Abstract
Objective:
To understand how longitudinal serum neurofilament light chain (sNfL) patterns can inform its use as a prognostic biomarker in multiple sclerosis (MS) and evaluate whether sNfL reflects MS disease activity and disease-modifying therapy usage.
Methods:
This was a post hoc analysis of longitudinal data and samples from the ADVANCE trial (NCT00906399) of patients with relapsing–remitting MS (RRMS). sNfL was measured every 3 months for 2 years, then every 6 months for 4 years. Regression models explored how sNfL data predicted 4-year values of brain volume, expanded disability status scale score, and T2 lesions. sNfL levels were assessed in those receiving placebo, peginterferon beta-1a, and those with disease activity.
Results:
Baseline sNfL was a predictor of 4-year brain atrophy and development of new T2 lesions. Clinical (p = 0.02) and magnetic resonance imaging (MRI) (p < 0.01) outcomes improved in those receiving peginterferon beta-1a whose sNfL decreased to <16 pg/mL after 12 months versus those whose sNfL remained ⩾16 pg/mL. Mean sNfL levels decreased in peginterferon beta-1a-treated patients and increased in placebo-treated patients (–9.5% vs. 6.8%; p < 0.01). sNfL was higher and more variable in patients with evidence of active MS.
Conclusion:
These data support sNfL as a prognostic and disease-monitoring biomarker for RRMS.
Keywords: Biomarker, serum neurofilament light chain, magnetic resonance imaging, prognosis, brain atrophy, multiple sclerosis
Introduction
Sensitive biomarkers for disease prognosis and monitoring are an unmet need in multiple sclerosis (MS).1,2 Clinicians use magnetic resonance imaging (MRI) and disease progression measures such as the expanded disability status scale (EDSS) and multiple sclerosis functional composite (MSFC) scores to evaluate a patient’s disease progression and response to treatment, but these approaches have limited sensitivity by themselves.3 Classification of patients, as showing no evidence of disease activity (NEDA) or exhibiting evidence of MS disease activity (EDA), requires further refinement and consensus.4,5 Clinicians and researchers would benefit from sensitive, quantifiable, and specific biomarkers to monitor MS progression and treatment response.
Neurofilaments, comprising neurofilament light (NfL), medium, and heavy chains, are scaffolding neuronal proteins that are released upon neuronal injury.2 Elevated NfL levels have been detected in the cerebrospinal fluid and sera of persons with MS (PWMS) and other neurodegenerative diseases.2,6–8 Recent reports support the utility of NfL as a prognostic marker for MS;9,10 higher serum NfL (sNfL) levels are positively correlated with clinical and imaging measures of disease severity in PWMS.2,11–13
We assessed the potential of sNfL as a biomarker for patients with relapsing–remitting MS (RRMS) in the pivotal ADVANCE (NCT00906399) trial. This longitudinal data set permitted the exploration of sNfL kinetics in patients achieving NEDA status compared with those exhibiting EDA.
Materials and methods
Study design
This was a post hoc analysis of data from ADVANCE, a randomized, multicenter, double-blind, placebo-controlled study assessing the efficacy and safety of peginterferon beta-1a for patients with RRMS, conducted from June 2009 to October 2013. Detailed methods and results for ADVANCE have been previously published.14,15
Adults with RRMS, an EDSS score of 0–5 and ⩾2 clinically documented relapses in the previous 3 years (including 1 within prior 12 months), were randomized to placebo or peginterferon beta-1a, 125 µg every 2 or 4 weeks for the first year of the study. For the second year of the study, patients who had received placebo were randomized to peginterferon beta-1a 125 µg every 2 or 4 weeks, and patients who had received active treatment during the initial study year continued on the same regimen for the second study year. Throughout ADVANCE, patients provided blood samples at baseline and every 3 months for 2 years. Upon completion of the trial, patients could continue in ATTAIN, an open-label extension study.16 At this point, patients provided blood samples every 6 months up to 4 years. Collected samples were stored at –70°C. ATTAIN included 1076 (71%) of the 1512 patients randomized in ADVANCE (859 with baseline sNfL data available).16 Patients with sufficient sample volume for the sNfL assay at ⩾4 time points were included in our analysis.
