Abstract
Background. Exercise is increasingly becoming recognized as an important adjunct to medications in the clinical management of Parkinson’s disease (PD). Boxing and sensory exercise have shown immediate benefits, but whether they continue beyond program completion is unknown. This study aimed to investigate the effects of boxing and sensory training on motor symptoms of PD, and whether these benefits remain upon completion of the intervention. Methods. In this 20-week double-blinded randomized controlled trial, 40 participants with idiopathic PD were randomized into 2 treatment groups, (n = 20) boxing or (n = 20) sensory exercise. Participants completed 10 weeks of intervention. Motor symptoms were assessed at (week 0, 10, and 20) using the Unified Parkinson’s Disease Rating Scale (UPDRS-III). Data were analyzed using SPSS, and repeated-measures ANOVA was conducted. Results. A significant interaction effect between groups and time were observed F(1, 39) = 4.566, P = .036, where the sensory group improved in comparison to the boxing group. Post hoc analysis revealed that in comparison to boxing, the effects of exercise did not wear off at washout (week 20) P < .006. Conclusion. Future rehabilitation research should incorporate similar measures to explore whether effects of exercise wear off post intervention.
Keywords: rehabilitation, boxing, Parkinson’s disease SAFEx, Parkinson’s disease, high-intensity, sensory, exercise
Introduction
Exercise is often prescribed as an adjunct therapy to medications to improve the debilitating motor symptoms of Parkinson’s disease (PD). Improving the symptoms of PD may have additional benefits to independence and improved quality of life (QOL) for those with PD. Research suggests exercise is a valid adjunct therapy to medications as some exercise rehabilitation programs have been shown to acutely improve disease severity of PD immediately after treatment completion.1 However, it is equally important to investigate whether the benefits of treatment continue beyond program cessation, if there is potential for the interventions to improve the underlying mechanisms of disease.
There are 2 therapeutic approaches that have been widely highlighted in PD rehabilitation literature. Currently, as a high-intensity form of exercise, boxing has gained immense popularity to treat symptoms of PD.2-4 High intensity exercise, such as boxing, has been suggested to promote the release of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which supports the survival and production of dopaminergic neurons in the basal ganglia, improving overall disease severity.5-10 Interestingly, animal models have suggested that increased BDNF levels after high-intensity exercise are correlated to increased motor improvement. This adds to the possibility that boxing is beneficial for PD motor symptoms.11-13 Similarly, sensory exercise can target potential executive function and sensory deficits that may underlie the motor symptoms of PD.15-20 Sensory exercise is low intensity and requires participants to complete exercise with their eyes closed.16 Previous studies have suggested sensory allows for sustained levels of dopamine in the basal ganglia, improving overall disease severity of PD.14,15 Similar to high-intensity exercise, animal models that have explored sensory training have also shown that rodents experience improvements to motor symptoms which are maintained post-intervention using a handgrip test.21-23 Together, these therapeutic approaches have the potential to have long-lasting improvement based on their ability to improve acute disease severity.
The previous studies conducted on boxing and sensory exercise have 3 major limitations. First, washout benefits were rarely accounted for.2,3,15-20 Most studies only included a short washout period, and while these studies can conclude immediate improvements may be achievable, lasting effects of exercise may be missed. It has been suggested that a sufficient washout period (∼more than 8 weeks) must be employed to account for any potential long-term impact of treatment.24 Second, even if a washout period is employed, most studies only use 1 assessment tool, the Unified Parkinson’s Disease Rating Scale (motor subsection) (UPDRS-III) to assess symptom severity. While this assessment tool has high concurrent validity, reliability, and interrater reliability,25,26 it is a subjective assessment tool, leading researchers to advise objective measures to accompany disease severity scores.27 When examining the lasting effects of an exercise therapy, small changes to disease severity may be better captured using an objective assessment tool and may further validate the findings.28 Finally, previous studies conducted on boxing2,3 and sensory exercise15-20 did not account for external physical activity levels. Similar to drug trials, it is important to ensure the results of a rehabilitation therapy which can be attributed solely to the intervention. Potential confounding factors which may affect the results would be participation in other exercise programs (e.g., Tai Chi), learning a new skill (e.g., needlework), or seasonal activities (e.g., snowboarding and golf) that may affect a person’s overall activity level, thereby influencing the motor outcomes measured in a rehabilitation study.20,29,30 Oftentimes, in current PD rehabilitation literature, these factors are unaccounted for, making it difficult to confirm whether the results of the exercise are only due to the treatment itself.32,33 As such, it is important to monitor everyday activities using a comprehensive physical activity questionnaire such as the Community Health Activities Model Program for Seniors.
Although both boxing and sensory exercise present with disease severity improvement immediately after intervention,2,3,15-20 studies have yet to investigate how long the benefits of these programs last after program cessation, which would be more indicative of the programs ability to impact the underlying disease. As such, this study aimed to compare the effect of high-intensity boxing and sensory exercise on Parkinson’s disease severity and whether or not these effects wear off. As both boxing5-10 and sensory exercise18 may impact the underlying neural mechanisms of Parkinson’s, it was hypothesized that both programs would improve disease severity postexercise and the benefits would not wear off after the cessation of exercise.
