min at 30°C, reactions were quenched by addition of 10 mM EDTA–0.1% SDS and DNA products were resolved on 1% agarose–Tris-borate-EDTA gels. Agarose gels were stained with ethidium bromide, and fluorescence was quantified by digital imaging.
RESULTS
Topoisomerase IIα and ERK2 associate in cell extracts and purified preparations.
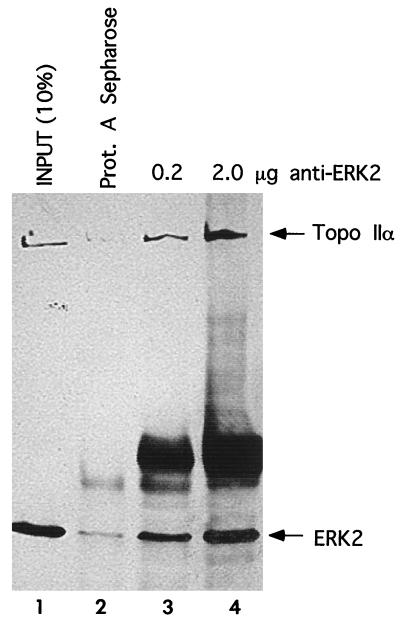
Interactions between endogenous ERK2 and topoisomerase IIα proteins were examined by coimmunoprecipitation from nuclear extracts prepared from nocodazole-treated cells. Topoisomerase IIα coimmunoprecipitated with ERK2 (Fig. 1, lanes 3 and 4) but not with protein A-Sepharose alone (Fig. 1, lane 2).
FIG. 1.
Coimmunoprecipitation of endogenous topoisomerase IIα and ERK2 from nuclear extracts. ERK2 was immunoprecipitated from nuclear extracts prepared from nocodazole-treated NIH 3T3 cells; this was followed by immunoblotting to visualize both ERK2 and coimmunoprecipitating topoisomerase (Topo) IIα. Lanes 3 and 4 show increasing amounts of immunoprecipitated ERK2 and coimmunoprecipitated topoisomerase IIα. A small amount of topoisomerase IIα nonspecifically bound to the protein A-(Prot. A)-Sepharose resin (lane 2). Ten percent of the extract volume used for the immunoprecipitations served as a loading control (lane 1).
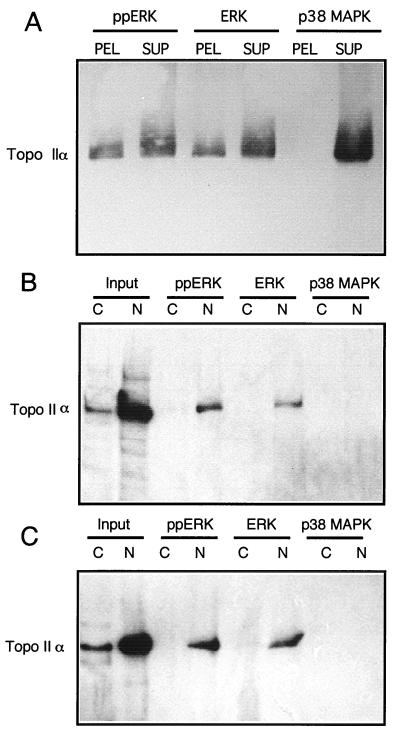
To evaluate whether the phosphorylation state of ERK2 affected its interactions with topoisomerase IIα, phosphorylated or unphosphorylated GST-ERK2 was expressed and purified from bacteria and tested for binding with purified topoisomerase IIα. In vitro, purified human topoisomerase IIα bound to GST-ERK2 immobilized on glutathione-Sepharose, as visualized by immunoblotting (Fig. 2A). Comparisons between the pelleted fraction of topoisomerase IIα and the fraction remaining in the supernatant showed that approximately 20% of the topoisomerase IIα had bound to either active diphosphorylated or inactive unphosphorylated forms of GST-ERK2 (Fig. 2A). Controls showed no evidence of interaction between topoisomerase IIα and GST-p38 MAPK, a kinase with homology to ERK (Fig. 2A). In addition, no binding of topoisomerase IIα to glutathione-Sepharose resin or to immobilized GST-PAK was observed (data not shown).
FIG. 2.
Specific binding between topoisomerase IIα and GST-ERK2. (A) Active diphosphorylated GST-ERK2 (ppERK), inactive unphosphorylated GST-ERK2 (ERK), or unphosphorylated GST-p38 MAPK was bound to glutathione-Sepharose and incubated in vitro with purified human topoisomerase IIα. The resin was washed, and topoisomerase IIα within immobilized complexes (PEL) or in equal proportions of the unbound pool (SUP) was resolved by SDS-PAGE and visualized by immunoblotting. (B and C) Cytosolic (C) and nuclear (N) extracts were prepared from (B) HEK293 or NIH 3T3 (C) cells; this was followed by incubation with inactive or active GST-ERK2 or GST-p38 MAPK immobilized on glutathione-Sepharose. After being washed, proteins were resolved by SDS-PAGE, and topoisomerase IIα was examined by immunoblotting. Lanes 1 and 2 show equivalent volumes of cytosolic and nuclear extracts prior to incubation with GST proteins. Topoisomerase IIα coprecipitated with both forms of GST-ERK2, but not with p38 MAPK. Results were reproduced in three separate experiments.
Topoisomerase IIα derived from nuclear pools of human kidney 293 or mouse NIH 3T3 cells was also tested for binding with GST-ERK2. As shown in Fig. 2B and C, active and inactive forms of GST-ERK2 were able to bind significant fractions of topoisomerase IIα from nuclear extracts. Binding of cytosolic topoisomerase IIα was minimal, reflecting the small amounts of enzyme in this pool. In controls, topoisomerase IIα failed to bind to GST-p38 MAPK (Fig. 2B and C), GST-PAK, or glutathione-Sepharose resin (data not shown). Topoisomerase IIα from nuclear extracts of other cell lines, including A431 and CC19, also bound to GST-ERK2 but not to GST-p38 MAPK (data not shown).
Phosphorylation and activation of topoisomerase IIα by ERK2 in vitro.
