Abstract
Background:
Treatment of children and adolescents with alveolar rhabdomyosarcoma (ARMS) and regional nodal involvement (N1) have been approached differently by North American and European cooperative groups. In order to define the better therapeutic strategy, we analyzed two studies conducted between 2005 and 2016 by the European paediatric Soft tissue sarcoma Study Group (EpSSG) and Children’s Oncology Group (COG).
Methods:
We retrospectively identified patients with ARMS-N1 enrolled in either EpSSG RMS2005 or in COG ARST0531. Chemotherapy in RMS2005 comprised IVADo (ifosfamide, vincristine, dactinomycin, doxorubicin), IVA and maintenance (vinorelbine, cyclophosphamide); in ARST0531 it consisted on either VAC (vincristine, dactinomycin, cyclophosphamide) or VAC alternating with VI (vincristine, irinotecan). Local treatment was similar in both protocols.
Results:
The analysis of the clinical characteristics of 239 patients showed some differences between study groups: in RMS2005, advanced IRS Group and large tumors predominated. There were no differences in outcomes between the two groups: 5-year event-free survival (EFS), 49%(95%CI=39–59) and 44%(95%CI=30–58), and overall survival (OS), 51%(95%CI=41–61) and 53.6%(95%CI=40–68), in RMS2005 and ARST0531, respectively. In RMS2005, EFS of patients with FOXO1-positive tumors was significantly inferior to those FOXO1-negative (49.3% vs 73%, p=0.034). In contrast, in ARST0531, EFS of patients with FOXO1-positive tumors was 45% compared with 43.8% for those FOXO1-negative.
Conclusions:
The outcome of patients with ARMS N1 was similar in both protocols. However, patients with FOXO1 fusion-negative tumors enrolled in RMS2005 showed a significantly better outcome, suggesting that different strategies of chemotherapy may have an impact in the outcome of this subgroup of patients.
Keywords: rhabdomyosarcoma, alveolar rhabdomyosarcoma, nodal involvement, prognostic factors, chemotherapy
1. INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and adolescents and comprises two major histologic subtypes: alveolar RMS (ARMS) and embryonal RMS (ERMS) [1–3].
The prognosis of RMS has improved considerably over time due to numerous clinical trials conducted by collaborative groups working in North America: Intergroup Rhabdomyosarcoma Study Group (IRS) and Children’s Oncology Group (COG), and in Europe: International Society of Paediatric Oncology-Malignant Mesenchymal Tumour Group (SIOP-MMT), Italian Soft Tissue Sarcoma Committee-STSC, Gesellschaft Cooperative Soft Tissue Sarcoma Study Group-CWS and more recently, EpSSG (European paediatric Soft tissue sarcoma Study Group). Despite this successful history, the improvement in the prognosis of patients with RMS has not been uniform. While the probability of cure in pediatric patients with localized disease is over 70%, the prognosis of those with distant metastatic disease remains poor [4–8], and the presence of disseminated disease at diagnosis continues to be the most powerful prognostic factor in this neoplasm.
For patients with localized RMS, clinical and tumor characteristics are used to classify RMS in different risk categories and to determine treatment intensity. These characteristics include histology, tumor invasiveness, tumor location, nodal involvement, tumor size and patient age [9–11] and they constitute the basis for the risk stratification system used in North America by COG and in Europe by EpSSG. Patients with ARMS and regional lymph node involvement represent approximately 5–10% of all cases of RMS in children and adolescents. Previous experience suggested that these patients represent a group with particularly poor prognosis [9].
In the EpSSG RMS2005 study, we stratified these patients into a very high risk (VHR) category and treated them with an intensified regimen of chemotherapy that included doxorubicin added to the standard schema of IVA (ifosfamide, vincristine, and dactinomycin) in the first 4 cycles, followed by 5 cycles of IVA and six cycles of intravenous vinorelbine and daily oral cyclophosphamide [12]. During the same period, COG conducted the ARST0531 study for intermediate risk RMS patients that included those with non-metastatic ARMS at any primary site without distant metastases; these patients were randomly assigned to receive the standard schema VAC (vincristine, dactinomycin, and cyclophosphamide) or VAC alternating with VI (vincristine, irinotecan) for 42 weeks [13].
Herein we report the results of a combined analysis of patients with ARMS and regional nodal involvement enrolled in the aforementioned studies, and we compare these results to determine which therapy optimizes survival.
2. PATIENTS AND METHODS
2.1. Patients
For the purpose of this analysis eligible patients were those with:
Alveolar rhabdomyosarcoma confirmed by histology and
Regional nodal involvement defined by clinical or radiological or pathologic criteria, without distant metastases, and
Enrolled in protocol EpSSG RMS2005, between November 1, 2005 and December 31, 2016 or
Enrolled in protocol COG ARST0531, between December 1, 2006 and December 31, 2012.