Standardized neurological assessments were completed by a blinded non-treating physician every 12 weeks and at the time of suspected relapse. Signif-icant clinical measurements included T2 lesion count and volume, gadolinium-enhancing (Gd+) lesion number, percent brain volume change (PBVC),17,18 MSFC and EDSS scores, and disease duration (measured from the onset of symptoms). Standardized MRI scans were obtained at baseline, 6 months, and 1, 2, 3, and 4 years.
sNfL levels
sNfL was measured in stored samples using a sensitive single molecule array (Simoa®, Quanterix, Lexington, MA) assay.7 The assay was analytically validated for the fit-for-purpose sNfL evaluation. The measurements were performed in one round of experiments using one batch of reagents.
Standard protocol approvals, registrations, and patient consents
Patients in ADVANCE and ATTAIN provided written consent to participate14,16 and provide serum samples for possible use in future MS research. The protocols were approved by all participating sites and the studies were conducted according to the International Council for Harmonization Guideline on Good Clinical Practice and the Declaration of Helsinki.
Statistics
Where indicated, sNfL was analyzed with clinical and MRI parameters using Spearman correlation, analysis of variance, and multivariate logistic regression. Logged values were based on the natural log scale.
Multivariate regression models were used to assess whether baseline sNfL and other baseline factors might be predictive of clinical and MRI outcomes. Independent variables for all models in this report included treatment arm, age, sex, number of relapses, years since disease onset, and baseline measures for sNfL, EDSS, MSFC, Gd+ lesion count, and T2 lesion volume. Variables showing significant associations (p ⩽ 0.05) in univariate analyses or those thought to be clinically important (e.g. sNfL, treatment) were included in multivariate models. Non-significant predictors (p > 0.05) were removed from the model to arrive at a final parsimonious model. A negative binomial regression model was used for the number of new or newly enlarging T2 lesions in 4 years, and a generalized linear regression model was used for 4-year PBVC and for 4-year change in EDSS.
These statistical approaches offer Type III sum of square estimates, which provide information on each predictor’s strength and contribution to the model in the presence or adjustment of others in the multivariate model. The estimates are derived from the proportion of variance that each predictor (e.g. sNfL, age, and T2 lesion volume) contributes to the outcome (e.g. PBVC and EDSS); higher χ2 estimates (for new T2 lesions) or F-values (for PBVC and EDSS) indicate a stronger predictor. Change in brain volume and EDSS outcome variables were treated as continuous, and the number of new or newly enlarged T2 lesions outcome was treated as a count variable.
The possible utility of, and optimal time frame for, using sNfL changes to monitor disease progression and inform decision on disease-modifying therapy (DMT) use was explored using cross-sectional analyses on data from treated patients to determine whether a decrease in sNfL at 3, 6, 9, and 12 months was predictive of 4-year outcomes in brain volume, EDSS score, and new or newly enlarged T2 lesions. Two groups of patients treated with peginterferon beta-1a were created: (1) those with a baseline and post-baseline sNfL of at least 16 pg/mL (a threshold that was previously shown to be associated with higher MS disease activity)19 and (2) those whose baseline sNfL was ⩾16 pg/mL but then decreased to <16 pg/mL post-baseline. These groups were compared using generalized linear regression models at each post-baseline time point for PBVC and EDSS score outcomes. New or newly enlarged T2 lesions were similarly assessed using a negative binomial regression model. Least square means estimates, along with 95% confidence intervals (CIs) and p values, were used to evaluate the “decreased sNfL” versus “no decreased sNfL” group comparisons.
An sNfL level ⩾16 pg/mL that we consider to be indicative of active disease comes from receiver operating characteristic analysis on 2000 bootstrapped baseline sNfL samples from nearly 500 PWMS pooled from three Biogen randomized controlled trials (sNfL distribution in these trials was similar).19 The sensitivity and specificity of predicting new T2 lesions over the subsequent year were determined, and the thresholds of 8 and 16 pg/mL were observed to be associated with at least 80% sensitivity and specificity, respectively, in the majority of these resampled iterations (data not shown).