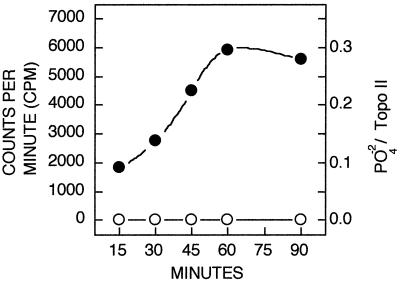
Topoisomerase IIα contains several potential phosphorylation sites for proline-directed protein kinases, some of which are targeted by cyclin B/cdc2 or sea star MAPK (57). We examined whether active mammalian ERK2 could recognize purified Drosophila topoisomerase II as a substrate in vitro and found that topoisomerase II could be phosphorylated to a stoichiometry of 0.3 mol/mol of enzyme after 90 min (Fig. 3). Topoisomerase II phosphorylation was not observed when inactive ERK2 was present (Fig. 3).
FIG. 3.
Phosphorylation of topoisomerase II by active ERK2 in vitro. Purified Drosophila topoisomerase (Topo) II was incubated with ERK2 in the presence of [γ-32P]ATP as described in Materials and Methods, and phosphate incorporation was monitored at various times. The levels of topoisomerase II phosphorylation in the presence of active ERK2 (closed circles) and inactive ERK (open circles) are shown. Phosphorylation stoichiometry shown on the right axis represents moles of phosphate per mole of topoisomerase.
Phosphorylation reactions were carried out in parallel, using human topoisomerase IIα purified from S. cerevisiae as the substrate (data not shown). Incorporation of radiolabelled phosphate was observed in the absence of ERK2, reflecting the presence of a contaminating kinase in these preparations, previously identified as CKII (4). Nevertheless, human topoisomerase IIα phosphorylation was further enhanced by active ERK2, with phosphate incorporation rates of 1.5 mol/mol of enzyme in the presence of active ERK2 and 0.7 mol/mol in the absence of ERK2. Thus, despite the high background, the phosphorylation stoichiometry of human topoisomerase IIα that can be attributed to ERK2 after 90 min is approximately 0.8 mol/mol.
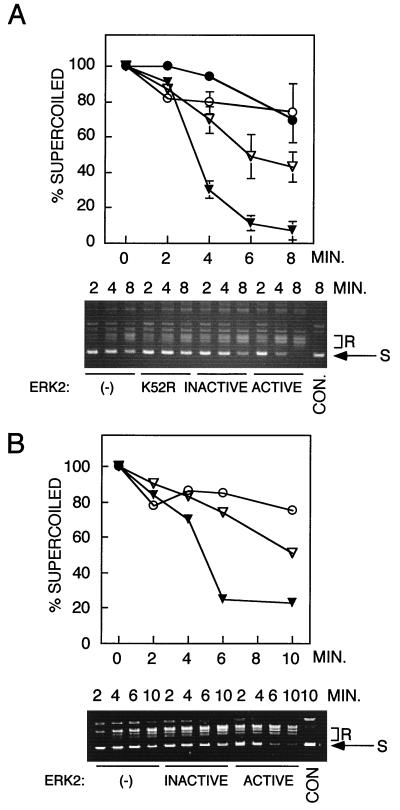
To evaluate the effects of ERK2 on topoisomerase II activity, purified Drosophila topoisomerase II or human topoisomerase IIα was incubated with ERK2 in the presence of Mg-ATP and topoisomerase II activity was evaluated by examining the steady-state relaxation of supercoiled DNA (40). Topoisomerase II activity, determined by measuring the rate of loss of supercoiled DNA, was enhanced by sevenfold following incubation with active ERK2 compared to the activity in the absence of ERK2 (Fig. 4). The lower panels in Fig. 4A and B show the conversion of supercoiled DNA to relaxed DNA on agarose gels. Control incubations with a nonactivable ERK2-K52R mutant (Fig. 4A) showed little evidence of an effect on topoisomerase II activity and supported a model in which topoisomerase II requires phosphorylation by ERK2 for enhancement of activity. However, incubation with inactive wild-type ERK2 (Fig. 4) resulted in twofold enhancement of activity in spite of the absence of phosphorylation under similar conditions (Fig. 3).
FIG. 4.
Activation of topoisomerase II by active ERK2. Drosophila topoisomerase II or human topoisomerase IIα was incubated for 1 h with inactive or active ERK2, and topoisomerase II activity was measured in relaxation assays, using supercoiled DNA as a substrate, by quenching reactions at the indicated time points. (A) Plasmid relaxation by Drosophila topoisomerase II following preincubation with active phosphorylated wild-type ERK2 (closed triangles), inactive unphosphorylated wild-type ERK2 (open triangles), unphosphorylated catalytically inactive mutant ERK2-K52R (closed circles), or no ERK2 (−) (open circles). (B) Activation of purified human topoisomerase IIα following preincubation with active phosphorylated wild-type ERK2 (closed triangles), inactive unphosphorylated wild-type ERK2 (open triangles), or no ERK2 (−) (open circles). Ethidium bromide-stained agarose gels in each panel show typical DNA profiles from relaxation assays with each topoisomerase. Results were reproduced in four separate experiments. S, supercoiled DNA substrate; R, relaxed DNA products; CON., plasmid DNA incubated in the absence of topoisomerase.
Activation of topoisomerase II does not depend on phosphorylation.