Date of data cutoff for the analysis were: Dec 31, 2019 for RMS2005 and Dec 31, 2018 for ARST0531.
2.2. Treatment
2.2.1. EpSSG RMS2005
Patients received intensified induction chemotherapy and additional maintenance chemotherapy with systematic local treatment to primary and nodal sites. Induction chemotherapy comprised four 21-day cycles of IVADo (ifosfamide 3 g/m2 on days 1–2 with MESNA, vincristine 1.5 mg/m2 [max dose 2 mg] on days 1, 8 and 15 in the first 2 cycles and on day 1 in cycles 3 and 4, dactinomycin 1.5 mg/m2 [max dose 2 mg] on day 1, and doxorubicin 30 mg/m2 on days 1–2) followed by five 21-day cycles of IVA and six 28-day cycles of maintenance chemotherapy comprising continuous daily oral cyclophosphamide 25 mg/m2 and intravenous vinorelbine 25 mg/m2 on days 1, 8 and 15 of each cycle. The total duration of chemotherapy was 50 weeks.
Response was evaluated at weeks 9, 27 and at the end of treatment. If free margins were achievable without organ or functional impairment, local treatment after the initial 4 cycles of IVADo (week 13) included delayed surgical resection. External beam radiotherapy was administered to the primary tumor area and the affected lymph node region, delivered using a daily dose per fraction of 1.8 Gy. Doses varied according to chemotherapy response and surgical results. The total dose to the primary tumor following delayed surgery with complete resection was 41.4 Gy. For IRS Group III with incomplete resection, or when delayed surgery was not feasible, total dose was 50.4 Gy with an optional additional boost of 5.4 Gy in 3 fractions for large tumors with poor response to chemotherapy. Radiotherapy (RT) was recommended for involved lymph nodes at a dose of 41.4 Gy regardless of the extent of surgical resection.
2.2.2. COG ARST531
Patients were randomly assigned to receive either VAC (vincristine 1.5 mg/m2 [max dose 2 mg], dactinomycin 0.045 mg/kg [max dose 2.5 mg], cyclophosphamide 1.2 g/m2 with MESNA) or VAC alternating with VI (vincristine 1.5 mg/m2, irinotecan 50 mg/m2 on 5 consecutive days) intravenously. During the first 12 weeks, the two treatment arms were identical in duration and schedule, with the exception of substituting irinotecan for dactinomycin and cyclophosphamide at week 4 and for cyclophosphamide at week 7 in VAC/VI. During the subsequent 30 weeks of therapy, irinotecan replaced dactinomycin and cyclophosphamide at weeks 16, 19, 25, 31, and 37 in VAC/VI. The schedule of vincristine differed slightly between the two treatment regimens, allowing for its administration during the weeks that followed all courses of irinotecan, but the total number of vincristine doses was the same in both regimens. The total duration of chemotherapy was 42 weeks.
Patients were evaluated for response at weeks 15 and 30 and at the end of therapy. For patients older than 24 months, definitive RT was the planned local control modality. Delayed primary resection was allowed but not encouraged. For patients younger than 24 months, individualized local control approaches, including delayed primary excision and response-adapted RT, were permitted. RT started at week 4, and the dose was determined by clinical group and histology at study entry: IRS Group II ARMS with regional lymph node involvement, 41.4 Gy; IRS Group III ARMS with orbital primary site, 45 Gy; and non-orbital primary sites, 50.4 Gy. RT was delivered using megavoltage photon, proton, and/or electron beams. For tumors with a rapid substantial decrease in tumor size, a volume reduction by cone down after 36 Gy was permitted, particularly for tumors with pushing rather than infiltrating margins.
2.3. Pathology:
The definition of ARMS was similar in both protocols and based on the International Rhabdomyosarcoma Classification [14]. Both protocols excluded the focal alveolar histology and included the solid variant of alveolar RMS.
2.4. Nodal status:
In both protocols nodal involvement was evaluated by either clinical (cN1) or pathologic (pN1) criteria.
2.5. Statistical analysis
Survival time was calculated from the date of diagnosis to the time of event or last follow-up. Tumor progression, relapse, occurrence of second malignancy, or death due to any cause were considered for event free survival (EFS). Overall survival (OS) was measured from the date of diagnosis to death from any cause. Patients still alive at the end of the study were censored at the date of the last observation. Survival probability was calculated by the Kaplan-Meier method and heterogeneity in survival among strata of selected variables was assessed with the Log-Rank test [15]. Five-year EFS and OS were reported along with their 95% confidence intervals (CI) as computed using the Peto-Peto method [16]. The Cox proportional hazards models were fit to compare EFS and OS while adjusting for potential confounders. Categorical variables were reported as counts and percentages and compared using Fisher’s Exact test. All data analyses were performed using the SAS statistical package (SAS, release 9.4; SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
The clinical characteristics of 154 patients enrolled in EpSSG RMS2005 and 85 patients enrolled in COG ARST0531 are presented in Table 1.