NEDA definition
Patients enrolled in ADVANCE were included in the EDA/NEDA analysis if they had more than one sNfL measurement (baseline data not required) and 4-year PBVC data (N = 897). PBVC does not factor into the EDA/NEDA definition we used but was superimposed on the data. NEDA was defined as exhibiting the following criteria at each available time point: no relapses, no new Gd+ lesions, no new or newly enlarged T2 lesions, and a <1-unit increase in EDSS score. Patients who only experienced a 1-unit increase in EDSS score from a baseline score of 0 or 1, however, were still considered NEDA if no other criterion was met. Patients who did not meet NEDA criteria were defined as EDA.
Data availability
To request access to data, please visit http://www.biogenclinicaldatarequest.com.
Results
Demographics
Baseline sNfL was measured in a total of 859 patients, representing both cohorts of the ADVANCE study: placebo (n = 393) and peginterferon beta-1a (n = 466). sNfL was also assayed at 1 year in 813 patients, and every ~3 months from baseline to 2 years in 511 patients. Of those 511 patients, 282 had sNfL measured at 2.5, 3, 3.5, and 4 years.
Baseline characteristics for patients in this study were similar between the treatment arms (Table 1). The mean (SD) age for the entire cohort was 36.9 (9.8) years and 70% of patients were female. The median (range) baseline EDSS score was 2.0 (0.0–5.5), and the mean (SD) baseline number of Gd+ lesions was 1.5 (4.0). Data are indicative of an MS cohort with mild-to-moderate disease activity.
Table 1.
Demographic and baseline characteristics for patients with available serum neurofilament light chain levels.a
| Baseline characteristics | All (N = 859) | Placebo (n = 393) | Peginterferon beta-1a (n = 466) |
|---|---|---|---|
| Mean (SD) age (years) | 36.9 (9.8) | 36.5 (9.6) | 37.2 (9.9) |
| Male/female (%) | 30/70 | 30/70 | 31/69 |
| Median (range) EDSS score | 2.0 (0.0–5.5) | 2.0 (0.0–5.0) | 2.0 (0.0–5.5) |
| Mean (SD) number of Gd+ lesions | 1.5 (4.0) | 1.6 (3.8) | 1.4 (4.1) |
| Mean (SD) T2 lesion volume (mm3) | 10.0 (12.1) | 10.4 (12.2) | 9.6 (12.0) |
EDSS: expanded disability status scale; Gd+: gadolinium-enhancing lesions; SD: standard deviation.
Sample time points: Baseline, every 3 months until 2 years, then every 6 months until 4 years.
sNfL and MRI outcomes
Univariate analyses demonstrated that baseline sNfL was a significant predictor of T2 lesions on MRI (Supplemental Table 1), PBVC (Supplemental Table 2), and EDSS score (Supplemental Table 3).
Multivariate analyses, adjusted as described, demonstrated that baseline sNfL was one of the strongest predictors of brain atrophy, as measured by PBVC over 4 years (Table 2). The generalized linear regression model estimated that if a patient’s sNfL were to increase 2.7-fold, brain volume would decrease by 0.58% over 4 years. When the data are visualized on a scatterplot or across sNfL tertiles (Supplemental Figure 1), it is evident that higher baseline sNfL predicts greater brain atrophy at 4 years.
Table 2.
Multivariate regression models over 4 years (PBVC, EDSS, and new T2 lesions).
| Variable label | Regression coefficient estimate
(95% CI); p; χ2
estimate/Fa |
||
|---|---|---|---|
| PBVCb,c | New T2 lesionsd,e | EDSSc,f | |
| Intercept | 0.594 (–0.094, 1.283); 0.091 | 1.939 (1.298, 2.580); <0.0001; NA | −0.383 (−0.782, 0.017); 0.060; NA |
| sNfL at baseline (log-transformed) | −0.579 (−0.782, −0.375); <0.0001; 31.21 | 0.734 (0.506, 0.962); <0.0001; 41.71 | 0.114 (–0.004, 0.231); 0.058; 3.63 |
| Age at reference start date (years) | −0.015 (−0.029, −0.001); 0.030; 4.71 | −0.037 (−0.050, −1.024); <0.0001; 28.48 | 0.012 (0.005, 0.020); 0.001; 10.56 |
| EDSS score at baseline | −0.119 (−0.231, −0.007); 0.037; 4.39 | NS | −0.130 (–0.193, –0.067); <0.0001; 16.62 |
| Duration of symptoms (years) | 0.034 (0.013, 0.055); 0.002; 10.02 | −0.037 (−0.056, −0.017); 0.0002; 12.71 | NS |
| T2 lesion volume at baseline (log-transformed) | −0.293 (−0.395, −0.191); <0.0001; 32.05 | 0.391 (0.279, 0.502); <0.0001; 44.03 | 0.062 (0.005, 0.119); 0.033; 4.55 |
| Treatment arm | NS | −0.549 (−0.791, −0.307); <0.0001; 19.48 | NS |
| MSFC score at baseline | NS | 0.536 (0.322, 0.750); <0.0001; 22.2 | NS |
CI: confidence interval; EDSS: expanded disability status scale; MSFC: multiple sclerosis functional composite; NA: not available; NS: not significant; PBVC: percent brain volume change; sNfL: serum neurofilament light.