Methods
Participants
Participants with idiopathic Parkinson’s were recruited from the Movement Disorders Research and Rehabilitation Centre at Wilfrid Laurier University. To be eligible to participate, participants could not have any additional clinical diagnoses of another neurological disease other than Parkinson’s. Prior to evaluation, informed consent was obtained according to the Declaration of Helsinki. Full ethics approval was obtained from the Ethics Review Board of Wilfrid Laurier (REB #5801). This RCT study was registered with the US National Institutes of Health (ClinicaTrials.gov Identifier: NCT03618901). Participants also completed a Physical Activity Readiness Medical Examination (PARMED-X) form that was signed by a physician (if required). It should be noted that participant recruitment and assessments are ongoing for this study.
Blinding, Study Design, and Randomization
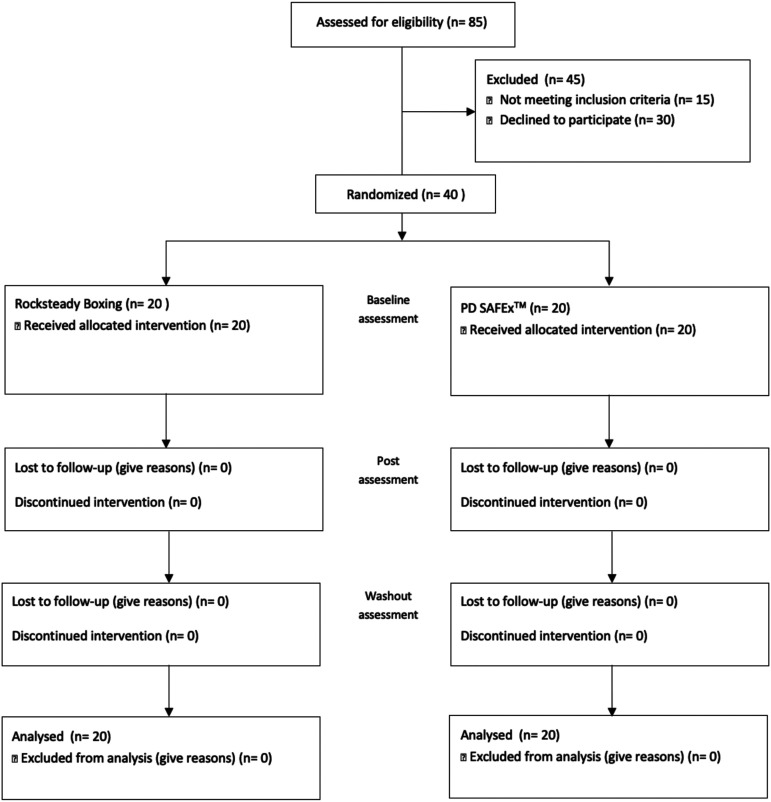
In order to minimize a source of bias, this is a double-blinded study. Assessors were blinded to the group allocation. Participants were blinded to the benefits of each intervention and the study hypothesis.28 The present study is a 20-week double-blinded parallel group randomized controlled trial. In this study, individuals participated in an exercise intervention for the first 10 weeks followed by a 10-week washout period (to assess whether the benefits wear off). Throughout the 20 weeks, participants were instructed to maintain their usual levels of physical activity and avoid changes to medications. Further, participants were not asked to modify their medication schedule to accommodate for exercise timing. Power analysis based on data from previous published rehabilitation study2,13,16,17,19,34 and the minimal clinically important difference in the UPDRS-III indicated a need for approximately 32 total (16 per group) to have 80% power, assuming a 5% type I error (α value). We have satisfied these requirements. We assessed the eligibility of 85 participants, and 40 participants (20 per group) were randomized into 1 of 2 intervention groups using a computer-based number generator: boxing or Parkinson’s disease sensory attention focused exercise (PD SAFExTM) (see Figure 1).
Figure 1.
CONSORT flow diagram illustrating participant recruitment, randomization and flow over the course of the study.
Boxing
As there is no set protocol for boxing, an established Rock Steady Boxing, Inc (RSB) certified coach’s exercise protocol was adapted for this study. The RSB exercise leaders were taught by a RSB certified coach (KS), each class was delivered 3 times a week for 10 weeks and lasted 60 minutes. Each boxing class consisted of a warm-up, boxing specific exercise (high-intensity boxing drills, shadow boxing, jumping jacks, and speedbag drills) and a cool down.
As each week progressed, the exercises became more challenging, and participants were continuously encouraged to push themselves to work harder. Intensity was measured using a self-perceived exertion scale.
Parkinson’s disease sensory attention focused exercise
Sensory exercise was taught by a senior kinesiology undergraduate student, and each class was delivered 3 times a week for 10 weeks and lasted 60 minutes. Each sensory class consisted of a warm-up, sensory specific exercise (stretches, walking, and chair exercises where participants were encouraged to complete the exercises slowly, in a controlled manner and with their eyes closed) and a cooldown.14 As each week progressed, the exercises became more challenging.
