Summary
Genetic dissection of neural circuits has been accelerated by recent advances in viral-based vectors. This protocol describes an effective approach to performing intraspinal injections of adeno-associated viruses, which can be used to label, manipulate, and monitor spinal and supraspinal neurons. By avoiding invasive laminectomies and restrictive spinal-clamping and by adopting injectable anaesthetics and tough quartz glass micropipettes, our protocol presents a time-saving and efficient approach for genetic manipulation of neural circuits nucleated in the spinal cord.
For complete details on the use and execution of this protocol, please refer to Sathyamurthy et al. (2020).
Subject areas: Microscopy, Model organisms, Neuroscience
Graphical abstract
Highlights
-
•
Protocol for introducing AAVs into specific segments of the adult mouse spinal cord
-
•
Spinal injections (SI) offer an efficient way to target specific groups of spinal neurons
-
•
SI can be combined with brain injections for manipulating ascending/descending pathways
-
•
Protocol can be optimized for delivering other viruses and chemical tracers
Before you begin
The spinal cord plays a pivotal role in integrating, gating, and relaying somatosensory information, orchestrating movements, and controlling visceral functions. To accomplish these diverse functions, the spinal cord relies on a broad array of functionally specialized neurons. To determine the spinal correlates of any behavior, it is necessary to be able to specifically label and manipulate distinct populations of spinal neurons so as to unravel their connectivity and to test their contributions to specific aspects of sensorimotor and autonomic control.
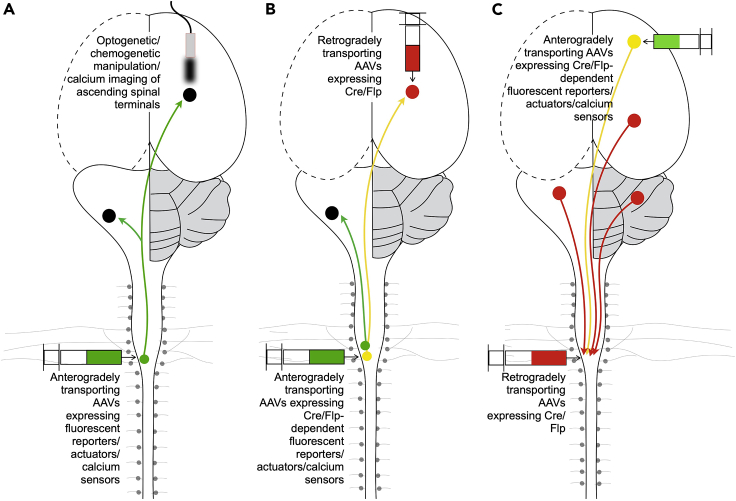
Over the last decade, advances in AAV technology have significantly enhanced our understanding of the circuit mechanisms underlying spinal cord function by enabling the labelling and manipulation of specific populations of spinal neurons. Some advantages of AAVs over other anterogradely transporting viruses such as lentiviruses, canine adenovirus type 2 (CAV-2), and herpes simplex viruses, are the high transduction efficiency, low toxicity, and stable transgene expression of AAVs (Li et al., 2020). Additionally, a huge library of ready-to-use AAVs expressing a wide variety of proteins, including Cre and Flp recombinases and Cre- and Flp-dependent and independent fluorescent reporters, optogenetic and chemogenetic actuators, and genetically encoded calcium indicators, are available from viral cores, including those at Addgene and several research institutions. Alternatively, custom-packaged recombinant AAV plasmids containing specific genes of interest can be obtained commercially (for example, Vigene Biosciences, Addgene, and Vector Biolabs), or can be produced in the lab (Fripont et al., 2019). A critical factor for successful delivery and expression of fluorescent proteins is the serotype of virus. In spinal cord research, anterogradely transporting AAVs containing the genome of serotype 2 packaged in capsids from serotypes 1, 2, 5, 8, or 9 (AAV2/1, AAV2/2, AAV2/5, AAV2/8, AAV2/9) have been routinely used for the manipulation of spinal neurons or ascending pathways (Dougherty et al., 2013; Fink et al., 2014; Bourane et al., 2015; Foster et al., 2015; Ruder, Takeoka and Arber, 2016; Choi et al., 2020; Sheahan et al., 2020; Barik et al., 2021; Gatto et al., 2021) (Figures 1A and 1B), while AAV retro (Tervo et al., 2016), which is a capsid variant of AAV2, is used for targeting descending neurons (Esposito et al., 2014; Basaldella et al., 2015; Murray et al., 2018; Sathyamurthy et al., 2020; Usseglio et al., 2020) (Figure 1C). For more information on AAVs, please refer to the following reviews (Li et al., 2020; Naso et al., 2017; Samulski and Muzyczka, 2014; Wang et al., 2019).
Figure 1.