The regression coefficient represents the change in value of the outcome, per unit change in each predictor. Higher χ2 estimates or F-values indicate a stronger predictor.
n = 473 and R2 = 0.23.
Results from generalized linear regression model.
n = 505 and scaled deviance = 1.2.
Results from a negative binomial regression model.
n = 550 and R2 = 0.06.
The F-value for sNfL predicting PBVC over 4 years was 31.21, which was similar to that for baseline T2 lesion volume (32.05) and higher than that for duration of symptoms (10.02), age (4.71), or baseline EDSS score (4.39). In these analyses, baseline sNfL was also a significant predictor of new T2 lesions at 4 years (Table 2 and Supplemental Figure 2). The model suggests that a 2.7-fold increase in sNfL would yield twice the number of T2 lesions in 4 years. The χ2 estimate was 41.71 for sNfL at baseline, predicting new T2 lesions at 4 years. χ2 estimates for other significant covariates for predicting new T2 lesions at 4 years were baseline T2 lesion volume (44.0), age (28.5), baseline MSFC score (22.2), treatment arm (19.5), and disease duration (12.7).
Adjusting for baseline EDSS score, age and baseline T2 lesion volume were the strongest predictors of EDSS score at 4 years, whereas baseline sNfL was not a significant predictor (Table 2). F-values for the covariates for EDSS score at 4 years were baseline EDSS score (16.6), age (10.7), baseline T2 lesion volume (4.6), and baseline sNfL (3.6).
Relationship of sNfL with disease activity and treatment
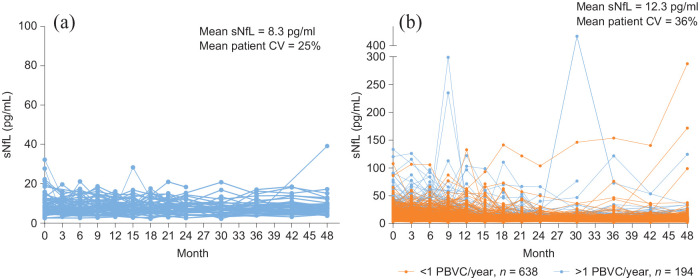
In the 65 patients with NEDA across 4 years, consistently low (mean 8.3 pg/mL across all time points) and stable (25% average patient coefficient of variation) sNfL was observed (Figure 1(a)). However, patients with EDA (n = 832) over 4 years had higher (mean 12.3 pg/mL) and more variable (36% average patient coefficient of variation) sNfL (between-group p < 10–7 for both sNfL levels and patient sNfL coefficient of variation) (Figure 1(b)). sNfL was especially high and variable in patients exhibiting EDA whose average annualized decrease in PBVC across 4 years was >1% (Figure 1(b)).
Figure 1.
sNfL levels across time in patients classified as exhibiting NEDA or EDA: (a) patients classified as exhibiting NEDA for the entire duration of the study, n = 65 (NEDA for 4 years) and (b) patients classified as exhibiting EDA at any time during the study, n = 832 (EDA over 4 years). The average annualized PBVC is based on data collected at 6 months and at 1, 2, 3, and 4 years. Each line represents the sNfL data for an individual patient.
CV: coefficient of variation; EDA: evidence of disease activity; NEDA: no evidence of disease activity; PBVC: percent brain volume change; sNfL: serum neurofilament light chain.