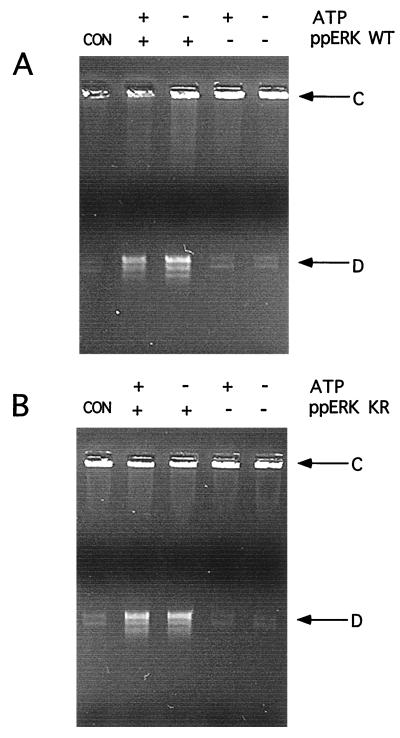
The effects of wild-type ERK on topoisomerase II activity suggested that regulation of topoisomerase II activity might occur through phosphorylation-independent mechanisms. Therefore, we tested the requirement for phosphorylation by including or omitting Mg-ATP and by comparing catalytically active and inactive forms of ERK2 in preincubation reactions. Human topoisomerase IIα activity was measured by decatenation of kinetoplast DNA as a specific assay for double-strand cleavage. As shown in Fig. 5 by the increased formation of decatenated DNA compared to catenated DNA, topoisomerase IIα activity was enhanced following preincubation with active, diphosphorylated ERK2 (Fig. 5A). Interestingly, activation was also observed with catalytically inactive, diphosphorylated mutant ERK2 (Fig. 5B, ppERK KR), indicating that the activation was not correlated with topoisomerase IIα phosphorylation. Both forms of ERK2 were able to activate topoisomerase IIα, whether ATP was present in or absent from the preincubation buffer (Fig. 5), confirming this observation. Similar results were also observed with Drosophila topoisomerase II (data not shown). In control reactions with diphosphorylated wild-type ERK2 in the absence of topoisomerase, no effect on kinetoplast DNA was observed, indicating that decatenation was due to topoisomerase IIα (data not shown). In addition, no difference in the topoisomerase II activities measured before and after preincubation was observed (Fig. 5), indicating that the activity was stable over the time course of preincubation with ERK2. These data thus support a mechanism in which phosphorylated ERK2 interacts with topoisomerase II in a manner that enhances the rate of catalysis by the topoisomerase.
FIG. 5.
ERK activation of topoisomerase IIα occurs independently of ATP and ERK activity. (A) Active phosphorylated wild-type ERK2 (ppERK WT) (A) or catalytically inactive ERK phosphorylated mutant ERK2 (ppERK KR) (B) was preincubated with human topoisomerase IIα in the presence (+) or absence (−) of ATP. The topoisomerase was removed and assayed by decatenation of kinetoplast DNA. The formation of decatenated DNA (D) from catenated DNA (C) was enhanced by preincubation with phosphorylated ERK2, regardless of whether ERK2 was active or ATP was present during the preincubation. Comparison of control reactions, which were not subjected to preincubation (lanes 1), to reactions which were preincubated without ERK or ATP (lanes 5) demonstrated that preincubation alone does not alter topoisomerase activity.
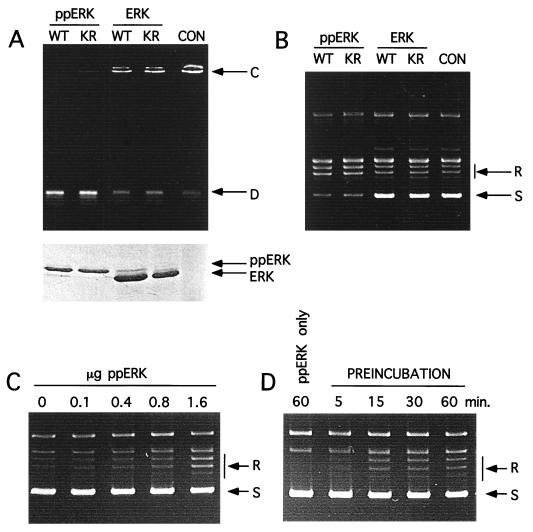
Next, the dependence of ERK phosphorylation on topoisomerase II activation was tested. Although both diphosphorylated active wild-type and inactive mutant ERK2 enhanced topoisomerase IIα activity, parallel reactions with unphosphorylated forms of the same kinases showed no effect on activity, as measured by kinetoplast DNA decatenation (Fig. 6A). Coomassie staining of ERK proteins used in each set of preincubation conditions (Fig. 6A, lower panel) confirms a retardation of the mobility of phosphorylated ERK compared to that of the unphosphorylated forms (Fig. 6A). Similar observations were made when topoisomerase IIα activity was measured by relaxation assays (Fig. 6B). Diphosphorylated ERK in its active and inactive forms equally increased topoisomerase IIα activity compared to reactions with unphosphorylated ERKs or in the absence of ERK (Fig. 6B). These data argue against phosphorylation as the mechanism of ERK activation of topoisomerase II.
FIG. 6.
Enhancement of topoisomerase IIα activity is dependent on the phosphorylation state of ERK2. (A) DNA decatenation assays with human topoisomerase IIα were performed following preincubation with phosphorylated wild-type (WT) or mutant (KR) ERK2 (ppERK), as well as with unphosphorylated wild-type or mutant ERK2, in the presence of ATP. The results of a control reaction (CON) involving incubation with topoisomerase IIα in the absence of ERK2 are also shown. The gel in the lower panel shows Coomassie-stained ERK2 proteins in each assay. D, decatenated DNA product; C, catenated kinetoplast DNA substrate. (B) DNA relaxation assays were performed following preincubation of human topoisomerase IIα with phosphorylated wild-type or mutant ERK2, as well as with unphosphorylated wild-type or mutant ERK2, in the presence of ATP. (C) DNA relaxation assays were performed by preincubating topoisomerase IIα (1.6 μg) with the indicated amounts of phosphorylated wild-type ERK. (D) DNA relaxation assays were performed after the indicated time points of preincubation, using phosphorylated wild-type ERK2 (1.6 μg) and topoisomerase IIα (1.6 μg) in the preincubation mixture. As a control, diphosphorylated ERK2 was used in the absence of topoisomerase IIα protein; no effect on DNA relaxation was seen (lane 1). S, supercoiled DNA substrate; R, relaxed DNA products.
Topoisomerase II activation was examined with respect to stoichiometry and time of preincubation. As shown in Fig. 6C, DNA relaxation was significant only after the ERK/topoisomerase molar ratio exceeded 2:1 to 4:1. In addition, a 15-min preincubation with diphosphorylated ERK2 was needed before significant topoisomerase activation was observed (Fig. 6D). Control reactions performed with diphosphorylated ERK2 in the absence of topoisomerase IIα did not affect DNA relaxation, ruling out the possibility that ERK2 preparations were contaminated with topoisomerase activity (Fig. 6D).