TABLE 1.
Characteristics of the 239 patients enrolled in the study
| ARST0531 No. (%) | RMS2005 No. (%) | P-value1 | |
|---|---|---|---|
| Total | 85 | 154 | |
| Sex | 1.0 | ||
| Female | 38 (44) | 68 (44) | |
| Male | 47 (56) | 86 (56) | |
| Age at diagnosis | 0.14 | ||
| 0–1 | 5 (6) | 3 (2) | |
| 1–10 | 34 (40) | 77 (50) | |
| 10+ | 46 (54) | 74 (48) | |
| Tumor size | 0.0029 | ||
| ≤5 cm | 40 (47) | 42 (27) | |
| >5 cm | 0 | 110 (71) | |
| Unknown | 45 (53) | 2 (2) | |
| Histology | 0.14 | ||
| Alveolar RMS | 75 (96) | 145 (94) | |
| Mixed Embryonal/Alveolar | 3 (4) | 3 (2) | |
| Solid Alveolar RMS | 0 | 6 (4) | |
| Nodal status (N1)* | 85 | 154 | |
| Clinical (cN1) | 0.21 | ||
| Positive | 81 (95) | 139 (90) | |
| Pathologic (pN1) | 0.0035 | ||
| Analyzed | 69 (81) | 77 (50) | |
| Positive | 51(74) | 71 (92) | |
| IRS Group | 0.0090 | ||
| I | 1 | 0 | (0.0027)2 |
| IIa | 1 | 0 | |
| IIb | 6 (7) | 5 (3.5) | |
| IIc | 9 (10) | 5 (3.5) | |
| III | 68 (80) | 144 (93) | |
| T-stage | 0.58 | ||
| T-1 | 30 (35) | 61 (40) | |
| T-2 | 55 (65) | 92 (60) | |
| Unknown | 0 | 1 | |
| FOXO1 fusion status | 0.64 | ||
| Negative | 17 (20) | 30 (19) | (0.73)3 |
| PAX3+ | 41 (48) | 55 (36) | |
| PAX7 + | 8 (9) | 11 (7) | |
| FOXO1 + | 13 (15) | 28 (18) | |
| Unknown | 6 (7) | 30 (19) | |
| Primary site | 0.83 | ||
| Orbit | 0 | 1 | |
| Head-neck non-PM | 11 (13) | 27 (17) | |
| Parameningeal (PM) | 29 (34) | 39 (25) | |
| Bladder-Prostate (BP) | 2 (2) | 5 (3) | |
| Genito-Urinary non-BP | 2 (2) | 4 (2) | |
| Extremities | 27 (32) | 54 (35) | |
| Other sites | 14 (16) | 24 (15) |
T1, tumor localized to the organ or tissue of origin; T2, tumor extending beyond the tissue of origin.
N1 defined by either clinical or pathologic criteria in both protocols
Fisher’s exact test
Comparing Group I, II (IIa+IIb+IIc) and III
Comparing FOXO1− and FOXO1+ (PAX3+PAX7+FOXO1)
Adverse prognostic factors including tumor size > 5 cm, advanced IRS Group and tumor location at unfavorable sites, predominated in both cohorts. Large tumors (>5 cm) were more frequent in EpSSG than in COG patients: 71% vs 53%, respectively, (p-value 0.0029). IRS Group III tumors were also more frequent in the EpSSG than in the COG cohort: 93% vs 80%, respectively, (p-value 0.0027). Tumor location at unfavorable sites (parameningeal, limbs, bladder-prostate, other sites) (79% in EpSSG and 84% in COG) and invasiveness (T-stage 2, 60% in EpSSG and 65% in COG), were similar in both cohorts.
Fusion status of tumors, determined by the presence of FOXO1 translocation (regardless of the PAX fusion partner), was analyzed in 81% of patients in EpSSG and in 93% in COG. FOXO1 translocation, when FOXO1 fusion status was known (76% in EpSSG and 79% in COG), was similar in both series.
Histological analysis of nodal involvement was performed less frequently (50%) in patients enrolled in EpSSG (nodal biopsy was not mandatory in RMS2005, except for tumors arising in extremities), as compared to COG (81%) (p-value 0.0035).
3.2. Treatment
Systemic and local treatment given to patients in both protocols are detailed in Table 2. One hundred and forty-five (145) patients who were enrolled in RMS2005 (94%) received chemotherapy according to protocol, 8 patients received IVA without doxorubicin, and 1 patient received IVA followed by VA. All patients included in ARST0531 received chemotherapy according to protocol: 44 received VAC and 41 VAC/VI.