Intraspinal delivery of viral vectors presents a powerful approach for addressing basic questions regarding the organization and function of spinal circuits
Genetically-defined groups of spinal neurons and the circuits that they are embedded in can be manipulated using two different groups of AAVs – anterogradely transporting (A and B) and retrogradely transporting AAVs (C). By injecting recombinant anterogradely transporting AAVs into different segments of the spinal cord in wildtype mice or genetic reporter lines, it is possible to acutely manipulate select spatially defined populations of spinal neurons, specifically in adulthood (Dougherty et al., 2013; Fink et al., 2014; Bourane et al., 2015; Foster et al., 2015; Ruder, Takeoka and Arber, 2016; Choi et al., 2020; Sheahan et al., 2020; Barik et al., 2021; Gatto et al., 2021) (A). By combining spinal injections of anterogradely transporting AAVs with stereotactic injections of retrogradely transporting AAVs in brain regions that are targeted by ascending spinal neurons, and/or by the placement of optic fibers or drug cannulae in specific brain regions, it is possible to manipulate ascending pathways in a circuit-specific manner (Conner et al., 2021; Fink et al., 2014; Bouvier et al., 2015; François et al., 2017; Sheahan et al., 2020) (B). Spinal injections of retrogradely transporting AAV (AAVretro) (Tervo et al. 2016) combined with stereotactic injections of anterogradely transporting viral vectors in the brain enables the targeting of functionally and somatotopically distinct groups of supraspinal neurons that communicate with specific segments of the spinal cord (Esposito, Capelli and Arber, 2014; Basaldella et al., 2015; Murray et al., 2018; Sathyamurthy et al., 2020; Usseglio et al., 2020).
Here we describe a streamlined technique for intraspinal injection of recombinant viral vectors into the spinal cord. While the protocol focuses on the injection of adenovirus associated viral vectors, it can be used to deliver other viruses and chemical tracers such as fluorogold, cholera toxin B, and retrobeads.
Part A: Preparation of the surgical setup
Timing: 45–60 min
-
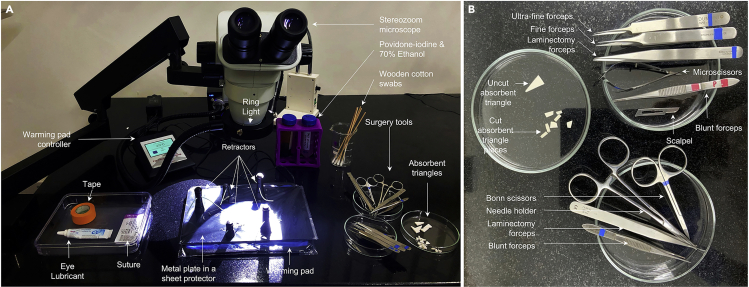
1.Disinfect the surgery bench housing the microscope (henceforth referred to as “surgery area”) with 70% ethanol, and arrange the following items on the surgery bench such that they are easily accessible during the surgery (Figures 2A and 2B).
-
a.Petri dish containing surgery tools (refer to step 2).
-
b.Petri dish containing absorption triangles (refer to step 3).
-
c.15 or 50 mL or tubes containing povidone-iodine and 70% ethanol solutions.
-
d.A tray containing wound clips and applier, adhesive tape, eye lubricant, and sutures.
-
e.Infrared warming pad and regulator.
-
f.Metal surgery plate and retractors (for cervical surgery) (part of the Magnetic Fixator Retraction System, see key resources table) for holding the mouse and the retracted skin and muscle in place, respectively.
-
a.
-
2.Sterilize surgical tools and supplies, including forceps, fine scissors, micro scissors, 100 mm glass Petri dish, and retractor hooks in an autoclave before transferring them to the surgery bench. Wrap all the items in aluminum foil and autoclave them at 132°C [270°F] (temperature) and 207 kPa (pressure) for 10 min. Once the items have been autoclaved, transfer the items to the surgery area. Alternatively, surgical equipment can be autoclaved in advance and stored for later use.
-
a.If using unsterilized wooden cotton swabs, place cotton swabs in a clean glass beaker with the cotton tip facing down, cover the beaker with aluminum foil, and sterilize in an autoclave. Transfer the required number of swabs to a clean Petri-dish or glass beaker in the surgery area. The rest can be covered and stored in a glass beaker for later use.
-
a.
Alternatives: Surgical tools can also be sterilized by incubating them in a glass bead sterilizer. If sterilizing tools in a hot bead sterilizer, spray surgical items with 70% ethanol, and once the tools are dry, insert the working tip of the tools into the pool of hot beads. Once the beads reach 233°C (464°F) (indicated by a green light), incubate tools for at least 20 seconds, wait for items to cool down, and then lay them out in a sterile glass Petri-dish such that they can be easily accessed during the surgery.
-
3.
Cut absorption triangles into 4–6 roughly equal sized pieces (∼20 mm2), as shown in Figure 2B, using sterile scissors, and lay out the pieces in a sterile Petri dish.
-
4.
Laminate the metal plate and the warming pad by wrapping them individually in commercially available A4-sized plastic sheet protectors (Figure 2A). Fold over any overhanging plastic, tuck it under the metal plate, and secure it firmly with tape so that the metal plate snugly fits in the plastic sheet.
Note: Lamination will prevent the metal plate and the warming pad from being soiled during surgery. Furthermore, during surgery, the mouse will be placed on the laminated window of the metal plate, which will facilitate easy transfer of the mouse from the surgery area to the injection area (Figure 2A).
-
5.
Place the metal plate on the warming pad and position the metal plate such that it can be easily viewed through the microscope (Figure 2A).
-
6.
Set the temperature on the warming pad to ∼30°C.
Note: Ensure that the temperature sensor is firmly attached to the pad and insulated with tape to prevent erratic readings, which might otherwise cause sub-optimal heating of the pad.
-
7.
Clean and disinfect the laminated metal plate, the magnetic retractors, and the warming pad by swabbing the surfaces with 70% ethanol.
Figure 2.
Photograph of the surgery set-up
(A) Photograph showing the surgery bench. (B) Photograph showing the surgery tools.
Part B: Preparation of the injection station
Timing: 15 min
-
8.
Pull quartz glass capillary tubes using a laser micropipette puller to produce micropipettes with long, sharp tips (parameters: heat – 650, filament – 4, velocity – 60, delay – 145, and pull – 185).