Monomeric ERK2 promotes topoisomerase II activation.
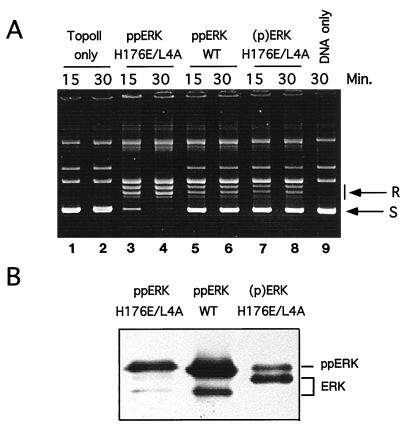
A recent study demonstrated that diphosphorylation of ERK2 promotes homodimerization, driven by a 2,800-fold reduction in Kd (23). Dimerization can be disrupted by mutation of residues in helices αC and αL16 of ERK2, consistent with subunit packing interactions observed in the X-ray structure of phosphorylated ERK. To test the influence of ERK dimerization on topoisomerase II activation, the mutant ERK2-H176E/L4A, which is impaired in terms of dimerization ability (23), was phosphorylated and tested in relaxation assays. Diphosphorylated ERK2-H176E/L4A in fact enhanced topoisomerase activity (Fig. 7A, lanes 3 and 4) compared to that of diphosphorylated wild-type ERK2 (Fig. 7A, lanes 5 and 6). This difference could not be accounted for by differences in ERK phosphorylation, since diphosphorylated ERK2-H176E/L4A and wild-type ERK2 showed similar degrees of gel mobility retardation on immunoblots (Fig. 7B). An incompletely phosphorylated form of ERK2-H176E/L4A (Fig. 7A, lanes 7 and 8) activated topoisomerase IIα to a degree comparable to that of wild-type diphosphorylated ERK2. Controls showed that neither form of ERK2 affected DNA relaxation in the absence of topoisomerase IIα (data not shown). Together, these data suggest that catalytic activation of topoisomerase II is favored by interaction with monomeric, diphosphorylated ERK2.
FIG. 7.
Dimerization defective mutants of ERK2 activate topoisomerase IIα. (A) Human topoisomerase IIα was preincubated in the presence of ATP with no ERK2 (lanes 1 and 2), with diphosphorylated dimerization-defective mutant ERK2 (ppERK H176E/L4A) (lanes 3 and 4), with phosphorylated wild-type ERK2 (ppERK WT) (lanes 5 and 6), or with incompletely phosphorylated mutant ERK2 [(p)ERK H176E/L4A] (lanes 7 and 8). Relaxation assays were performed for 15 or 30 min as indicated. Lane 9 shows a control reaction performed in the absence of topoisomerase. (B) ERK2 was immunoblotted to reveal gel mobilities, reflecting its state of phosphorylation. Note that diphosphorylated ppERK2 H176E/L4A and ppERK WT show nearly complete shifts toward slower-migrating form whereas incompletely phosphorylated (p)ERK H176E/L4A shows significant levels of the faster-migrating unphosphorylated ERK.
Activation of topoisomerase II via ERK activation in intact cells.
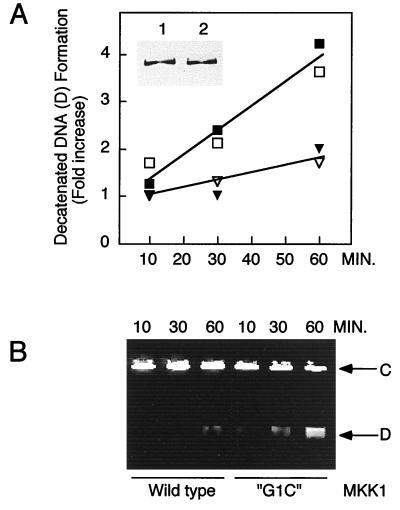
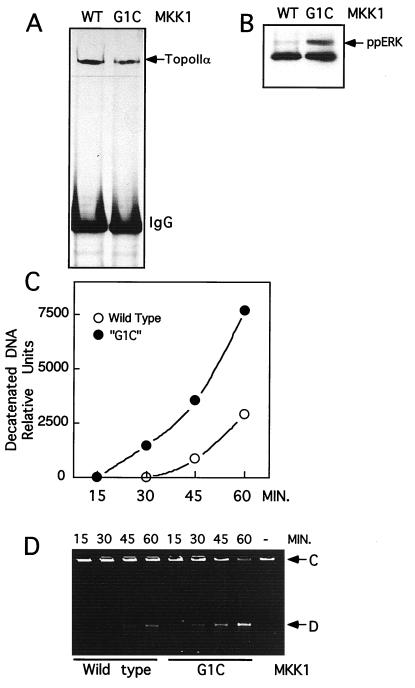
To test the physiological relationship between ERK activation and topoisomerase II activity, NIH 3T3 cells were transiently transfected with active mutant MKK1-G1C, which elevates ERK1 and ERK2 activity (35, 58). Nuclear extracts were prepared from these cells, and topoisomerase II activity was measured by decatenation of kinetoplast DNA. As shown in Fig. 8A, nuclear topoisomerase II activity was enhanced fourfold in cells transfected with constitutively active MKK1 compared to that in cells transiently transfected with wild-type MKK1. A typical experiment comparing the conversion of catenated DNA to decatenated DNA is shown in Fig. 8B. Topoisomerase II activity, determined by measuring the relaxation of supercoiled DNA, showed a similar enhancement in response to MKK1-G1C transfection (data not shown). Control immunoblots verified that topoisomerase IIα levels were unaltered following transfection (Fig. 8A, inset). Similar results were obtained with extracts prepared from transfected Chinese hamster ovary cells (data not shown).
FIG. 8.