Table 2.
Treatment details of patients by protocol
| ARST0531 Total=85 No. (%) | RMS2005 Total=154 No. (%) | P-value1 | |
|---|---|---|---|
| Chemotherapy | |||
| IVA | 0 | 8 (5) | |
| IVA/VA | 0 | 1 | |
| IVADo | 0 | 145 (94) | |
| VAC | 44 (52) | 0 | |
| VAC/VI | 41 (48) | 0 | |
| RT on primary tumor | 0.0081 | ||
| Not done | 3 (3) | 22* (14) | |
| Done | 82 (97) | 132 (86) | |
| RT on nodes | |||
| Not done | 3 (3) | 18* (11) | |
| Done | 82 (97) | 136 (89) | |
| RT_type | 0.25 | ||
| External photon | 73 (89) | 125 (92) | |
| Brachytherapy | 2 (2) | 0 | |
| Proton | 7 (8) | 8 (6) | |
| External PT, brachy N | 0 | 1 | |
| External N, brachy PT | 0 | 2 (1) | |
| Resection of primary tumor | 0.30 | ||
| Primary or delayed | 22 (26) | 75 (49) | |
| R0 | 20 (91) | 55 (73) | |
| R1 | 2 (9) | 14 (18) | |
| R2 | 0 | 6 (8) | |
Fisher’s exact test
4 on nodes only, 18 did not receive RT: 6 early progression, 2 amputation, 1 very young, 2 parent refusal, 7 center decision
Radiotherapy to the site of primary tumor and nodes was administered in 132 (86%) and 82 (97%) patients enrolled in RMS2005 and ARST0531, respectively (Table 2). A significantly higher proportion of patients in EpSSG did not receive RT for the following reasons: parent refusal (2), early progression (6), amputation (2), very young age (1) and treatment center decision (7). Four patients received nodal RT only. The most common type of radiotherapy in both series was photon external beam, and a minority of patients in both protocols received proton beam radiotherapy.
In the EpSSG cohort, 75 out of 154 patients (49%) underwent surgical resection of the primary tumor: up-front primary resection in 10/75 (13%) and delayed primary resection in 65/75 (87%). Results of surgery included complete local resection (R0) in 55 (73%), microscopic residual disease (R1) in 14 (19%), and macroscopic residual (R2) in 6 (8%). In the COG cohort, 22 out of 85 patients (26%) underwent surgical resection of the primary tumor: up-front primary resection in 17/22 (77%) and delayed primary resection in 5/22 (23%). R0 was achieved in 20 (91%) and R1 in 2 (9%).
3.3. Outcome and prognostic factors
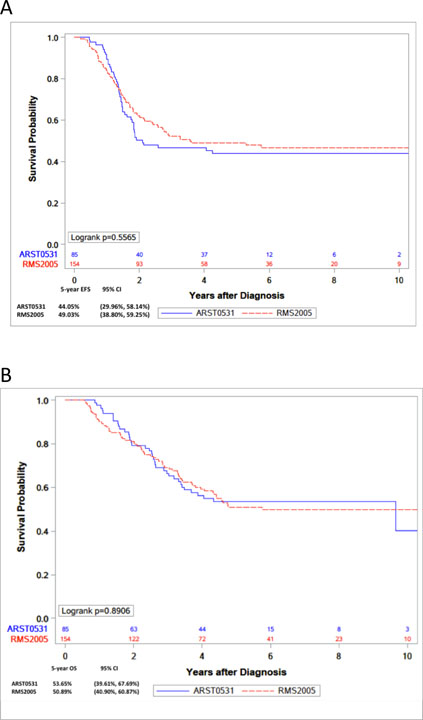
The median follow-up of patients was 5.2 years in COG study and 5.4 years in EpSSG. At the time of the analysis, 45 patients in the COG cohort had relapsed, of whom, 38 died (37 by disease progression and 1 by an unrelated disease). Seventy-eight patients in the EpSSG cohort had relapsed, 69 of whom died (66 by disease progression, 2 by toxic deaths and 1 by a second neoplasm). Outcomes of patients (EFS and OS) included in the study are detailed in Table 3. The 5-year EFS of the COG cohort was 44% (95% CI: 30.0–58.1) compared with 49% (95% CI 38.8–59.3) of the EpSSG, The 5-year OS was 53.6% in COG (95% CI 39.6–67.7) and 51% in EpSSG (95% CI 40.9–60.9) (Figure 1). There were no differences in EFS or OS between the analytic cohort of RMS2005 and ARST0531. These results held when EFS (p=0.2) and OS (p=0.28) were compared while adjusting for tumor size, nodal status and IRS Group (I/II vs III). In the combined analysis of both cohorts, outcomes by FOXO1 fusion status showed 5-year EFS of 63% for patients with fusion-negative tumors (95% CI 44.6–81.0) vs 47.6% for fusion-positive (95% CI 37.2–58.1). The 5-year OS for patients with fusion-negative tumors was 69% (95% CI 51.7–86.3) compared with 52.7% (95% CI 42.6–62.9), for fusion-positive.