Alternatives: If a laser micropipette puller is not available, borosilicate glass capillary tubes could be used to make micropipettes (parameters: heat – 554, time – 200, pressure – 500, velocity – 70, delay – 145, and pull – 60) (Methods video S1).
Note: The parameters for pulling micropipettes depend on the heating filament in the micropipette puller machine and will have to be optimized each time the heating filament is changed.
-
9.
Move the plunger up and down through the Hamilton syringe a few times to eject any residual oil or dirt. Wipe away any oil that is ejected using soft tissue paper.
-
10.
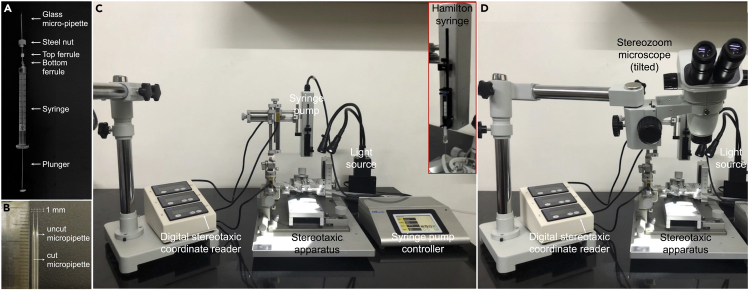
Remove the steel nut from the Hamilton syringe and thread it over the glass micropipette. Next, insert the unpulled end of the glass micropipette into the narrow end of the top ferrule, then insert the top ferrule into the narrow end of the bottom ferrule, and finally, thread the metal nut back onto the syringe to fix the micropipette in place (Figure 3A, Methods video S3).
-
11.
Carefully clip the tip of the glass micropipette using ultra-fine forceps (#5SF) to obtain a sharp tip (final tip diameter of around ∼20 μm). This will allow the virus solution to flow freely through the tip (Figure 3B, Methods video S2).
Figure 3.
Setup of the spinal injection station
Picture showing assembly of the Hamilton syringe and micropipette (A), cut and uncut micropipettes (B), and the injection set-up without (C) and with (D) the tilted stereozoom microscope.
Note: Tip diameter can be assessed under the microscope by placing the needle tip against a micro-meter scale.
-
12.
Fill a 10 mL syringe with mineral oil and fit it with a 26-gauge needle (priming syringe).
-
13.
Remove the plunger from the Hamilton syringe, and insert the needle of the priming syringe into the barrel. Slowly inject the mineral oil into the Hamilton syringe until the syringe and the attached micropipette are completely filled with oil and a drop of expelled oil can be seen at the tip of the micropipette. Slowly reinsert the plunger all the way into the syringe barrel till about only 1–2 ul of oil is left in the syringe barrel (Methods video S3).
Note: The Hamilton syringe needs to be primed to reduce the dead volume in the syringe and prevent the virus solution in the syringe from being wasted.
CRITICAL: While reinserting the plunger of the Hamilton syringe, expel any air bubbles that may have formed within the micropipette or the syringe by holding the syringe upright and allowing the bubble(s) to slowly rise while gently pushing the plunger. This ensures smooth flow of the virus solution during injections.
-
14.
Once the syringe and the attached micropipette have been primed with mineral oil, transfer a small aliquot of the desired virus from the −80°C freezer into an ice box filled with ice. Briefly spin down the tube containing the virus aliquot so that the solution settles down at the bottom of the tube. Dilute the stock solution with saline such that the physical viral titer is 1–5× 1012 genome copies/mL. Then add the fast green solution to track the flow of viral solution through the syringe. Once again spin down the tube to thoroughly mix the contents of the virus solution.
CRITICAL: Ensure that the fast green solution does not exceed 10%–15% of the final volume.
Note: Avoid multiple freeze-thaw cycles of AAVs as this may reduce the transduction efficiency of the virus. Upon receiving the AAV, we typically freeze the tube at −80°C. When the virus is to be injected the first time, we thaw the virus by placing the tube in ice and then aliquot the viral solution into individually labelled PCR tubes (1 ul per tube). We ensure that the PCR tubes are tightly sealed and then store the PCR tubes in 50 mL tubes.
Note: Wear gloves and adhere to institutional safety protocols while handling tubes and syringes containing AAVs.
-
15.
Using a 10 μL pipette, transfer ∼4–8 ul of the working solution of the virus onto a piece of parafilm (∼1 cm2).
-
16.
Insert the tip of the micropipette into the drop of viral solution on the parafilm, and slowly withdraw the solution into the Hamilton syringe while ensuring that no air bubbles are formed (Methods video S4)
Alternatives: Set the syringe pump controller on the stereotaxic injector to the “withdraw only” setting, place the syringe onto the syringe holder on the injector, and then run the syringe pump at a suitably slow rate (∼4 ul per minute) to withdraw the solution without forming air bubbles. Ensure that the micropipette tip does not get inadvertently blunted during this step.
-
17.
Fix the Hamilton syringe containing the virus solution onto the syringe holder on the stereotaxic injector (inset in Figures 3C and 3D, and Methods video S5).
Note: Raise the syringe holder to prevent the micropipette tip from breaking when transferring the mouse from the surgical area to the stereotaxic injector.
-
18.
Ensure that the solution can smoothly flow through the micropipette by running the syringe at a speed of 100–200 nl/min till a drop of the viral solution can be seen forming at the tip of the micropipette.
Part C: Setting up the mouse preparation station
Timing: 20 min
-
19.Prepare the appropriate volume of one of the following anaesthesia cocktails to anaesthetize mice. Please refer to Table S1 for calculating the concentration and volume of drugs.