Outcome Measures
Primary
The primary outcome measure in this study was disease severity and it was measured using the UPDRS-III. The UPDRS-III assessment was used as it is the gold standard assessment tool to measure disease severity in Parkinson’s in both rehabilitation and pharmaceutical trials.25,26,28 All assessments were conducted by the same, blinded, experienced assessor. Participants were assessed in the ON medication state (approximately 1 hour after administration of medication) to study the effects of exercise in adjunct to dopaminergic therapy.
Secondary
Several secondary outcome measures were employed to control for factors that may influence the effects of the exercise program on the primary outcome measure. In addition to UPDRS-III, gait parameters were used as an instrumental measure of disease severity. Specifically, stride length and stride velocity have been suggested to be closely linked to overall motor symptom severity.29 Both of these parameters have been widely used in Parkinson’s literature alongside UPDRS-III to monitor disease severity.28,29 As such if the exercise interventions have a lasting effect on Parkinson’s, it is important to evaluate whether these effects are present in these specific gait parameters. To do this, participants were asked to walk at a comfortable pace across a 10 m long .61 m wide electronic walkway (Zeno Walkway-ProtoKinetics). In this study, 2 walking tasks were assessed. The first task was baseline walking where participants began walking 2 m before the start of the walkway and walked 2 m beyond the end of the walkway to avoid collecting acceleration and deceleration. In the second task, participants began walking 2 m before the start of the walkway (to avoid acceleration), walked to the end of the walkway, turned (180°) on the walkway, and walked back 2 m beyond the end (to avoid deceleration). Further, the Community Health Activities Model Program for Seniors (CHAMPS) was used to monitor physical activity throughout the study. This was important because physical activity levels prior and during participation may influence the benefit the exercise interventions provide.20,30,31 While CHAMPS is not specifically designed for the PD population, it acts as a questionnaire addressing various types of physical activity a senior may participate in. As there is currently no specific developed questionnaire to monitor physical activity levels for those with Parkinson’s, CHAMPS was used in this study. Additionally, previous studies have indicated that CHAMPS has high reliability, validity, and sensitivity to change.32,33 Finally, to assess the effects of exercise on participants’ perceived QOL, the Parkinson’s Disease Questionnaire-39 (PDQ-39) was used.
Data Analysis
Statistical Program Software for Social Sciences (SPSS) was used to analyze the data. Independent T-tests were conducted to assess if groups were comparable at pre-assessment (levodopa equivalent dosage, disease severity, age, and years of diagnosis). The primary outcome measure (UPDRS-III) was explored using a 2-factor mixed repeated-measures ANOVA (2 groups × 3 evaluation times). The secondary outcome measures were also explored using a repeated-measures ANOVA. To further explore the data, pairwise comparisons of within (differences between each timepoint) and between (differences between RSB and PD SAFExTM) were explored. Significant interactions were followed up with Tukey’s post hoc, and alpha level was set at .05 for all analyses.
Results
Demographics
Disease severity for participants randomized to the RSB and PD SAFExTM groups at pre-assessment were equal t(39) = 1.7, P = .097. Additionally, neither group differed significantly in physical activity levels t(39) = 2.04, P = .072, nor medication dosage (Levodopa equivalent dosage) pre- and post-intervention. All participants can be seen in Table 1.
Table 1.
Demographic Characteristics (Standard Deviation in Parentheses).
| RSB | PD SAFEx™ | |
|---|---|---|
| Number of participants (n) | 20 | 20 |
| Age (years) | 64.2 (9.8) | 65.1 (9.2) |
| Number of years since diagnosis | 6.38 (4.9) | 7.82 (5.2) |
| Program adherence (%) | 96% (2.26) | 98% (1.65) |
| Baseline disease severity | 28.38 (11.41) | 28.44 (14.23) |
| Hoehn and Yahr stage | 2.5 | 2.5 |
| Levodopa equivalent dose (mg/d) | ||
| Pre | 612.13 (220.75) | 608.11 (238.44) |
| Post | 613.13 (232.12) | 608.23 (258.25) |
| Washout | 602.13 (225.60) | 599.01 (242.42) |
Abbreviations: RSB, Rock Steady Boxing, Inc; PD SAFExTM, Parkinson’s disease sensory attention focused exercise.
Any significant findings will be marked with an asterisk (*).