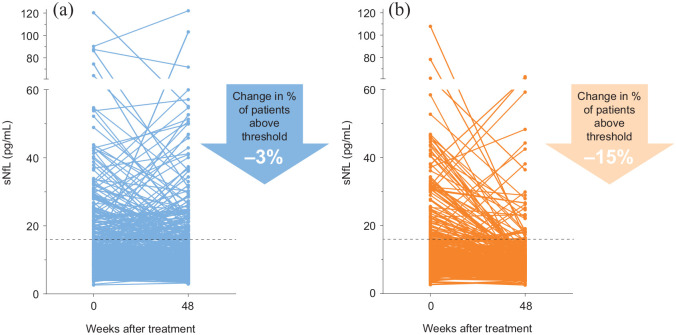
Similarly, the reduction in sNfL at 1 year compared with baseline was numerically greater with peginterferon beta-1a than with placebo. Patients receiving peginterferon beta-1a every 2 weeks exhibited an average decrease in 9.5% in sNfL after 48 weeks, whereas those receiving placebo exhibited an average increase in 6.8% (p < 0.01). Furthermore, 3% of the placebo cohort exhibited a reduction of sNfL to below the 16 pg/mL threshold, compared with 15% of patients receiving peginterferon beta-1a every 2 weeks (Figure 2).
Figure 2.
sNfL levels measured at baseline and 48 weeks. (a) Placebo, n = 373. (b) Peginterferon beta-1a cohorts, n = 350.
sNfL: serum neurofilament light chain.
Dotted horizontal lines indicate 16 pg/mL, which we consider to be the threshold for active MS disease.
Predictive value of sNfL
Table 3 provides data on the groups with baseline sNfL ⩾ 16 pg/mL and post-baseline sNfL: ⩾16 pg/mL or <16 pg/mL. A decrease in sNfL to <16 pg/mL at 6 months was associated with a significant (p = 0.05) reduction in 4-year PBVC (0.89%). Although significance of this association was not achieved at 9 months (p = 0.07), the difference between the groups’ PBVC was large (1.6%) and significant (p < 0.01) by 12 months. The first indication that a decrease in sNfL is associated with a 4-year change in EDSS (p = 0.04) is evident at 9 months; however, the average difference between the two groups’ EDSS scores is only 0.5 units; the results are similar for the 12-month sNfL readout (p = 0.02 for an EDSS difference of 0.5).
Table 3.
Association between short-term (up to 12 months) sNfL change and long-term MRI/clinical outcomes, using sNfL = 16 pg/mL as the threshold.
| Comparison | Patients (n) | Month | Change from baseline to 4 years,
LSM difference (95% CI); p |
||
|---|---|---|---|---|---|
| PBVC | New T2 lesions | EDSS | |||
| No sNfL decreasea versus sNfL decreaseb | 58 38 |
3 | −0.564 (−1.440, 0.312); 0.205 | 1.071 (0.455, 2.521); 0.875 | 0.321 (−0.149, 0.791); 0.179 |
| No sNfL decreasea versus sNfL decreaseb | 42 49 |
6 | −0.891 (−1.783, 0.002); 0.050 | 1.455 (0.620, 3.415); 0.389 | 0.237 (−0.271, 0.746); 0.357 |
| No sNfL decreasea versus sNfL decreaseb | 29 68 |
9 | −0.882 (−1.845, 0.080); 0.072 | 1.839 (0.727, 4.651); 0.198 | 0.530 (0.019, 1.041); 0.042 |
| No sNfL decreasea versus sNfL decreaseb | 33 87 |
12 | −1.560 (−2.401, −0.718); 0.0003 | 3.067 (1.342, 7.011); 0.008 | 0.513 (0.072, 0.954); 0.023 |
CI: confidence interval; EDSS: expanded disability status scale; LSM: least square means; PBVC: percent brain volume change; sNfL: serum neurofilament light.
Generalized linear regression models were used for the PBVC and EDSS outcomes, and a negative binomial regression model was used to assess T2 lesions.
sNfL levels remained ⩾16 pg/mL.
sNfL levels decreased from ⩾16 pg/mL to <16 pg/mL.
Finally, a decrease in sNfL to <16 pg/mL after 12 months of treatment was associated with an average threefold decrease in the number of new T2 lesions at 4 years (p < 0.01), compared with PWMS who did not exhibit a decrease in sNfL.