-
a.Ketamine (100–200 mg/kg body weight) and xylazine (5–16 mg/kg body weight)
-
b.Fentanyl (0.05 mg/kg), dexmedetomidine (1 mg/kg), and midazolam (5 mg/kg) dissolved in salineNote: Also prepare the appropriate volume of the following reversal drug cocktail to awaken the mice after surgery (in order).
-
c.Buprenorphine (0.1 mg/kg), atipamezole (2.5 mg/kg)
-
d.Buprenorphine (0.1 mg/kg), atipamezole (2.5 mg/kg), and flumazenil (0.2 mg/kg) in saline.
-
a.
-
20.
Draw the drug cocktails in labelled 1 mL syringes fitted with a 26-gauge needle.
-
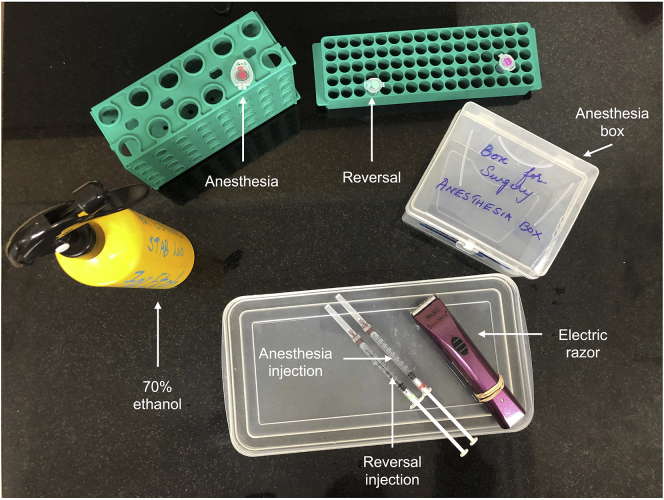
21.Gather the following items and lay them on a clean bench/surface (that has been decontaminated with 70% ethanol) for easy access during the mouse preparation step (Figure 4):
-
a.An empty pipette tip box or an empty jar in which mice can be placed following administration of anaesthesia.
-
b.Electric hair clipper/trimmer.
-
c.Shallow plastic box in which the mouse can be placed for shaving the back. If required, line the box with a paper towel, which can then be disposed of along with the shaved hair.
-
d.The syringes that contain the drug cocktails for anaesthetizing the mice and reversing the anaesthesia.
-
a.
Figure 4.
Photograph of the mouse preparation station
Part D: Preoperative procedures common to cervical and lumbar surgeries
Timing: 15 min
-
22.
Weigh the mouse (> 3 weeks old, either sex) and anesthetize the mouse by intraperitoneally injecting an appropriate volume of the anaesthetic drug cocktail in one of the lower quadrants of the mouse’s abdomen using a 1mL syringe fitted with a 26-gauge needle.
-
23.
Following injection, place the mouse in an empty cage or a vented container (such as an empty pipette tip box or a cylindrical jar), and ensure that it is covered so that the mouse does not escape (Figure 4).
-
24.
After 3–5 min, determine if the mouse is anesthetized by pinching one of the hind paws to trigger the pedal reflex response. Once the mouse is unresponsive, proceed to the next step.
Note: A fully anesthetized mouse should show no pedal reflex.
-
25.
Place the mouse on a clean surface. Using an electric shaver, shave the area spanning the neck and the upper back for cervical injections and the area around the hump at the middle/lower back for lumbar injections so as to remove any hair that might otherwise contaminate the surgical wound.
-
26.
Spray the exposed skin with 70% alcohol and gently wipe it with a paper towel to remove any loose hair that may have settled on the skin during shaving.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| pAAV1-hSyn-EGFP | Addgene | 50465-AAV1 |
| Chemicals, peptides, and recombinant proteins | ||
| Fentanyl∗ | Rusan Pharma Ltd. | Fenstud |
| Dexmedetomidine Hydrochloride | TCI chemicals | D5062 |
| Midazolam∗ | Aesmira | Napro Mida 1 |
| Atipemazole | TCI chemicals | A2956 |
| Buprenorphine hydrochloride (0.3mg/mL)∗ | Farbe Firma | Fargesic1 |
| Flumazenil | Neon | Fludot |
| Ketamine∗ | Aesmira | Zokent |
| Xylazine | Indian Immunologicals | Xylaxin |
| Meloxicam (5mg/mL) | Intas Pharmaceuticals | Melonex |
| Saline (Sodium Chloride 0.9% w/v) | Otsuka pharmaceutical | NS |
| Povidone-iodine (5% solution) | Cipla | Cipladine 500ML |
| Eye lubricant | Sun Pharma | Eyemist Gel |
| Mineral oil | Genei | FC21L |
| Fast Green FCF | TCI chemicals | F7258 |
| Paraformaldehyde | Sigma | P6148 |
| Ethanol | generic | generic |
| Experimental models: Organisms/strains | ||
| Crl:CD1(ICR) | Charles River Laboratories | Strain code 022 |
| CB6F1/ Crl | Charles River Laboratories | Strain code 176 |
| Other | ||
| Maxiamp PCR tube | Tarsons | 510053 |
| 50 mL tube (centrifuge tube conical bottom) | Tarsons | 546041 |
| Electric shaver | Wahl | 41590-0438 |
| Far Infrared Warming Pad Controller with warming pad | Kent-Scientific | RT0515 |
| Magnetic Fixator Retraction System | Fine Science Tools | 18200-20 |
| Tape (Shamrock Multipurpose Labelling Tape) | Tarsons | 680000 |
| Aluminum foil | Solimo | SFoil500gm18mFBA |
| Sheet protector | Avery | 74203 |
| 1 mL Syringe | Hindustan Syringes & Medical Devices | Dispo Van 1 mL |
| 3 mL Syringe | Hindustan Syringes & Medical Devices | Dispo Van 3 mL |
| 10 mL Syringe | Hindustan Syringes & Medical Devices | Dispo Van 10 mL |