Primary Outcome Measure
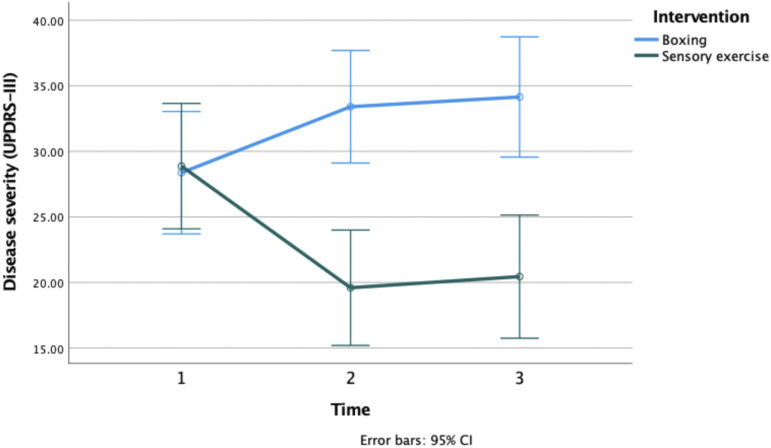
Disease severity was measured using the UPDRS-III scale (see Table 2). A significant main effect of group on motor symptom severity was discovered F(1, 39) = 4.566, P = .036. A significant group by time interaction was also discovered F(1, 39) = 19.566, P < .0001. Post hoc revealed that the PD SAFExTM group presented with greater improvements to motor symptoms compared to the RSB group at post-assessment (MD = 13.8, SE = 3.1, and P < .0001) and at washout (MD = 13.7, SE = 3.3, and P < .006). Whereas symptoms in the RSB group worsened at post assessment (MD = −5.04, SE = .8, and P < .033) and washout (MD = −5.77, SE = .9, and P < .04) compared to baseline (see Figure 2).
Table 2.
Primary Outcome Measure (UPDRS-III Scores) as Group Averages (Standard Deviation in Parentheses).
| Within-group differences (pre-post): mean ± SE (95% CI) (pre-washout): mean ± SE (95% CI) | Between-group differences mean ± SE (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Groups | MDC* (greater than 5 points)28 | MDC* (greater than 5 points)28 | |||||
| PD SAFExTM | RSB | PD SAFExTM | RSB | PD SAFExTM vs RSB | |||
| UPDRS-III | |||||||
| Pre | 28.8 (10.19) | 28.37 (11.22) | .5 ± 3.3 (−6.3 to 7.3) | ||||
| Post | 19.60 (10.03) | 33.41 (9.67) | 9.2 ± 1.1 (.4 to 6.1) | −5.04 ± .8 (−.9 to −3.4) | 13.8 ± 3.1 (−20.1 to −7.4) | ||
| Washout | 20.45 (10.95) | 34.14 (10.69) | 8.35 ± 1.2 (.2 to 4.2) | −5.77 ± .9 (−.76 to −3.49) | 13.7 ± 3.3 (−20.4 to −6.98) | ||
Abbreviations: RSB, Rock Steady Boxing, Inc; PD SAFExTM, Parkinson’s disease sensory attention focused exercise; UPDRS-III, Unified Parkinson’s Rating Scale subsection III; MDC, minimal detectable change.
Figure 2.
Change in disease severity (UPDRS-III) at pre-assesment, post-assessment, and washout for the boxing and sensory exercise groups. UPDRS-III, Unified Parkinson’s Rating Scale subsection III.
Secondary Outcome Measures
Disease severity was objectively monitored using gait parameters (see Table 3). A significant main effect of group was found F(1, 39) = 1396.309, P < .044 for stride length. A significant interaction between group and time was identified for stride length F(2, 39) = 5.307, P < .007. Stride length increased from 1.46 m to 1.73 m (+.27 m change) immediately following PD SAFExTM and was 1.76 m at the end of washout. No significant difference was seen in stride length for the RSB group post-intervention and decreased by .09 m at washout. A main effect of group was seen for stride velocity F(1, 39) = 8.184, P < .007. Where the PD SAFExTM group had experienced an increase in stride velocity (MD = .144, SE = .05, and P < .007). An interaction effect between group and time was also found F(1, 39) = 9.825, P < .0001. Stride velocity increased by .97 m/s from baseline to washout for the PD SAFExTM group. Total stride velocity decreased by .08 m/s for the RSB group at washout from baseline.
Table 3.
Secondary Outcome Measures and Significant Main Effects. (Standard Deviation in Parentheses).
| RSB | PD SAFEx™ | Effect | |
|---|---|---|---|
| Stride length (m) | |||
| Pre | 1.48 (.24) | 1.46 (.13) | Main effect of group: F(1, 39) = 1396.309, P < .044* |
| Post | 1.47 (.22) | 1.73 (.52) | Interaction effect of group ∗ time: F(2, 39) = 5.307, P < .007* |
| Washout | 1.39 (.22) | 1.76 (.38) | |
| Stride velocity (m/s) | |||
| Pre | 1.40 (.17) | 1.433 (.13) | Main effect of group: F(1, 39) = 8.184, P < .007* |
| Post | 1.36 (.18) | 1.53 (.20) | Interaction effect of group ∗ time: F(2, 39) = 9.825, P < .0001* |
| Washout | 1.32 (1.18) | 1.55 (.21) | |
| CHAMPS | |||
| Pre | 3149.82 (2040.11) | 3844.71 (2963.80) | No significant effects or interactions |
| Post | 3146.17 (2059.17) | 3847.99 (2483.90) | |
| Washout | 3147.20 (2102.12) | 3850.9 (2863.80) | |
| PDQ-39 | |||
| Pre | 31.4 (21.97) | 35.33 (23.52) | Significant effect of time: F(2, 39) = 56.533, P < .0001* |
| Post | 26.20 (30.62) | 30.62 (21.75) | |
| Washout | 26.35 (21.53) | 30.71 (21.29) | |
Abbreviations: RSB, Rock Steady Boxing, Inc, PD SAFExTM, Parkinson’s disease sensory attention focused exercise; UPDRS-III, Unified Parkinson’s Rating Scale subsection III; PDQ-39, Parkinson’s Disease Questionnaire-39; CHAMPS, Community Health Activities Model Program for Seniors. Note: Significance is indicated by an ∗.