Discussion
Currently, MRI and clinical scoring scales, such as EDSS, are recommended for monitoring disease activity in PWMS.20–22 These outcome measures cannot detect subclinical changes, and they reflect pathological changes to different extents, which may explain the intra-patient lack of alignment that is often observed. Both of these measures reflect disease activity downstream from molecular mechanisms of disease.
Similarly, predicting disease course has proven elusive; and, indeed, the evolution of symptoms in PWMS across time is highly heterogenous.20,22 Identifying an earlier, real-time measure of neurodegeneration could complement the current arsenal of tools used by neurologists. sNfL shows promise as one such biomarker; as a molecular marker of axonal degeneration resulting from inflammation and demyelination, elevated sNfL can be surmised to indicate neurodegeneration before it manifests as severe and irreversible symptoms in the clinic.
In this study, we used longitudinal data to examine how sNfL tracks with MRI and clinical outcomes in MS. We confirmed earlier reports that suggest that a patient’s baseline sNfL is prognostic of disease outcomes and reduced with DMTs.11,13,23,24 We showed that a baseline level of sNfL is a significant predictor of both brain atrophy and the development of new T2 lesions at 4 years; these results support previous reports demonstrating that high sNfL predicted a more rapid decrease in brain volume25 and, more recently, that sNfL tracks with brain atrophy in normal aging.26 Our model also confirmed the findings of an earlier study that age, baseline EDSS score, disease duration, and baseline T2 lesion volume were predictive of 4-year PBVC.27
Baseline sNfL was only weakly predictive of EDSS outcome. This finding may be due to measurement characteristics of EDSS, as it has been repeatedly noted that EDSS is nonlinear and somewhat coarse, and changes in the lower part of the scale may not necessarily be indicative of disease progression. On average, this cohort experienced a net increase in 1 EDSS unit from baseline to 4 years; this narrow range limits the outcomes that the model could span. These data, in conjunction with the strong predictive value for MRI metrics, suggest that monitoring sNfL in the clinic might supplement current approaches to prognosis and tracking disease course or activity.
The heterogeneous clinical presentation of MS may be explained with quantitative data obtained through measuring sNfL. The sNfL profile across time was consistently low and stable for patients exhibiting NEDA at 4 years. However, PWMS and EDA exhibited a heightened, 50% higher (on average), and erratic sNfL profile across time, especially if they were also experiencing a >1% annualized decrease in PBVC. These data suggest that a transitory, and especially prolonged, increase in sNfL is a harbinger of clinical disease activity.
On average, PWMS receiving peginterferon beta-1a every 2 weeks exhibited a 9.5% decrease in sNfL after 48 weeks, whereas the sNfL for those receiving placebo increased by 6.8% over the same time frame. As reported in ADVANCE, by 48 weeks, this same cohort of patients had a significantly reduced annualized relapse rate and risk of disability progression. Taken together, these data offer further evidence that sNfL can reflect DMT efficacy as well as disease activity.
Beyond cross-sectional associations, we explored how monitoring short-term changes in sNfL might predict long-term clinical and MRI outputs. At 3-month intervals, we examined how a reduction in sNfL might align with 4-year MRI and EDSS outcomes. A decrease in sNfL to <16 pg/mL as early as 6 months differentiated patients in 4-year PBVC values. At 12 months, however, the difference in the populations with NfL < 16 pg/mL versus ⩾ 16 pg/mL was significantly associated with 4-year changes in PBVC, EDSS, and new T2 lesions. Therefore, if sNfL readouts were to be implemented in the clinic for PWMS taking peginterferon beta-1a, a 12-month follow-up might be sufficient to assess whether a more aggressive treatment regimen is necessary for these patients. Timings for other DMTs will need to be investigated.
This study has limitations. As a post hoc study, interpretation of results is limited, but the results are in line with observations from other studies in PWMS.24,28–33 Because the study included only 1 year of placebo data, we were unable to compare the predictive effects of sNfL across patients receiving peginterferon beta-1a and placebo. Also, as with all extension studies, there is a possibility of ascertainment bias in the population of patients who continued in ATTAIN on completion of ADVANCE; however, there was <30% attrition from ADVANCE to ATTAIN. Finally, data from this trial may not be fully extrapolated into the clinical setting due to known effects of age11 and comorbid illnesses34 on sNfL, and future studies are needed to establish normative reference ranges.