Table 3.
Five-year EFS and OS estimate with 95% CI by protocol
| 5-year EFS (95% CI) | 5-year OS (95% CI) | ||
|---|---|---|---|
| Cohort RMS2005 | 49% (38.80%–59.25%) | 51% (40.90%–60.87%) | |
| Cohort ARST0531 | 44% (29.96%–58.14%) | 53.6% (39.61%–67.69%) | |
| FOXO1 fusion negative (cohorts combined) | 63% (44.61%–81.03%) | 69% (51.71%–86.26%) | |
| FOXO1 fusion positive (cohorts combined) | 47.6% (37.21%–58.06%) | 52.7% (42.62%–62.93%) | |
| FOXO1 fusion status for RMS2005 | Negative | 73 % (53.00%–92.76%) | 76% (57.22%–94.89%) |
| Positive | 49.3 % (35.80%–62.79%) | 51.6% (38.57%–64.71%) | |
| FOXO1 fusion status for ARST0531 | Negative | 43.8% (6.62%–80.88%) | 56.2% (19.79%–92.71%) |
| Positive | 45% (28.76%–61.53%) | 53.7% (37.74%–69.79%) | |
| Primary site (cohorts combined) | Extremities | 38% (23.77%–52.22%) | 50% (35.87%–64.17%) |
| Parameningeal | 46.5% (30.79%–62.22%) | 47.4% (31.51%–63.27%) | |
| All other sites | 55.5% (42.51%–68.58%) | 57% (44.19%–69.69%) | |
Figure 1.
Kaplan-Meier curves representing (A) 5-year EFS and (B) 5-year OS, by protocol.
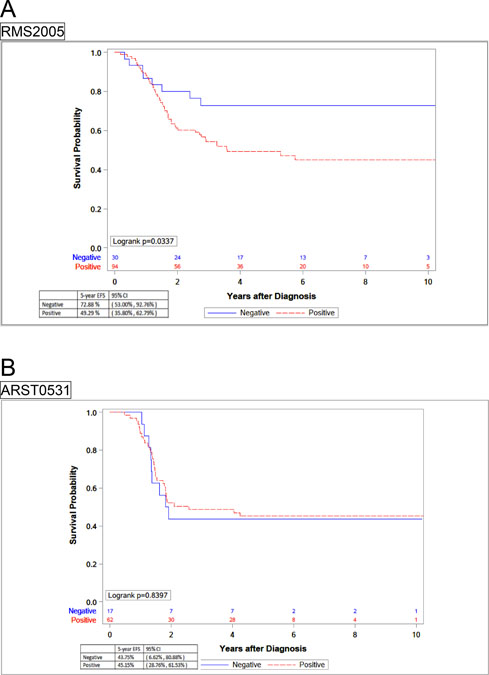
However, results from each cohort showed striking differences on the impact of the presence of FOXO1 fusion on prognosis. In EpSSG, the 5-year EFS of patients with FOXO1 fusion-positive tumors (49.3%, 95% CI 35.8–62.8) was significantly inferior to those with FOXO1 fusion-negative ones (73%, 95% CI 53.0–92.8) (p=0.034). In contrast, COG patients showed no differences between these two groups: The EFS of COG patients with FOXO1 fusion-positive tumors was 45.1% (95% CI 28.8–61.5) compared with 43.8% (95% CI 6.6–80.9%) of FOXO1 fusion-negative (Figure 2).
Figure 2.
Kaplan-Meier curves representing 5-year EFS by fusion status in separated cohorts, (A) EpSSG RMS2005 and (B) COG ARST0531.
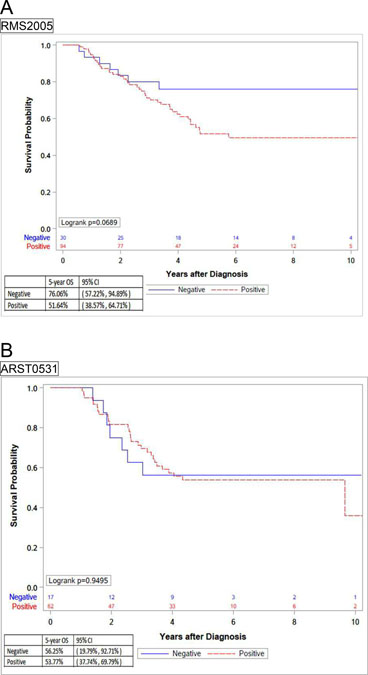
Five-year OS of patients with FOXO1 fusion-positive tumors in the EpSSG cohort was 51.6%, compared with 76% of FOXO1 fusion-negative and approached significance (p=0.069). In contrast, there were no differences in the outcomes between COG patients with fusion-positive or fusion-negative tumors (53.7% and 56.2%, respectively) (Figure 3).