Physical activity levels were monitored at the start and throughout the intervention. No significant effect of group or time was observed for CHAMPS for both groups.
PDQ-39 results revealed a significant difference between groups F(1, 39) = 1.27, P = .049) from pre-assessment to washout. Post hoc revealed both PD SAFExTM and RSB maintained improvements to QOL.
Discussion
This was the first RCT to compare boxing and sensory exercise for Parkinson’s disease. In this study, it was found that in comparison to boxing, sensory exercise improved disease severity that did not wear off at washout. Therefore, the hypothesis was not supported.
Immediately after intervention, the sensory exercise group indicated improvements to disease severity in comparison to the boxing group. This was further supported by gait parameters (improved stride length and velocity) linked to disease severity. These findings are consistent with previous literature suggesting that sensory exercise can improve the motor symptoms of PD.18-20 In comparison, immediately after the intervention, boxing did not improve disease severity. This is in line with a previous study that assessed disease severity and boxing, where researchers indicated motor scores fluctuated after boxing, where 2 of the 6 participants experienced improvements to symptoms.3 Of the gait parameters assessed, a slight increase in stride length was seen. This is contrary to a previous study that had reported improvements to gait velocity post-boxing.3 However, it is unclear whether the previous study measured stride or step velocity, and therefore, it is possible that an increased stride length was a contributing factor to the improved gait velocity previously reported. Moreover, it should be noted that in this previous study, participants took part in 12 weeks of boxing exercise. As such, even though participants in the current study only took part in 10 weeks of exercise, the motor outcome was not significantly different.
Once immediate effects were established, this study aimed to assess whether these effects wore off. At the end of the washout period, in comparison to the boxing group, the sensory group maintained improved levels of disease severity. Importantly, improvement in UPDRS-III scores from pre- to washout assessment for the sensory group is double the change needed to reach minimal clinical importance (greater than a 5-point change).35,36 This is line with previous research utilizing similar exercise protocols involving slow and controlled moment (external focused exercise,17 blindfolded balance training,37 balance training HIBalance,38 progressive modular rebalancing,39 and Tai Chi exercises40). The lasting improvements to disease severity post-sensory may have been due to the improved functioning of the remaining dopaminergic neurons as a result of the increased sensory stimulation passing through the basal ganglia during exercise.11,14,29,34,41,42 In comparison to sensory exercise, boxing did not have a lasting effect on disease severity. This is contrary to recent rehabilitation literature that have stated high-intensity exercise (e.g., treadmill training,43 tandem cycling,44 multidisciplinary intensive rehabilitation treatments,45 and aerobic exercise46) can greatly improve disease severity as well as potentially have a neuroprotective effect. One difference between these studies and the current one is that intensity was not quantified. The results of the current study may have been different if a heart rate monitor was utilized during exercise to assess maximum heart rate to determine whether high-intensity exercise was achieved47 instead of using self-perceived exertion ratings to judge intensity.8,30 Or it may have been useful to incorporate VO2 testing or handgrip tests to monitor the effects of the high-intensity exercise.48,49
Improvements to disease severity were also seen in gait parameters. Specifically, stride length and stride velocity remained increased (improved) at washout for the sensory group. This may be because sensory exercise has the ability to improve underlying symptoms such as rigidity and postural instability.15 Previous studies have noted that rigidity and postural instability may reduce forward propulsion and thereby negatively affect stride length and stride velocity.23,50-53 As such, sustained improvements to rigidity and postural instability due to sensory exercise may then translate to improvements in gait parameters.54,55 In comparison, stride length and stride velocity did not change at washout for the boxing group. This may be because boxing did not influence the underlying neurological disease, and as such, participants continued to have impaired gait. It should be noted that no significant changes were seen in physical activity levels or medications. This lack of change in external physical activity levels and medications suggests that the changes seen throughout the study are a result of the intervention and not of external confounding factors such as exercise.
Despite these differing results at washout, both boxing and sensory exercise improved self-perceived QOL, which was maintained throughout the duration of the study. The maintenance of the improved self-perceived QOL despite exercise intervention was to be expected as participation in any exercise is likely to lead to an improved perception of QOL.56-58While improvements to QOL reflect disease severity improvement seen in sensory exercise, QOL improvements were also maintained throughout boxing despite lack of improvement to disease severity. This may be because participants experienced increased musculoskeletal gains during boxing rather than improvements to the underlying neurological disease. A past study conducted on boxing discussed that during informal interviews participants described boxing training as enjoyable and were happy to be a part of this group.59,60 The fun, enjoyable nature of boxing allows participants to feel as though they are part of a community, which may allow them to have a positive outlook on the severity of their disease.3,61 Recent literature has suggested that perceived QOL may have a more significant effect on a person’s well-being in comparison to clinical outcomes, indicating that this may be an important consideration when comparing boxing and sensory exercise.58 This would be an important consideration for future research.