Expression of constitutively active MKK1 stimulates topoisomerase II activity in nuclear extracts. NIH 3T3 cells transiently transfected with wild-type or constitutively active MKK1-G1C were used to prepare nuclear extracts. Kinetoplast decatenation assays were used to selectively measure topoisomerase II activity. (A) Decatenation of DNA versus time, measured with nuclear extracts from cells transfected with constitutively active MKK1 (squares) or wild-type MKK1-transfected cells (triangles). Open and closed symbols represent two independent transfections. The inset shows immunoblotting of topoisomerase IIα from equivalent volumes of nuclear extracts prepared from cells transfected with wild-type MKK1 (lane 1) or constitutively active MKK1 (lane 2). (B) Representative decatenation assay used to quantify the amount of decatenated kinetoplast DNA in panel A. Shown are catenated DNA substrate (C) and decatenated DNA product (D) on an ethidium bromide-stained agarose gel. Data were reproduced in four separate experiments.
To eliminate the possibility that these effects were due to contaminating topoisomerase IIβ, immunoprecipitations from nuclear extracts were performed with topoisomerase IIα-specific antibodies and topoisomerase activity was measured in decatenation assays. Figure 9A shows the amount of topoisomerase IIα which immunoprecipitated from nuclear extracts prepared from cells transfected with wild-type MKK1 or MKK1-G1C. In the latter case, immunoblots of ERK2 demonstrated gel mobility retardation indicative of activation (Fig. 9B). In decatenation assays, topoisomerase IIα activity in extracts of cells transfected with MKK1-G1C was elevated compared to that in extracts of cells transfected with wild-type MKK1 (Fig. 9C). A typical experiment shows the conversion of catenated DNA to decatenated DNA (Fig. 9D).
FIG. 9.
Expression of constitutively active MKK1 enhances the activity of topoisomerase IIα immunoprecipitated from nuclear extracts. NIH 3T3 cells transiently transfected with wild-type MKK1 or MKK1-G1C were used to prepare nuclear extracts. Topoisomerase IIα was immunoprecipitated from these extracts, and its activity was determined by measuring kinetoplast decatenation over time. (A) Immunoblots of topoisomerase IIα immunoprecipitated from nuclear extracts transfected with wild-type (WT) or constitutively active MKK1. (B) Immunoblots of ERK2 in nuclear extracts, showing gel mobility retardation of ERK2 (ppERK) in cells transfected with MKK1-G1C. (C) Decatenation of DNA versus time, measured after immunoprecipitation of topoisomerase IIα from nuclear extracts of cells transfected with MKK1-G1C (closed circles) or wild-type MKK1 (open circles). (D) Decatenation assay used to quantify the amount of decatenated kinetoplast DNA in panel C. Shown are catenated DNA substrate (C) and decatenated product (D) on an ethidium bromide-stained agarose gel. Data are representative of two independent transfections.
Because topoisomerase IIα protein levels are known to increase during mitosis (46), it could be argued that the enhanced topoisomerase activity might be caused by an increased proportion of mitotic cells resulting from transfection with MKK1-G1C. Three observations eliminated this possibility. First, topoisomerase IIα protein levels did not significantly differ between transfections or immunoprecipitations, indicating that the effect occurs at the level of topoisomerase II specific activity (Fig. 8A, inset, and Fig. 9A). Second, the level of histone IIIS kinase activity measured in cdc2 immunoprecipitates was the same or slightly lower in cells transiently transfected with MKK1-G1C than in cells transfected with wild-type MKK1 (data not shown). Third, the number of mitotic cells, identified by 4′,6-diamidino-2-phenylindole (DAPI) staining, did not significantly differ between transfections; mitotic indices in two separate transfections were 5.6 and 3.2% in untransfected cells, 4.4 and 3.5% in wild-type MKK1-transfected cells, and 3.6 and 3.5% in mutant-MKK1-transfected cells. Therefore, MKK- or ERK-dependent enhancement of topoisomerase II specific activity was not due to enrichment of mitotic cells.
It was also possible that the activity of topoisomerase IIα from cells transfected with MKK1-G1C was elevated in vitro in proportion to the amount of active ERK contained in the nuclear extracts. To control for this possibility, purified recombinant diphosphorylated wild-type ERK2 was added to nuclear extracts of cells transfected with wild-type MKK1 to a level comparable to that seen in G1C-transfected cell extracts. No elevation of topoisomerase II activity was observed either in crude nuclear extracts or in topoisomerase IIα immunoprecipitates (data not shown), indicating that the effect of MKK1-G1C on topoisomerase activity occurs in intact cells prior to cell disruption.
DISCUSSION
In this article, we present several lines of evidence demonstrating regulation of topoisomerase IIα by the MKK/ERK pathway. First, ERK2 enhances topoisomerase IIα activity in vitro in a manner that correlates with the phosphorylation state of ERK. Second, nuclear topoisomerase II activity is significantly enhanced on activation of ERK in intact cells by transient transfection of constitutively active MKK1. Finally, the lack of dependence of topoisomerase activation on ERK activity suggests that physical interactions between ERK and topoisomerase IIα are relevant to the mechanism of activation; this is substantiated by coimmunoprecipitations and interactions between GST-ERK and topoisomerase II derived from purified protein mixtures or nuclear extracts.
Topoisomerase IIα is a highly abundant chromatin-associated protein which appears to be a major constituent of nuclear scaffold preparations, showing preferential association with A-T-rich sequences within chromatin and colocalizing with scaffold attachment regions (16, 47). Both topoisomerases IIα and IIβ become highly phosphorylated in G2/M (6, 20, 21, 51). Whereas a large fraction of topoisomerase IIα remains chromatin bound, topoisomerase IIβ appears to be released into cytoplasmic pools during mitosis (38). Thus, topoisomerase IIα is presumably the major form regulating mitotic chromosome events (21, 39). Changes in the association of topoisomerase IIα with centromeres and condensed chromosomes during mitotic progression (38, 42) suggest structural and possibly noncatalytic functions for this enzyme in chromatin organization, which might be controlled by phosphorylation.
The regulation of topoisomerase II activity by phosphorylation is poorly understood, with most of the information being based on in vitro data. Topoisomerase II has been shown to be a substrate for CKII, PKC, cyclin B/cdc2, and sea star MAPK (1, 8, 21, 45, 57), and our present study demonstrates phosphorylation by mammalian ERK2. At high stoichiometries, phosphorylation by either CKII or PKC occurs concomitantly with a threefold enhancement of lower-eukaryotic topoisomerase II specific activity. Mechanistic studies attribute this effect to enhancement of ATP hydrolysis and enzymatic turnover (9, 10).