Figure 3.
Kaplan-Meier curves representing 5-year OS by fusion status in separated cohorts (A) EpSSG RMS2005 and (B) COG ARST0531.
The clinical characteristics of patients with fusion-negative tumors (size, site, age and pathologic nodal status) did not differ between both groups (Supplementary Table S1).
The pooled COG and EpSSG 5-year EFS and OS did not show statistically significant differences by location of primary tumor.
The site of the first relapse was also similar in both cohorts (Supplementary Table S2). Metastatic relapses with or without local recurrence represented half of the relapses in both protocols. Loco-regional relapses represented 35% and 29% of all recurrences in COG and EpSSG cohorts, respectively. Tumor progression during treatment occurred in 13% of the patients in COG and 18% in EpSSG.
4. DISCUSSION
The combined analysis of the results of these two cooperative studies suggests that different strategies of chemotherapy may have an impact in the outcome of a subgroup of patients with ARMS and regional nodal involvement.
The prognosis of patients with ARMS N1 has been reported to be poor in the historical series of European co-operative studies. In the CWS-86 study, 3-year EFS was 25% and OS 29% [11]. In the SIOP MMT84 study, 5-year EFS was 31% comparable to that of stage IV disease [17].
The experience of similar patients in North American cooperative groups has been better than that of the European studies. Of 125 patients with localized RMS and nodal involvement enrolled in the IRS-IV study, Rodeberg et al [18] reported 43% five-year failure-free survival of patients with ARMS and nodal involvement. The overall outcome of patients with alveolar histology and N1 disease reported in that study was similar to that of patients with metastatic disease at a single site.
We recently reported the experience of EpSSG RMS2005 [12] in patients with ARMS N1 and showed results similar to the IRS-IV study. The reasons for this apparent improvement in the outcome of these patients, compared to those treated within previous European studies, could be due in part to better risk stratification, more adequate treatment with intensified chemotherapy, systematic local treatment and/or improvement in supportive care.
Failures among patients reported in the present study were predominantly with metastatic disease and was similar in both cohorts. Their tumors frequently presented with advanced IRS Group and unfavorable location, characteristics related to an increased risk of distant metastatic disease [19–21]. Local recurrences represented one-third of all relapses in both protocols and local treatment in RMS2005 and in ARST0531 was identical. However, Casey et al [22] reported more loco-regional relapses in patients enrolled in ARST0531 compared with the previous COG intermediate-risk RMS study, D9803. The reasons for this increase in local relapses were not clear but may have been related to changes in cyclophosphamide dosing, changes in RT administration and fewer surgical procedures.
Nodal involvement (N1) was defined in both protocols either by clinical or pathologic criteria. Although histologic analysis was performed more frequently in patients of the COG cohort, pathologic evaluation of sampled lymph nodes was negative in a significant number of patients of both protocols. These discrepancies between clinical (cN1) and pathologic (pN1) involvement has been reported in patients with different types of soft tissue sarcomas reflecting variability in the obtaining nodal samples, i.e. fine needle aspiration, surgical excision, lymphadenectomy, or removal of peri-tumoral lymph nodes during resection of the primary tumor [23].
The most important difference between EpSSG and COG protocols was the type of chemotherapy. Patients received three different regimens based on the use of the standard combination (IVA in EpSSG and VAC in COG) to which was added doxorubicin and 6 months of maintenance chemotherapy with vinorelbine and cyclophosphamide in the European study or irinotecan in the North American study.
In RMS2005, doses of alkylating agents included 54 g/m2 of ifosfamide plus 4.5 g/m2 of cyclophosphamide. COG patients received either 16.8 g/m2 (VAC) or 8.4 g/m2 of cyclophosphamide (VAC/VI). The cumulative doses of alkylators were similar in the EpSSG cohort (equivalent to 17.5 g/m2 of cyclophosphamide) and COG cohort in the VAC arm, and twice the dose in the VAC/VI arm [24].
Moreover, patients in EpSSG received doxorubicin at a total dose of 240 mg/m2. One-half of the patients in COG received irinotecan. Total duration of chemotherapy was 42 weeks in COG protocol vs 50 weeks in EpSSG.