| 26-Gauge needle | Hindustan Syringes & Medical Devices | Dispo Van Hypodermic Needle (0.5 inch) |
| 10 μL Hamilton syringe | Hamilton company | 7635-01 |
| Quartz Glass with Filament - 10 cm | Sutter instruments | QF100-70-10 |
| Borosilicate Glass with Filament - 10 cm | Sutter instruments | BF100-50-10 |
| Micropipette Puller (Flaming/Brown style) | Sutter instruments | P-1000 |
| Micropipette Puller (Laser-Based) | Sutter instruments | P-2000 |
| RN Compression Fitting 1 mm | Hamilton company | 55750-01 |
| Parafilm (Parafilm M) | Ted Pella | 807-5 |
| Micrometer Scale, 1mm in 0.01mm divisions | Ted Pella | 2285-13 |
| Scalpel Blades - #11 | Fine Science Tools | 10011-00 |
| Student Dumont #5 forceps | Fine Science Tools | 91150-20 |
| Laminectomy forceps (Dumont#2) | Fine Science Tools | 11223-20 |
| Extra fine forceps (Dumont#5SF) | Fine Science Tools | 11252-00 |
| Extra Fine Graefe Forceps | Fine Science Tools | 11150-10 |
| Scissors (Extra fine Bonn scissors) | Fine Science Tools | 14083-08 |
| Glass Bead Sterilizer | Cell Point | Germinator 500™ |
| Cotton swabs | Dealmed | 782010 |
| Glass Petri dish | Eisco Labglass | CH0370C |
| Glass beaker | Borosil | 1060D24 |
| McPherson-Vannas Micro Dissecting Spring Scissors | Roboz Surgical Instrument | RS-5600 |
| Absorption Triangles - Unmounted | Fine Science Tools | 18105-03 |
| Halsey needle holder | Fine Science Tools | 12001-13 |
| Wound Clips; Stainless Steel (Reflex7mm) | Roboz Surgical Instrument | RS-9255 |
| Wound Clip Applier (Reflex 7mm) | Roboz Surgical Instrument | RS-9250 |
| Suture 6-0 (polyglactin 910) | Dolphin sutures | DS 2670 |
| Stereo Zoom Microscope (OptoMag) | Khush Enterprises | KOM23SB |
| Standard LED Ring Light with Dimmer 64 LEDs 6/8cm | Khush Enterprises | KMA105 |
| Microscope Eyepiece 10× | Khush Enterprises | KMA121 |
| KDS Legato 130 Syringe Pump | RWD | KDS LEGATO® 130 |
| 69100 Rotational Digital Stereotaxic Instruments for Mice and Rat | RWD | K69100 |
| Light source | Khush Enterprises | Dual Gooseneck TT FO Point Light – 10w |
| Temperature-controlled incubator (ThermoCare® Water Jacketed Warmer ICU Base with Clear Dome Cover) | Thermocare | DW-1 |
| Hotpack (hot and cold softgel) | HealthClues | ASIN: hotcold |
| Handwarmer | Hothands | ASIN:B00VPO5EEC |
| Cryo-embedding compound | Pelco | 27300 |
Materials and equipment
CRITICAL:
-
•
Since some drugs (marked with an asterisk in the key resources table) may be classified as controlled substances based on local laws and regulations, please follow appropriate regulations on storage, use, and disposal of these substances.
-
•
Please wear gloves while handling drugs and biological materials and adhere to institutional safety protocols.
-
•
Please comply with institutional guidelines on the care and use of animals in research.
Alternatives:
-
•
Any standard surgical/dissection microscope (5–10× zoom) can be used for the surgery. For spinal injections, the microscope body needs to be tilted so that there is enough clearance for the syringe to be manipulated and be viewed as it is being lowered into the spinal cord.
-
•
If an infrared warming pad is not available, commercially-available hand warmers or gel-based heating pads can be used. However, it is important to note that this method might be less efficient, and since the temperature cannot be finetuned, it is critical to periodically ensure that the warmer is neither too hot nor too cold.
-
•
If the “Magnetic Fixator Retraction System” is unavailable, any sturdy metal plate (10–20 cm × 30cm) with a window (5 cm × 7.5 cm) of the appropriate size can be used for holding the mouse, and custom-made retractors as shown in Figure 8 can be taped to the plate and be used for holding the retracted skin and muscles in place.
-
•
Instead of glass Petri-dishes, any sterile surface can be used for placing surgery tools.
-
•
While CB6F1J mice have been used in this paper, authors could extend this protocol to ICR and C57BL/6 mice without any need for optimization.
-
•
For injection of more viscous substances such as retrobeads, it is important to use a micropipette with a larger tip diameter so that the solution can easily flow through the micropipette.
Figure 8.