This analysis of sNfL, in conjunction with MRI and clinical outcomes for MS in patients with RRMS in ADVANCE, supports sNfL as a promising candidate biomarker for assessing MS disease severity and treatment monitoring. Further studies are needed to understand how sNfL varies with age and other comorbidities; these data would inform, for example, an sNfL cutoff for NEDA in PWMS. If validated, sNfL could complement clinical and MRI measures in guiding decisions for treatment regimens.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_1352458520972573 for Temporal profile of serum neurofilament light in multiple sclerosis: Implications for patient monitoring by Peter A Calabresi, Douglas L Arnold, Dipen Sangurdekar, Carol M Singh, Arman Altincatal, Carl de Moor, Bob Engle, Jaya Goyal, Aaron Deykin, Suzanne Szak, Bernd C Kieseier, Richard A Rudick and Tatiana Plavina in Multiple Sclerosis Journal
Acknowledgments
This study was sponsored by Biogen. Biogen provided funding for medical writing support in the development of this manuscript: Linda Wagner, PharmD, from Excel Scientific Solutions provided medical writing support, and Nathaniel Hoover from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content.
Footnotes
Author Contributions: P.A.C. was global PI of the ADVANCE study and participated in the design and biomarker component. D.S., A.A., and C.d.M. analyzed the data. C.M.S., J.G., A.D., and R.A.R. designed and conceptualized the study. P.A.C. D.L.A., D.S., C.M.S., A.A., C.d.M., B.E., A.D., S.S., B.C.K., R.A.R., and T.P. interpreted the data. A.D., S.S., and R.A.R. drafted the manuscript for intellectual content. A.A., D.S., C.d.M., B.E., A.D., S.S., B.C.K., R.A.R., and T.P. revised the manuscript for intellectual content and approved the final version. P.A.C., D.L.A., C.M.S., and J.G. revised the manuscript for intellectual content; had a major role in acquisition of the data; and approved the final version.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.A.C. received consulting fees from Biogen and Disarm and grants from Annexon and Biogen. D.L.A. reports equity interest in NeuroRx during the conduct of the study and consulting activities with Biogen, Celgene, Frequency, Genentech, GeNeuro, Genzyme, MedImmune, Merck Serono, Novartis, Receptos, Roche, and Sanofi-Aventis, and received grants from Biogen, Immunotec, and Novartis. D.S., J.G., and T.P. were employees of Biogen at the time of this study. C.M.S., A.A., C.d.M., B.E., A.D., S.S., B.C.K., and R.A.R. are employees of and hold stock/stock options in Biogen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The original study and these analyses were supported by Biogen.
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Peter A Calabresi, Department of Neurology, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Douglas L Arnold, Montreal Neurological Institute, McGill University, Montreal, QC, Canada/NeuroRx, Montreal, QC, Canada.
Dipen Sangurdekar, Biogen, Cambridge, MA, USA.
Carol M Singh, Biogen, Cambridge, MA, USA.
Arman Altincatal, Biogen, Cambridge, MA, USA.
Carl de Moor, Biogen, Cambridge, MA, USA.
Bob Engle, Biogen, Cambridge, MA, USA.
Jaya Goyal, Biogen, Cambridge, MA, USA.
Aaron Deykin, Biogen, Cambridge, MA, USA.
Suzanne Szak, Biogen, Cambridge, MA, USA.
Bernd C Kieseier, Department of Neurology, Medical Faculty, Heinrich Heine University, Dusseldorf, Germany/Biogen, Cambridge, MA, USA.
Richard A Rudick, Biogen, Cambridge, MA, USA.