There is no evident advantage of adding doxorubicin or irinotecan to the standard chemotherapy in these patients. Hawkins et al [13] have previously reported a lack of improvement with the addition of irinotecan to VAC in the same of group of patients as the present analysis. The European experience demonstrated that the addition of doxorubicin to IVA in patients with high-risk disease also failed to improve outcome when compared to standard chemotherapy [25]. Moreover, previous experiences in Europe demonstrated that the addition of carboplatin, etoposide, and epirubicin did not improve the outcome for patients with high-risk disease enrolled in the SIOP MMT-95 study [8]. In RMS2005, two toxic deaths and one secondary neoplasm occurred, so that when designing future studies, we consequently should search for a balance between the total burden of chemotherapy and the risk of toxicity.
Numerous studies have reported the impact of fusion status on the outcome of patients with ARMS and suggested that the presence of PAX3/7-FOXO1 fusion genes have prognostic significance [26–28]. However, this impact could be different in patients with other adverse prognostic factors. For example, in patients classified as low or intermediate risk in COG studies, the presence of FOXO1 fusion has a strong impact in prognosis [28,29], while in patients with metastatic disease, the clinical prognostic factors have a stronger impact than the fusion status [30]. Hibbitts, et al recently reported the results of a survival tree regression in a large group of RMS patients treated in 6 different COG trials, and demonstrated that after metastatic status, FOXO1 fusion status is the most important prognostic factor. They concluded that these results support the incorporation of fusion status in future risk-stratified trials [31]. Similarly, as proposed in our previous report [12], fusion status has been incorporated into the risk stratification of patients participating in the ongoing EpSSG protocol. Patients with fusion-negative N1 RMS are assigned to the high-risk group, while patients with fusion-positive N1 RMS are kept as very-high-risk group and treated with a strategy similar to that of metastatic patients.
The impact of FOXO1 fusion status in the outcome of patients in both cohorts showed important differences. In the European study, fusion-positive tumors had significantly worse EFS as well as a trend toward inferior OS, while the EFS/OS of patients enrolled in the COG study was the same whether they had fusion-positive or fusion-negative tumors. These contradictory results between our two homogeneous cohorts are intriguing.
We can hypothesize that those patients with fusion-negative tumors benefit from a more intense chemotherapy. Patients in ARST0531 received a lower cumulative dose of cyclophosphamide than in previous COG RMS studies, with a possible negative impact on outcome [13]. In the European study the EFS of patients with fusion-negative tumors was 73%, similar to those of high-risk patients enrolled in this protocol who received maintenance chemotherapy [25]. However, in the cohort of patients analyzed in this report, the addition of maintenance chemotherapy was not randomized, limiting our ability to determine its contribution to the overall EpSSG strategy.
Our study presents some limitations, such as the relatively small number of patients with fusion-negative tumors in both cohorts, and the higher proportion of patients with fusion status unknown in the EpSSG cohort, which could reduce the precision of the EFS estimations.
In conclusion, there are some lessons learned from the present combined analysis that should be further explored in the upcoming studies of both cooperative groups: First, very different treatment strategies in two concurrent clinical trials generated similar outcomes for patients with ARMS and regional lymph node involvement. Second, among patients with FOXO1 fusion-negative ARMS and regional lymph node involvement, differences in chemotherapy between both groups may influence outcome and, third, a need persists for innovative therapeutic strategies for those patients with fusion-positive tumors.
Supplementary Material
Acknowledgements
We are indebted to all children and families that participated in the trials.
Funding sources:
Children’s Oncology Group Grants U10CA180886, U10CA180899, U10CA098543, and U10CA098413 and St. Baldrick’s Foundation; EpSSG funding: Fondazione Città della Speranza.
Abbreviations:
- ARMS
alveolar rhabdomyosarcoma
- COG
Children’s Oncology Group
- EFS
event-free survival
- EpSSG
European paediatric Soft tissue sarcoma Study Group
- ERMS
embryonal rhabdomyosarcoma
- IRS
Intergroup Rhabdomyosarcoma Study
- OS
overall survival
- MMT
Malignant Mesenchymal Tumour
- RMS
rhabdomyosarcoma
- VHR
very high risk
Footnotes
Potential conflicts of interest: The author(s) indicated no potential conflicts of interest.