Picture showing a custom-built retractor
Step-by-step method details
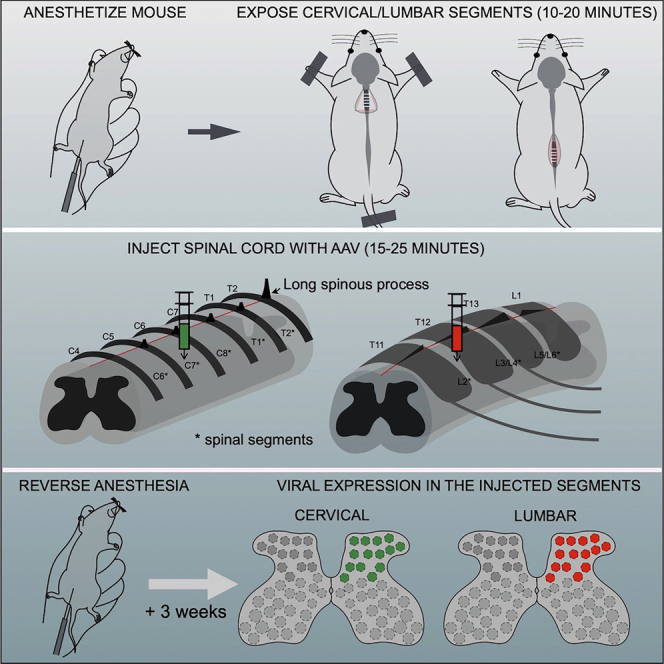
This section provides detailed instructions for (a) performing surgeries for exposing the cervical (part 1) and lumbar segments (part 2) of the spinal cord, (b) intraspinal injection of the cervical and lumbar segments (part 3), and (c) reversing anesthesia and post-operative care following surgery (part 4). If performing cervical injections alone, skip part 2 and proceed directly to part 3. If performing lumbar injections alone, skip part 1 and proceed directly to part 2. If performing both cervical and lumbar injections on the same mouse, finish injecting one region, close the wound, and then proceed to perform surgery and injections on the second region.
Part 1: Operative procedure for cervical spinal injections
Timing: 10–20 min
Here we describe how to perform surgery for exposing cervical segments of the spinal cord for intraspinal injections. Upon completion of the following steps, the cervical segments should be readily accessible for intraspinal injections (Figure 5 and Methods video S6).
-
1.Transfer the mouse to the surgical area and place it on the window of the metal plate such that the underside of the mouse is exposed to the heating pad, then prepare the mouse for surgery.
-
a.Tape the forelimbs to the metal plate (Figure 5A).
 CRITICAL: Take care not to tape the whiskers while taping the mouse.
CRITICAL: Take care not to tape the whiskers while taping the mouse. -
b.Place a 3 mL syringe or a similarly sized wedge under the lower neck/upper back (Figure 5B).Note: This will help lift the upper back of the mouse, providing easier access to the cervical spinal cord during surgery.
-
c.Finally, tape the hindlegs and the tail at two points as shown in Figure 5A such that the mouse remains taut along the rostro-caudal axis throughout the procedure (Troubleshooting 1).
 CRITICAL: Ensure that the mouse is not pressed against the plate too tightly, which might otherwise lead to death or respiratory distress.
CRITICAL: Ensure that the mouse is not pressed against the plate too tightly, which might otherwise lead to death or respiratory distress. -
d.Once the mouse has been satisfactorily positioned, apply eye lubricant on both eyes to prevent drying of the eyes during surgery.
-
a.
-
2.
Sterilize the surgical site by swabbing the skin with three alternating rubs of povidone-iodine and 70% ethanol.
-
3.
Using a scalpel, make a 2 cm long incision starting at the base of the head and extending through the midline of the back (Figure 5C). Slide a pair of blunt forceps under the rostral margin of the incision and use the retractor at the mouse’s head to retract the skin. Using the other retractors on the sides, retract the skin along the caudal margin on either side to create a triangular surgical window (Figure 5D).
-
4.
In the exposed tissue, locate the margin (along the mediolateral axis) between the rostrally located trapezius muscle and the caudally located, lighter coloured adipose tissue. Using micro scissors, gently cut any fascia that might hold these two pieces of tissue along this margin. Adjust the rostrally placed retractor to tuck away the musculature, and similarly, adjust the other two retractors to tuck away the adipose tissue.
-
5.
Locate the prominent, long spinous process of the second thoracic vertebra (T2 vertebra), which protrudes dorsally from the spinal cord (Figure 5E). Using micro scissors, snip the muscles that are attached rostrally to this vertebra. Gently slide the micro scissors underneath the rostrally located musculature and cut it along the midline to detach it from the underlying cervical vertebrae. Use two new retractors, one on either side of the mouse, to retract the midline muscles (Figure 5F).
-
6.
Use the spinous process of the second thoracic vertebra (T2) as a landmark and count up from T2 to locate the appropriate cervical segments for injection (Harrison et al., 2013).
Note: The number and identity of segments to be injected depend on the research question. Typically, to target neurons in the lower cervical regions, spinal segments between C5-C6, C6-C7, and C7-C8 vertebrae can be injected (Figure 5F).
-
7.
Using an absorption triangle, gently swab the exposed vertebrae and remove any residual tissue so as to obtain a clear view of the spinal cord segments.
-
8.
To facilitate smooth entry of the micropipette into the spinal cord, puncture the meninges using a fine-tipped forceps (preferably Dumont #5SF Forceps) and peel it away (Troubleshooting 2) (Methods video S6).
Note: If both sides of the spinal cord are to be injected, remove the meninges on either side of the midline.