Some limitations were present in this study. PD SAFExTM was created by one of the authors of this study (QJA); however, there is no financial benefit or gain associated with PD SAFExTM. QJA was completely blinded to the allocation of the participants and in order to control for any potential unconscious bias gait parameters were employed. Our results provide striking similarities between the computerized sensor carpet (used for gait) and the disease severity scores (UPDRS-III).
Conclusions
In conclusion, we have found sensory exercise in comparison to boxing had an effect on disease severity, where the improvements were sustained beyond cessation of the program. This was reflected in UPDRS-III scores as well as unbiased computerized gait data linked to disease severity. Future studies, should incorporate similar methods to explore PD disease severity and whether or not effects of a treatment wear off.
Acknowledgments
We would like to thank all of the individuals that participated in this study, without whom this study would not be possible.
Footnotes
Author Contributions: Research project: (A) conception: Kishoree Sangarapillai, (B) organization: Kishoree Sangarapillai and Quincy J. Almeida, and (C) execution: Kishoree Sangarapillai and Quincy J. Almeida; statistical analysis: (A) design: Kishoree Sangarapillai and Quincy J. Almeida, (B) execution: Kishoree Sangarapillai, and (C)review and critique: Quincy J. Almeida and Benjamin M. Norman; manuscript: (A) writing of the first draft: Kishoree Sangarapillai and (B) review and critique: Benjamin M. Norman and Quincy J. Almeida.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kishoree Sangarapillai was the lead boxing instructor and is a certified RSB instructor. PD SAFExTM was designed by Quincy J. Almeida, and a trademark on the name is pending, but there is no financial relationship or gain associated with either of these programs.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Quincy J. Almeida https://orcid.org/0000-0002-0806-4397
References
- 1.Agosta F, Roberto G, Sarasso E, et al. Brain plasticity in Parkinson’s disease with freezing of gait induced by action observation training. J Neurol Sci. 2017;264:88-101. doi: 10.1007/s00415-016-8309-7 [DOI] [PubMed] [Google Scholar]
- 2.Combs SA, Diehl MD, Chrzastowski C, et al. Community-based group exercise for persons with Parkinson disease: A randomized controlled trial. NeuroRehabilitation. 2013;32(1):117-124. doi: 10.3233/NRE-130828 [DOI] [PubMed] [Google Scholar]
- 3.Combs SA, Diehl MD, Staples WH, et al. Boxing training for patients with Parkinson disease: A case series. Phys Ther. 2011;91(1):132-142. doi: 10.2522/ptj.20100142 [DOI] [PubMed] [Google Scholar]
- 4.Morris ME, Ellis TD, Jazayeri D, et al. Boxing for Parkinson’s disease: Has implementation accelerated beyond current evidence? Front Neurol. 2019;10(December):1222. doi: 10.3389/fneur.2019.01222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250-4258. doi: 10.1523/JNEUROSCI.3920-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benisty S, Boissiere F, Faucheux B, Agid Y, Hirsch EC. trkB messenger RNA expression in normal human brain and in the substantia nigra of Parkinsonian patients: An in situ hybridization study. Neuroscience. 1998;86:813-826. doi: 10.1016/S0306-4522(98)00126-2 [DOI] [PubMed] [Google Scholar]
- 8.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72-83. doi: 10.1016/j.brainres.2010.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou L, Chen W, Liu X, Qiao D, Zhou FM. Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front Aging Neurosci. 2017;9:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Numan S, Seroogy KB. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: A double-label in situ hybridization study. J Comp Neurol. 1999;403:295-308. [DOI] [PubMed] [Google Scholar]
- 11.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tillerson JL, Caudle WM, Reverón ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002178:80-90. doi: 10.1006/exnr.2002.8021 [DOI] [PubMed] [Google Scholar]
- 13.Tillerson JL, Caudle WM, Reverón ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899-911. doi: 10.1016/S0306-4522(03)00096-4 [DOI] [PubMed] [Google Scholar]
- 14.Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson’s disease. Mov Disord. 2009;24:1132-1138. [DOI] [PubMed] [Google Scholar]
- 15.Sangarapillai K, Norman BM, Almeida QJ. Analyzing the effects of PDSAFExTM on the motor symptoms of Parkinson’s disease: A retrospective study. NeuroRehabilitation. 2020;46:589-593. [DOI] [PubMed] [Google Scholar]
- 16.Sage MD, Almeida QJ. A positive influence of vision on motor symptoms during sensory attention focused exercise for Parkinson’s disease. Mov Disord. 2010;25(1):64-69. [DOI] [PubMed] [Google Scholar]
- 17.Lefaivre SC, Almeida QJ. Can sensory attention focused exercise facilitate the utilization of proprioception for improved balance control in PD? Gait Posture. 2015;41(2):630-633. [DOI] [PubMed] [Google Scholar]