More-recent studies have found that the activation of mammalian topoisomerase IIα by CKII is probably independent of topoisomerase phosphorylation and may instead be accounted for by two alternative mechanisms. One study found that following phosphorylation and activation by CKII, topoisomerase IIα activity was unchanged upon phosphatase-catalyzed dephosphorylation (26). In fact, topoisomerase IIα activity was enhanced following incubation with low concentrations of glycerol in the absence of kinase, suggesting that the activation mechanism involves topoisomerase autoactivation independent of CKII. A second study found that preincubation with CKII protected topoisomerase IIα from thermal inactivation and that the apparent activation by CKII was actually due to stabilization against activity loss during the preincubation (43). This protective effect was specific for CKII and could be ascribed to stable interactions previously demonstrated between kinase and topoisomerase IIα (4), suggesting a potential physiological role for kinase-topoisomerase interactions.
Our results contrast both of these mechanisms. In our hands, topoisomerase IIα activity was stable throughout the time of preincubation (Fig. 5); thus, neither autoactivation nor thermal inactivation appears to be important, while preincubation with phosphorylated ERK2 clearly enhances topoisomerase IIα activity. The lack of any effect with unphosphorylated ERK indicates that topoisomerase IIα is somehow able to distinguish between related forms of ERK, most likely through direct interactions with ERK2. Furthermore, topoisomerase appears to be able to distinguish between monomeric and dimeric forms of ERK2, given our finding that diphosphorylated ERK2 dimerization mutants preferentially activate topoisomerase IIα compared to wild-type ERK2 (Fig. 7A).
During mitosis, phosphorylation of topoisomerase IIα increases significantly, suggesting a mechanism for controlling DNA unwinding in chromatin condensation and/or separation (6, 8, 20, 25, 46, 51). Phosphopeptide mapping studies have shown increased occupancy at Ser1212 and Ser1246 in mitotic cells; both are proline-directed sites that have also been found to be phosphorylated by cdc2 or sea star MAPKs in vitro (57). In vivo phosphorylation sites have also been mapped at Ser1392 and Ser1353, targeted by cdc2 and MAPK, as well as at thr1342, Ser1360, Ser1376, and Ser1524, targeted by CKII (22, 57). None of these sites shows clear changes in occupancy during mitosis, as measured by phosphopeptide mapping of enzyme from 32P-labelled cells. Importantly, specific activity measurements on topoisomerase II extracted from cells have so far not demonstrated an increase occurring during mitosis (27, 38). It therefore seems likely that phosphorylation regulates topoisomerase IIα by mechanisms other than enhancement of catalysis. For example, phosphorylation may well regulate topoisomerase interactions with nuclear targets, perhaps through enhancement of enzyme oligomerization (53).
The fact that ERK translocates to nuclei following stimulation of the MKK/ERK pathway suggests that enhancement of topoisomerase IIα activity by specific interactions with phosphorylated forms of ERK may be physiologically significant. For example, mitotic phosphorylation may indirectly regulate topoisomerase IIα activity by modulating its interaction with activating nuclear proteins. In addition, the abundance of topoisomerase IIα in nuclei suggests that this enzyme may be a potential anchor involved in stabilizing nuclear retention of phosphorylated ERK in response to cell stimulation or during prophase (23, 32, 48, 60).
Cells treated with various topoisomerase II inhibitors arrest in G2/M under conditions of suppressed cdc2 activity, indicating a requirement for chromatin decatenation in mitotic entry (2, 15). Compelling evidence supporting a requirement for ERK activation for M phase entry has been demonstrated by Wright et al., who found that synchronized cells undergo arrest at G2/M in response to dominant-negative MKK1 or the MKK1 inhibitor PD98059 (59). We speculate that the dual requirement for MKK/ERK and topoisomerase II activities in early mitosis may reflect a regulatory interaction between topoisomerase IIα and ERK. Conceivably, ERK may play an essential role, via topoisomerase II activation, during M phase entry as well as in later mitotic events. We have demonstrated by indirect immunofluorescence of PtK1 cells that active ERK and topoisomerase IIα colocalize during prophase, when the levels of both enzymes are elevated in the nucleoplasm, and that they specifically colocalize at kinetochore regions of condensed chromatin (data not shown). Interactions between topoisomerase IIα and ERK2 in nuclei and at kinetochores may represent events during mitosis in which ERK2 regulates topoisomerase II activity and chromatin remodeling.
Our findings also suggest a potential mechanism by which the ERK pathway may contribute to tumorigenesis. Activation of ERK by expression of constitutively active MKK mutants in NIH cells results in transformed morphology, enhanced focus formation, and solid-tumor formation in nude mice (5, 11, 36), although the transformed phenotype is less pronounced due to the requirement for multiple pathways downstream of Ras (24, 41, 44). The ability of oncogenic Ras and Mos to compromise the chromosomal stability of somatic cells (12, 19) implies a similar function for MKK/ERK. A link between topoisomerase II and cellular transformation through Ras is further suggested by findings that tumor cell lines containing oncogenic Ras are more sensitive to topoisomerase II inhibitors (29). Conceivably, unregulated forms of ERK could promote genomic instability by enhancing the frequency of chromosomal translocations due to double-stranded DNA cleavage by topoisomerase II.
ACKNOWLEDGMENTS
We are indebted to Daniel Bogenhagen, University of Colorado Health Sciences Center, Denver, for providing kinetoplast DNA and advice on decatenation assays; to Melanie Cobb, University of Texas Southwestern Medical Center, Dallas, for providing ERK2 and PAK constructs; to Roger Davis for providing p38 MAPK constructs; and to Ian Macara for providing green fluorescent protein constructs. We also thank Jocelyn Wright and Edwin Krebs, University of Washington, for sharing results prior to publication.
This study was supported by the Searle Scholars Program (N.G.A.) and by grants RO1 GM48521 (N.G.A.), F32 GM18151 (P.S.), and RO1 GM33944 (N.O.) from the National Institutes of Health.