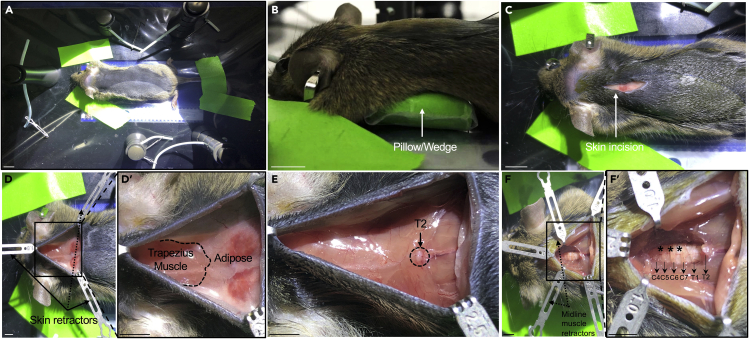
Figure 5.
Representative images of a mouse at different stages of cervical injection
(A) Mouse is kept taut along the rostro-caudal axis by taping the limbs and tail to the metal plate. Scale bar – 1 cm.
(B) A wedge is inserted below the mouse to make access to the spinal cord easier. Scale bar – 1 cm.
(C) Image showing skin incision. Scale bar – 1 cm.
(D and D′) (D) Retractors are used to hold open the incision on the skin. A magnified image of the skin incision depicting the musculature and adipose tissue underlying the skin (D′). Scale bar – 3 mm.
(E) Image showing T2 vertebra. Scale bar – 3 mm.
(F and F′) (F) Image showing exposed cervical segments (magnified in F′). Scale bar – 3 mm.
(Skip to Part 3 if only cervical injections are being performed).
Part 2: Operative procedure for lumbar surgery
Timing: 10–20 min
Here we describe how to perform surgeries for exposing lumbar segments of the spinal cord for intraspinal injections. Upon completion of the following steps, the lumbar segments should be readily accessible for intraspinal injections (Figure 6 and Methods video S7).
-
9.
Using a scalpel, make an incision 2 cm in length, starting ∼1cm rostral to the middle of the hump (Figures 6A and 6B). Gently cut the musculature along the midline of the hump with micro scissors to expose the underlying vertebrae (Figure 6C).
-
10.
Locate the T12-T13 vertebral junction, which is usually centered at the peak of the hump. Locate the lumbar segments of interest by counting up or down from the identified vertebra.
Note: To target neurons in the lumbar cord (L1-L6 spinal segments), we typically inject the spinal segments between T11-12, T12-13, and T13-L1 vertebrae (Figure 6D).
Alternatives: Since the floating 13th rib (the lowest rib in mice) is attached to rostral end of the T13 vertebra, the latter can be identified by gently palpating the 13th rib and tracing its point of insertion into the spinal cord. To locate the 13th rib, pull down the incised skin along the left margin. This should expose 2–3 roughly parallel floating ribs of which the caudal most one is the 13th rib (Methods video S7). If required, two magnetic retractors can be used to increase the area exposed under the skin.
CRITICAL: While palpating make sure not to damage the rib or puncture the pleural space.
-
11.
Cut away the tissue that hold the vertebrae together to reveal the underlying spinal segments. (Troubleshooting 2)
Note: Unlike cervical surgery, moderate oozing or bleeding is common during lumbar surgeries, and so, periodically soak the excess blood using absorption triangles (Methods video S7).
-
12.
Gently swab the exposed vertebrae with absorption triangles to remove any residual tissue and to obtain a clear view of the spinal segments.
-
13.
Carefully puncture the meninges and peel it away to facilitate smooth entry of the micropipette into the spinal cord.
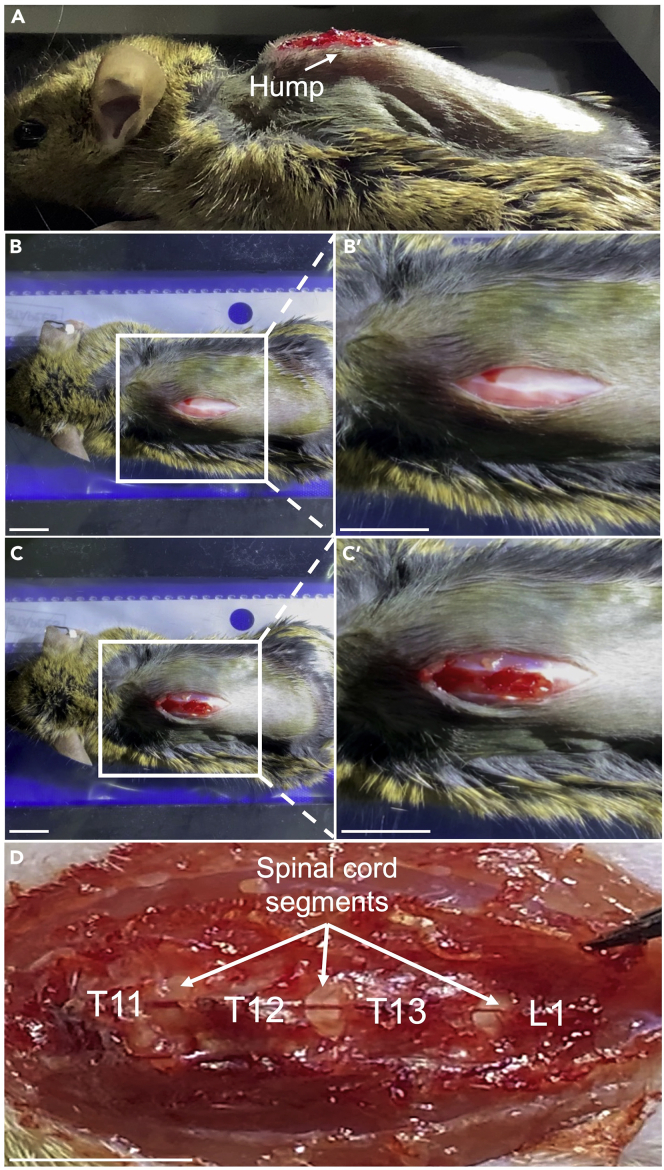
Figure 6.