- 18.Silveira CRA, Roy EA, Intzandt BN, Almeida QJ. Brain and cognition aerobic exercise is more effective than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain Cognit. 2018;122:1-8. [DOI] [PubMed] [Google Scholar]
- 19.Beck EN, Wang MTY, Intzandt BN, Almeida QJ, Martens KAE. Sensory focused exercise improves anxiety in Parkinson’s disease: A randomized controlled trial. PloS One. 2020;15(4):e0230803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck EN, Intzandt BN, Almeida QJ. Can dual task walking improve in Parkinson’s disease after external focus of attention exercise? A single blind randomized controlled trial. Neurorehabil Neural Repair. 2018;32:1-16. doi: 10.1177/1545968317746782 [DOI] [PubMed] [Google Scholar]
- 21.Whishaw IQ, Castañeda E, Gorny BP. Dopamine and skilled limb use in the rat: More severe bilateral impairments follow substantia nigra than sensorimotor cortex 6-hydroxydopamine injection. Behav Brain Res. 1992;47:89-92. [DOI] [PubMed] [Google Scholar]
- 22.Schallert T, Upchurch M. Posture-independent sensorimotor analysis of inter-hemispheric receptor asymmetries in neostriatum. Pharmacol Biochem Behav. 1983;18:753-759. [DOI] [PubMed] [Google Scholar]
- 23.Schallert T, Upchurch M, Lobaugh N, et al. Tactile extinction: Distinguishing between sensorimotor and motor asymmetries in rats with unilateral. Nigrostriatal Damage. 1982;16:55-462. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB. The delayed-start study design. N Engl J Med. 2009;361(13):304-1306. [DOI] [PubMed] [Google Scholar]
- 25.Siderowf A, McDermott M, Kieburtz K, Blindauer K, Plumb S, Shoulson I. Test-retest reliability of the unified Parkinson’s disease rating scale in patients with early Parkinson’s disease: Results from a multicenter clinical trial. Mov Disord. 2002;17:758-763. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Poewe W, Rascol O, Christina S. State of the art review the unified Parkinson ’s Disease Rating Scale (UPDRS): Status and recommendations. Society. 2003;18:738-750. [DOI] [PubMed] [Google Scholar]
- 27.AlMahadin G, Lotfi A, Zysk E, Siena FL, Carthy MM, Breedon P. Parkinson’s disease: Current assessment methods and wearable devices for evaluation of movement disorder motor symptoms - A patient and healthcare professional perspective. BMC Neurol. 2020;20(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-odasso M, Almeida QJ, Burhan AM, et al. SYNERGIC TRIAL (SYNchronizing exercises, remedies in gait and cognition) a multi- Centre randomized controlled double blind trial to improve gait and cognition in mild cognitive impairment. BMC. 2018;18(93):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirelman A, Bonato P, Camicioli R, et al. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019;18(7):697-708. doi: 10.1016/S1474-4422(19)30044-4 [DOI] [PubMed] [Google Scholar]
- 30.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126-1141. [DOI] [PubMed] [Google Scholar]
- 31.Stewart AL, Mills KM, Sepsis PG, et al. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med. 1997;19(4):353-361. doi: 10.1007/BF02895154 [DOI] [PubMed] [Google Scholar]
- 32.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33(6):962-970. doi: 10.1097/00005768-200106000-00016 [DOI] [PubMed] [Google Scholar]
- 33.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: A physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465-M470. doi: 10.1093/gerona/56.8.m465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sangarapillai K, Norman BM, Almeida QJ. Rehabilitation of falls in Parkinson’s disease: Self-perception vs. objective measures of fall risk. Brain Sci. 2021;11(3):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Ferro Á, Matarazzo M, Martínez‐Martín P, et al. Minimal clinically important difference for UPDRS-III in daily practice. Movement Disorders Clinical Practice. 2018;5(4):448-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clini- cally important change on the Unified Parkinson’s Disease Rating Scale. Mov Disord. 2006;21:1200-1207. doi: 10.1002/mds.20914 [DOI] [PubMed] [Google Scholar]
- 37.Bonnì S, Ponzo V, Tramontano M, et al. Neurophysiological and clinical effects of blindfolded balance training (BBT) in Parkinson’s disease patients: A preliminary study. Eur J Phys Rehabil Med. 2019;55(2):176-182. doi: 10.23736/S1973-9087.18.05126-2 [DOI] [PubMed] [Google Scholar]
- 38.Franzén E, Johansson H, Freidle M, et al. The EXPANd trial: Effects of exercise and exploring neuroplastic changes in people with Parkinson’s disease: A study protocol for a double-blinded randomized controlled trial. BMC Neurol. 2019;19(1):280. doi: 10.1186/s12883-019-1520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meloni M, Saibene FL, Di Tella S, et al. Functional and cognitive improvement after an intensive inpatient multidisciplinary rehabilitation program in mild to severe Parkinson’s disease: A retrospective and observational study. Front Neurol. 2021;12:626041. doi: 10.3389/fneur.2021.626041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao S, Kaudimba KK, Cai J, et al. A mobile phone app-based Tai Chi training in Parkinson’s disease: Protocol for a randomized controlled study. Front Neurol. 2021;11:615861. doi: 10.3389/fneur.2020.615861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shine JM, Matar E, Ward PB, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain. 2013;136(Pt 4):1204-1215. doi: 10.1093/brain/awt049 [DOI] [PubMed] [Google Scholar]