REFERENCES
- 1.Ackerman P, Glover C V C, Osheroff N. Phosphorylation of DNA topoisomerase II by casein kinase II: modulation of eukaryotic topoisomerase II activity in vitro. Proc Natl Acad Sci USA. 1985;82:3164–3168. doi: 10.1073/pnas.82.10.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreassen P, Lacroix F B, Margolis R L. Chromosomes with two intact axial cores are induced by G2 checkpoint override: evidence that DNA decatenation is not required to template the chromosome structure. J Cell Biol. 1997;136:29–43. doi: 10.1083/jcb.136.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beno W R, Brady L M, Bissonnette M, Davis B H. Protein kinase C and mitogen-activated protein kinase are required for 1,25-dihydroxy vitamin D3-stimulated Egr induction. J Biol Chem. 1995;270:3642–3647. doi: 10.1074/jbc.270.8.3642. [DOI] [PubMed] [Google Scholar]
- 4.Bojanowski K, Filhol O, Cochet C, Chambaz E M, Larsen A K. DNA topoisomerase II and casein kinase II associate in a molecular complex that is catalytically active. J Biol Chem. 1993;268:22920–22926. [PubMed] [Google Scholar]
- 5.Brunet A, Pagès G, Pouysségur J. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994;9:3379–3387. [PubMed] [Google Scholar]
- 6.Burden D A, Sullivan D M. Phosphorylation of the α- and β-isoforms of DNA topoisomerase II is qualitatively different in interphase and mitosis in Chinese hamster ovary cells. Biochemistry. 1994;33:14651–14655. doi: 10.1021/bi00253a001. [DOI] [PubMed] [Google Scholar]
- 7.Burden D A, Osherhoff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:139–154. doi: 10.1016/s0167-4781(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas M E, Dang Q, Glover C V C, Gasser S M. Casein kinase II phosphorylates the eukaryote-specific C-terminal domain of topoisomerase II in vivo. EMBO J. 1992;11:1785–1796. doi: 10.1002/j.1460-2075.1992.tb05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbett A H, DeVore R F, Osheroff N. Effect of casein kinase II-mediated phosphorylation on the catalytic cycle of topoisomerase II. J Biol Chem. 1992;267:20513–20518. [PubMed] [Google Scholar]
- 10.Corbett A H, Fernald A W, Osheroff N. Protein kinase C modulates the catalytic activity of topoisomerase II by enhancing the rate of ATP hydrolysis: evidence for a common mechanism of regulation by phosphorylation. Biochemistry. 1993;32:2090–2097. doi: 10.1021/bi00059a029. [DOI] [PubMed] [Google Scholar]
- 11.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 12.Denko N C, Giaccia A J, Stringer J R, Stambrook P J. The human Ha-ras oncogene induces genomic instability in murine fibroblasts within one cell cycle. Proc Natl Acad Sci USA. 1994;91:5124–5128. doi: 10.1073/pnas.91.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVore R F, Corbett A H, Osheroff N. Phosphorylation of topoisomerase II by casein kinase II and protein kinase C: effects on enzyme-mediated DNA cleavage/religation and sensitivity to the antineoplastic drugs etoposide and 4′-(9′-acridinylamino)methane-sulfon-m-anisidide. Cancer Res. 1992;52:2156–2161. [PubMed] [Google Scholar]
- 14.DiNardo S, Voelkel K, Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci USA. 1984;81:2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downes C S, Clarke D J, Mullinger A M, Giménez-Ablán J F, Creighton A M, Johnson R T. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature. 1994;372:467–470. doi: 10.1038/372467a0. [DOI] [PubMed] [Google Scholar]
- 16.Earnshaw W C, MacKay A M. Role of nonhistone proteins in the chromosomal events of mitosis. FASEB J. 1994;8:947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- 17.Englund P T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978;14:157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- 18.Froelich-Ammon S J, Osheroff N. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem. 1995;270:21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- 19.Fukasawa K, Vande Woude G F. Synergy between the Mos/mitogen-activated protein kinase pathway and loss of p53 function in transformation and chromosome instability. Mol Cell Biol. 1997;17:506–518. doi: 10.1128/mcb.17.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heck M M S, Hittelman W N, Earnshaw W C. In vivo phosphorylation of the 170-kDa form of eukaryotic DNA topoisomerase II. J Biol Chem. 1989;264:15161–15164. [PubMed] [Google Scholar]
- 21.Isaacs R J, Davies S L, Sandri M I, Redwood C, Wells N J, Hickson I D. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 22.Ishida R, Hamatake M, Wasserman R A, Nitiss J L, Wang J C, Andoh T. DNA topoisomerase II is a molecular target of bisdioxopiperazine derivatives ICRF-159 and ICRF-193 in Saccharomyces cerevisiae. Cancer Res. 1995;55:2299–2303. [PubMed] [Google Scholar]
- 23.Khokhlatchev A V, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb M H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 24.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura K, Saijo M, Ui M, Enomoto T. Identification of the nature of modification that causes the shift of DNA topoisomerase IIβ to apparent higher molecular weight forms in the M phase. J Biol Chem. 1994;269:24523–24526. [PubMed] [Google Scholar]
- 26.Kimura K, Saijo M, Tanaka M, Enomoto T. Phosphorylation-independent stimulation of DNA topoisomerase IIα activity. J Biol Chem. 1996;271:10990–10995. doi: 10.1074/jbc.271.18.10990. [DOI] [PubMed] [Google Scholar]
- 27.Kimura K, Nozaki N, Enomoto T, Tanaka M, Kikuchi A. Analysis of M phase-specific phosphorylation of DNA topoisomerase II. J Biol Chem. 1996;271:21439–21445. doi: 10.1074/jbc.271.35.21439. [DOI] [PubMed] [Google Scholar]
- 28.Kingma P S, Greider C A, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 29.Koo H-M, Monks A, Mikheev A, Rubinstein L V, Gray-Goodrich M, McWilliams M J, Alvord W G, Oie H K, Gazdar A F, Paull K D, Zarbl H, Vande Woude G F. Enhanced sensitivity to 1-β-d-arabinosfuranosylcytosine and topoisomerase II inhibitors in tumor cell lines harboring activated ras oncogenes. Cancer Res. 1996;56:5211–5216. [PubMed] [Google Scholar]