Tatiana Plavina, Biogen, Cambridge, MA, USA.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA.Multiple sclerosis. N Engl J Med 2018; 378(2): 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14(10): 577–589. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol 2014; 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huhn K, Senger D, Utz KS, et al. No evidence of disease activity status over 3 years in a real-world cohort of relapsing remitting MS patients in Germany. Mult Scler Relat Disord 2019; 27: 133–138. [DOI] [PubMed] [Google Scholar]
- 5.Lu G, Beadnall HN, Barton J, et al. The evolution of “no evidence of disease activity” in multiple sclerosis. Mult Scler Relat Disord 2018; 20: 231–238. [DOI] [PubMed] [Google Scholar]
- 6.Zetterberg H, Skillback T, Mattsson N, et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol 2016; 73(1): 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendricks R, Baker D, Brumm J, et al. Establishment of neurofilament light chain Simoa assay in cerebrospinal fluid and blood. Bioanalysis 2019; 11(15): 1405–1418. [DOI] [PubMed] [Google Scholar]
- 8.Sormani MP, Haering DA, Kropshofer H, et al. Blood neurofilament light as a potential endpoint in phase 2 studies in MS. Ann Clin Transl Neurol 2019; 6(6): 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 2020; 77(1): 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaetani L, Eusebi P, Mancini A, et al. Cerebrospinal fluid neurofilament light chain predicts disease activity after the first demyelinating event suggestive of multiple sclerosis. Mult Scler Relat Disord 2019; 35: 228–232. [DOI] [PubMed] [Google Scholar]
- 11.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81(6): 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141(8): 2382–2391. [DOI] [PubMed] [Google Scholar]
- 13.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92(10): e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): A randomised, phase 3, double-blind study. Lancet Neurol 2014; 13(7): 657–665. [DOI] [PubMed] [Google Scholar]
- 15.Kieseier BC, Arnold DL, Balcer LJ, et al. Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler 2015; 21(8): 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newsome SD, Scott TF, Arnold DL, et al. Long-term outcomes of peginterferon beta-1a in multiple sclerosis: Results from the ADVANCE extension study, ATTAIN. Ther Adv Neurol Disord 2018; 11: 1756286418791143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermel RA, Bakshi R.The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006; 5(2): 158–170. [DOI] [PubMed] [Google Scholar]
- 18.Rovaris M, Comi G, Rocca MA, et al. Short-term brain volume change in relapsing-remitting multiple sclerosis: Effect of glatiramer acetate and implications. Brain 2001; 124(pt 9): 1803–1812. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi PA, Arnold DL, Kinkel R, et al. Serum neurofilament light (NfL): Towards a blood test for prognosis and disease/treatment monitoring in multiple sclerosis patients. Neurology 2018; 90(15 Suppl): S24.003. [Google Scholar]
- 20.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014; 83(3): 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wattjes MP, Rovira A, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—Establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015; 11(10): 597–606. [DOI] [PubMed] [Google Scholar]
- 22.Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the consortium of MS centers task force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 2016; 37(3): 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020; 94(11): e1201–e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89(22): 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 2017; 88(9): 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020; 11(1): 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015; 84(8): 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amor S, van der Star BJ, Bosca I, et al. Neurofilament light antibodies in serum reflect response to natalizumab treatment in multiple sclerosis. Mult Scler 2014; 20(10): 1355–1362. [DOI] [PubMed] [Google Scholar]
- 29.Canto E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019; 76(11): 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakimovski D, Zivadinov R, Ramanthan M, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult Scler 2020; 26(13): 1670–1681. [DOI] [PubMed] [Google Scholar]
- 31.Kuhle J, Plavina T, Barro C, et al. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult Scler 2020; 26(13): 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sejbaek T, Nielsen HH, Penner N, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J Neurol Neurosurg Psychiatry 2019; 90(12): 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2018; 5(1): e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald K, Sotirchos E, De Moor C, et al. Serum neurofilament light chain is associated with MS outcomes and comorbidity in a large population of people with multiple sclerosis. ECTRIMS Online Library, 2019, https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/279375/kathryn.fitzgerald.serum.neurofilament.light.chain.is.associated.with.ms.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Adate%3D2019-04-17%2C2020-04-17%2Asearch%3Dneurofilament+light (accessed 30 April 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_1352458520972573 for Temporal profile of serum neurofilament light in multiple sclerosis: Implications for patient monitoring by Peter A Calabresi, Douglas L Arnold, Dipen Sangurdekar, Carol M Singh, Arman Altincatal, Carl de Moor, Bob Engle, Jaya Goyal, Aaron Deykin, Suzanne Szak, Bernd C Kieseier, Richard A Rudick and Tatiana Plavina in Multiple Sclerosis Journal
Data Availability Statement
To request access to data, please visit http://www.biogenclinicaldatarequest.com.