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data available on request from the authors The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Skapek SX, Ferrari A, Gupta AA et al. Rhabdomyosarcoma. Nat Rev Dis Primers 2019;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raney RB, Anderson JR, Barr FG et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of Intergroup Rhabdomyosarcoma Study Group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol 2001;23: 215–220. [DOI] [PubMed] [Google Scholar]
- 3.Sultan I, Qaddoumi I, Yaser S, et al. : Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2,600 patients. J Clin Oncol 2009;27:3391–3397. [DOI] [PubMed] [Google Scholar]
- 4.Carli M, Colombatti R, Oberlin O, et al. : European intergroup studies (MMT4–89 and MMT4–91) on childhood metastatic rhabdomyosarcoma: Final results and analysis of prognostic factors. J Clin Oncol 2004;22:4787–4794. [DOI] [PubMed] [Google Scholar]
- 5.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol 2008;26:2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Arush M, Minard-Colin V, Mosseri V, et al. Does aggressive local treatment have an impact on survival in children with metastatic rhabdomyosarcoma? Eur J Cancer 2015;51:193–201. [DOI] [PubMed] [Google Scholar]
- 7.Weigel BJ, Lyden E, Anderson JR, et al. Intensive multiagent therapy, including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation, in patients with high-risk rhabdomyosarcoma: A report from the Children’s Oncology Group J Clin Oncol 2016;34:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberlin O, Rey A, Sanchez de Toledo J, et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: long-term results from the International Society of Pediatric Oncology MMT95 study. J Clin Oncol 2012;30:2457–2465. [DOI] [PubMed] [Google Scholar]
- 9.Meza JL, Anderson J, Pappo AS, et al. : Analysis of prognostic factors in patients with non-metastatic rhabdomyosarcoma treated on Intergroup Rhabdomyosarcoma studies III and IV: The Children’s Oncology Group. J Clin Oncol 2006;24:3844–3851. [DOI] [PubMed] [Google Scholar]
- 10.Joshi D, Anderson JR, Paidas C, et al. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer 2004;42:64–73. [DOI] [PubMed] [Google Scholar]
- 11.Neville HL, Andrassy RJ, Lobe TE, et al. Preoperative staging, prognostic factors, and outcome for extremity rhabdomyosarcoma: a preliminary report from the Intergroup Rhabdomyosarcoma Study IV (1991–1997). J Pediatr Surg 2000;35:317–321. [DOI] [PubMed] [Google Scholar]
- 12.Gallego S, Zanetti I, Orbach D, et al. Fusion Status in patients with lymph node positive (N1) alveolar rhabdomyosarcoma is a powerful predictor of prognosis: Experience of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer 2018;124:3201–3209. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins DS, Chi YY, Anderson JR, et al. Addition of vincristine and irinotecan to vincristine, dactinomycin, and cyclophosphamide does not improve outcome for intermediate-risk rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol 2018;36:2770–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification – an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–1085. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn 1958;53:457–481. [Google Scholar]
- 16.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. Journal of the Royal Statistical Society A 1972; Series A135: 185–207. [Google Scholar]
- 17.Flamant F, Rodary C, Rey A, et al. Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescence. Results of the second study of the International Society of Paediatric Oncology MMT84. Eur J Cancer 1998;34:1050–1056. [DOI] [PubMed] [Google Scholar]
- 18.Rodeberg DA, Garcia-Henriquez N, Lyden ER et al. Prognostic significance and tumor biology of regional lymph node disease in patients with rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol 2011;29:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss AR, Lyden ER, Anderson JR, et al. : Histologic and clinical characteristics can guide staging evaluations for children and adolescents with rhabdomyosarcoma: A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. J Clin Oncol 2013;31:3226–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol 2008;26:2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chisholm J, Marandet J, Rey A, et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: A nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol 2011;29:1319–25 [DOI] [PubMed] [Google Scholar]
- 22.Casey DL, Chi YY, Donaldson SS, et al. Increased local failure for patients with Intermediate-risk rhabdomyosarcoma on ARST0531: A report from the Children’s Oncology Group. Cancer 2019;125:3242–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keung EZ, Chiang YJ, Voss R, et al. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur J Surg Oncol 2018;44:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure. A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisogno G, Jenney M, Bergeron C, et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol 2018; 19: 1061–1071. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen PH, Lynch JC, Qualman SJ, et al. : PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the Children’s Oncology Group. J Clin Oncol 2002;20:2672–2679. [DOI] [PubMed] [Google Scholar]
- 27.Missiaglia E, Williamson D, Chisholm J, et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J Clin Oncol 2012;30:1670–1677. [DOI] [PubMed] [Google Scholar]
- 28.Skapek SX, Anderson J, Barr FG, et al. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: A Children’s Oncology Group report. Pediatr Blood Cancer 2013;60:1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold MA, Anderson JR, Gastier-Foster JM, et al. Histology, Fusion Status and Outcome in Alveolar Rhabdomyosarcoma with Low-Risk Clinical Features: A Report from the Children’s Oncology Group. Pediatr Blood Cancer 2016; 63: 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudzinski ER, Anderson JR, Chi YY, et al. Histology, fusion status, and outcome in metastatic rhabdomyosarcoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer 2017; 64:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hibbitts E, Chi YY, Hawkins DS, et al. Refinement of risk stratification for childhood rhabdomyosarcoma using FOXO1 fusion status in addition to established clinical outcome predictors: A report from the Children’s Oncology Group. Cancer Med 2019;8:6437–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisogno G, De Salvo GL, Bergeron C, et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2019; 20: 1566–1575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.