- 30.Kortenjann M, Thomae O, Shaw P E. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994;14:4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouysségur J. Cyclin D1 expression is regulated positively by p42/p44mapk and negatively by the p38/HOGmapk pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 32.Lenormand P, Brondello J M, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 34.Luke M, Bogenhagen D F. Quantitation of type II topoisomerase in oocytes and eggs of Xenopus laevis. Dev Biol. 1989;136:459–468. doi: 10.1016/0012-1606(89)90271-6. [DOI] [PubMed] [Google Scholar]
- 35.Mansour S J, Candia J M, Matsuura J E, Manning M C, Ahn N G. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochemistry. 1996;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- 36.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 37.Mansour S J, Resing K A, Candia J M, Hermann A S, Gloor J W, Herskind K R, Wartmann M, Davis R J, Ahn N G. Mitogen-activated protein (MAP) kinase phosphorylation of MAP kinase kinase: determination of phosphorylation sites by mass spectrometry and site-directed mutagenesis. J Biochem. 1994;116:304–314. doi: 10.1093/oxfordjournals.jbchem.a124524. [DOI] [PubMed] [Google Scholar]
- 38.Meyer K N, Kjelden E, Straub T, Knudsen B R, Hickson I D, Kikuchi A, Kreipe H, Boege F. Cell cycle-coupled relocation of types I and II topoisomerases and modulation of catalytic enzyme activities. J Cell Biol. 1997;136:755–788. doi: 10.1083/jcb.136.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nitiss J L. Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochim Biophys Acta. 1998;1400:63–81. doi: 10.1016/s0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 40.Osheroff N, Shelton E R, Brutlag D L. DNA topoisomerase II from Drosophila melanogaster. J Biol Chem. 1983;258:9536–9543. [PubMed] [Google Scholar]
- 41.Qiu R G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 42.Rattner J B, Hendzel M J, Furbee C S, Muller M T, Bazett-Jones D P. Topoisomerase IIα is associated with the mammalian centromere in a cell cycle- and species-specific manner and is required for proper centromere/kinetochore structure. J Cell Biol. 1996;134:1097–1107. doi: 10.1083/jcb.134.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redwood C, Davies S L, Wells N J, Fry A M, Hickson I D. Casein kinase II stabilizes the activity of human topoisomerase IIα in a phosphorylation-independent manner. J Biol Chem. 1998;273:3635–3642. doi: 10.1074/jbc.273.6.3635. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytokeleton by ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 45.Sahyoun N, Wolf M, Besterman J, Hsieh T-S, Sander M, LeVine III H, Chang K-J, Cuatrecasas P. Protein kinase C phosphorylates topoisomerase II: topoisomerase activation and its possible role in phorbol ester-induced differentiation of HL-60 cells. Proc Natl Acad Sci USA. 1986;83:1603–1607. doi: 10.1073/pnas.83.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saijo M, Ui M, Enomoto T. Growth state and cell cycle dependent phosphorylation of DNA topoisomerase II in Swiss 3T3 cells. Biochemistry. 1992;31:359–363. doi: 10.1021/bi00117a007. [DOI] [PubMed] [Google Scholar]
- 47.Saitoh Y, Laemmli U K. Metaphase chromosome structure: bands arise from a differential folding path of the highly AT-rich scaffold. Cell. 1994;76:609–622. doi: 10.1016/0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro P S, Vaisberg E, Hunt A J, Tolwinski N S, Whalen A M, McIntosh J R, Ahn N G. Activation of the MKK/ERK pathway during somatic cell mitosis. Direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;142:1533–1545. doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shelton E R, Osheroff N, Brutlag D L. DNA topoisomerase II from Drosophila melanogaster. J Biol Chem. 1983;258:9530–9535. [PubMed] [Google Scholar]
- 50.Sif S, Stukenberg P T, Kirschner M W, Kingston R E. Mitotic inactivation of a human SWI/SNF chromatin remodeling complex. Genes Dev. 1998;12:2842–2851. doi: 10.1101/gad.12.18.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taagepera S, Rao P N, Drake F H, Gorbsky G J. DNA topoisomerase IIα is the major chromosome protein recognized by the mitotic phosphoprotein antibody MPM-2. Proc Natl Acad Sci USA. 1993;90:8407–8411. doi: 10.1073/pnas.90.18.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassetzky Y S, Dang Q, Benedetti P, Gasser S M. Topoisomerase II forms multimers in vitro: effects of metals, β-glycerophosphate, and phosphorylation of its C-terminal domain. Mol Cell Biol. 1994;14:6962–6974. doi: 10.1128/mcb.14.10.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vouret-Craviari V, Van Obberghen-Schilling E, Scimeca J C, Van Obberghen E, Pouysségur J. Differential activation of p44mapk (ERK1) by alpha-thrombin and thrombin-receptor peptide agonist. Biochem J. 1993;289:209–214. doi: 10.1042/bj2890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 56.Wells N J, Addison C M, Fry A M, Ganapathi R, Hickson I D. Serine 1524 is a major site of phosphorylation on human topoisomerase IIα protein in vivo and is a substrate for casein kinase II in vitro. J Biol Chem. 1994;269:29746–29751. [PubMed] [Google Scholar]
- 57.Wells N J, Hickson I D. Human topoisomerase IIα is phosphorylated in a cell-cycle phase-dependent manner by a proline-directed kinase. Eur J Biochem. 1995;231:491–497. doi: 10.1111/j.1432-1033.1995.tb20723.x. [DOI] [PubMed] [Google Scholar]
- 58.Whalen A M, Galasinski S C, Shapiro P S, Nahreini T S, Ahn N G. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright, J. H., E. Munar, P. Andreassen, R. Margolis, R. Seger, and E. G. Krebs. Unpublished data.
- 60.Zecevic M, Catling A D, Eblen S T, Renzi L, Hittle J C, Yen T J, Gorbsky G J, Weber M J. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J Cell Biol. 1998;142:1547–1558. doi: 10.1083/jcb.142.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]