- 42.Taub E, Uswatte G, Mark VW. The functional significance of cortical reorganization and the parallel development of CI therapy. Front Hum Neurosci. 2014;8:396. doi: 10.3389/fnhum.2014.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De Novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219-226. doi: 10.1001/jamaneurol.2017.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segura C, Eraso M, Bonilla J, et al. Effect of a high-intensity tandem bicycle exercise program on clinical severity, functional magnetic resonance imaging, and plasma biomarkers in Parkinson’s disease. Front Neurol. 2020;11:656. doi: 10.3389/fneur.2020.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrao M, Pierelli F, Sinibaldi E, et al. Progressive Modular Rebalancing System and Visual Cueing for Gait Rehabilitation in Parkinson’s disease: A pilot, randomized, controlled trial with crossover. Front Neurol. 2019;10:902. doi: 10.3389/fneur.2019.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van der Kolk NM, de Vries NM, Kessels R, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019;18(11):998-1008. doi: 10.1016/S1474-4422(19)30285-6 [DOI] [PubMed] [Google Scholar]
- 47.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13:496-502. doi: 10.1016/j.jsams.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 48.Combs-Miller SA, Moore ES. Predictors of outcomes in exercisers with Parkinson disease: A two-year longitudinal cohort study. NeuroRehabilitation. 2019;44(3):425-432. doi: 10.3233/NRE-182641 [DOI] [PubMed] [Google Scholar]
- 49.Borrero L, Miller SA, Hoffman E. The meaning of regular participation in vigorous-intensity exercise among men with Parkinson’s disease. Disabil Rehabil. 2020;1-7. Advance online publication. doi: 10.1080/09638288.2020.1836042 [DOI] [PubMed] [Google Scholar]
- 50.Hausdorff JM. Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19(2):026113. doi: 10.1063/1.3147408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warabi T, Furuyama H, Sugai E, Kato M, Yanagisawa N. Gait bradykinesia in Parkinson’s disease: A change in the motor program which controls the synergy of gait. Exp Brain Res. 2018;236(1):43-57. doi: 10.1007/s00221-017-5106-1 [DOI] [PubMed] [Google Scholar]
- 52.Wu Z, Xu H, Zhu S, et al. Gait analysis of old individuals with mild Parkinsonian signs and those individuals’ gait performance benefits little from Levodopa. Risk Manag Healthc Pol. 2021;14:1109-1118. doi: 10.2147/RMHP.S291669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamarajah K. Postural instability and gait disorder (PIGD) in Parkinson’s disease: Is there another target for deep brain stimulation? (P6.381). Neurology. 2016;86(16):6381. [Google Scholar]
- 54.Barbosa AF, Chen J, Freitag F, et al. Gait, posture and cognition in Parkinson’s disease. Dement Neuropsychol. 2016;10(4):280-286. doi: 10.1590/s1980-5764-2016dn1004005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siragy T, Nantel J. Quantifying dynamic balance in young, elderly and Parkinson’s individuals: A systematic review. Front Aging Neurosci. 2018;10:1-22. doi: 10.3389/fnagi.2018.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fayyaz M, Jaffery SS, Anwer F, Zil-E-Ali A, Anjum I. The effect of physical activity in Parkinson’s disease: A mini-review. Cureus. 2018;10(7):e2995. doi: 10.7759/cureus.2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dauwan M, Begemann MJH, Slot MIE, Lee EHM, Scheltens P, Sommer IEC. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J Neurol. 2021;168:1222. doi: 10.1007/s00415-019-09493-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho AOD, Souza A, Filho S, et al. Clinical practice & epidemiology in physical exercise for parkinson’s disease: Clinical and experimental evidence. Clin Pract Epidemiol Ment Health. 2018;14:89-98. doi: 10.2174/1745017901814010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermanns M, Mastel-Smith B, Donnell R, Quarles A, Rodriguez M, Wang T. Counterpunching to improve the health of people with Parkinson’s disease. J Am Assoc Nurse Pract. 2021. Advance online publication. doi: 10.1097/JXX.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 60.Brunet J, Price J, Wurz A, McDonough M, Nantel J. Boxing with Parkinson’s disease: Findings from a Qualitative Study Using Self-Determination theory. Disabil Rehabil. 2021. Advance online publication. doi: 10.1080/09638288.2021.1891465 [DOI] [PubMed] [Google Scholar]
- 61.Domingos J, Radder D, Riggare S, et al. Implementation of a community-based exercise program for Parkinson patients: Using boxing as an example. J Parkinsons Dis. 2019;9(3):615-623. doi: 10.3233/JPD-191616 [DOI] [PMC free article] [PubMed] [Google Scholar]