Representative images of a mouse at different stages of lumbar injection
(A and B) An incision is made on the dorsal hump skin to expose the underlying musculature.
(C) The musculature is gently separated to expose the vertebrae corresponding to lumbar spinal segments.
(D) The exposed spinal segments with the corresponding vertebrae labelled.
Scale bar – 1 cm.
Part 3: Procedure for cervical and lumbar injections
Timing: 15–25 min
Here we describe how to perform intraspinal injections.
-
14.
After the relevant spinal cord segments have been exposed, transfer the mouse along with the metal plate to the stereotaxic stage.
CRITICAL: Take care not to disturb the mouse or the retractors, which may otherwise impede clear view of the cervical segments. While positioning the mouse on the stereotaxic stage, ensure that there is adequate clearance between the mouse and the tip of the micropipette.
-
15.
Adjust the metal plate containing the mouse and the arms of the stereotaxic manipulator such that the micropipette tip is positioned directly above the surface of the first spinal segment to be injected. The micropipette should be 400–600 μm away from the midline.
Note: The midline along the rostro-caudal axis can be identified by the blood vessel that runs along the length of the spinal cord.
-
16.
Set the total volume and rate of release of viral particles on the syringe pump. Typically, 250 nl of the solution containing viral particles is injected in each of the cervical segments, while 300 nl is injected in each of the lumbar segments at a rate of 75–100 nl per minute.
CRITICAL: Before performing the injections, run the program till a tiny drop of the viral solution can be seen forming at the tip of the micropipette to ensure that the micropipette is not blocked by any debris that might impede solution flow.
-
17.
Use the axis manipulators on the stereotaxic injector to gently lower the micropipette (Methods video S8) so that it just about touches the surface of the spinal cord, and then tare the reading for the dorso-ventral coordinate.
CRITICAL: Taring the dorso-ventral coordinate is crucial since it enables accurate measurement of the depth of the micropipette tip while performing the injections.
Note: If using an injector that does not digitally display the coordinates, note the start position of the rotating wheel and refer to manufacturer instructions to determine the number of rotations of the wheel that is required to advance the syringe by a millimeter. Typically, one rotation of the wheel should advance the syringe by a millimeter.
-
18.
To infect neurons in the dorsal laminae of the spinal cord, gently lower the micropipette to a depth of 500 μm and pull back 200 μm, so that micropipette tip settles at ∼ 300 μm from the dorsal surface. To infect neurons in the ventral laminae of the spinal cord, gently lower the micropipette to a depth of 800–1000 μm and withdraw 200 μm, so that the micropipette tip settles at a depth of ∼600–800 μm from the dorsal surface (Troubleshooting 3).
Note: After a segment has been injected, wait one minute before pulling the micropipette out to ensure that the viral solution is efficiently absorbed by the tissue and does not leak out. Additionally, before proceeding to inject the next segment, run the program till a small drop of virus solution can be seen pooling at the tip (Troubleshooting 4).
-
19.
Repeat the preceding two steps to inject the remaining segments.
-
20.
Once the mouse is injected, move the metal plate containing the mouse to a flat surface. Wait for bleeding, if any, to stop. Gently remove the retractors holding the muscles and then suture the midline muscles using a 6-0 absorbable suture (Methods video S9).
-
21.
Next, remove the retractors holding the skin and use wound clips or 6-0 sutures to close the skin wound (Methods video S9).
Part 4: Post-operative care
Timing: 10–30 min
-
22.
Reverse the anesthesia by intraperitoneally injecting an appropriate volume of the reversal drug cocktail.
-
23.
Return the mouse to its home cage.
-
24.
The mouse should awaken within 2–4 min and ambulate within 15–20 min of administering the reverse cocktail.
-
25.
To counteract any loss in body temperature, place a hot pack or hand warmer on the cage floor overnight.
Alternatives: The cage containing the mouse can be placed in a temperature-controlled incubator for a few hours before being returned to the holding cage rack. In this case, remove the top lid or the air filter so that the cage is adequately vented during the time in the incubator. Follow manufacturer’s instructions to ensure that the ambient temperature in the incubator is around 25°C–27°C.
-
26.
Place a few food pellets on the floor of the cage for a day, so that the mice can easily access the food.
-
27.
Administer analgesics (Meloxicam) for two days following surgery using a 1mL syringe with a 26-gauge needle (please refer to Table S1 for calculating the concentration and volume of meloxicam). While handling mice for injecting analgesics, avoid scruffing the mouse as this may not only be painful for the mouse, but also irritate the wound and disturb the staples or sutures. Instead, gently place the mouse on the steel food rack and lift the base of the mouse away from the rack floor by lifting the tail to gain access to the intraperitoneal space and then swiftly inject the mouse (Methods video S10).
-
28.
The day after the surgery, monitor mice for any signs of spinal damage, including partial or complete paralysis in one or more limbs after surgery. While relatively uncommon (∼ < 5% of mice injected), varying degrees of paralysis might result from damage to the spinal cord during surgery depending on the extent and location of the injury on the spinal cord. Euthanize mice displaying paralysis to prevent further distress to the mice.
Expected outcomes
Some advantages of the technique we present over other methods of intraspinal injections are that: (a) laminectomies are avoided, making it less invasive; (b) the use of injectable anesthesia rather than isoflurane circumvents the need for a nose-cone, which allows greater flexibility in the handling and positioning of the animal during surgery; (c) the spinal cord does not have to be clamped during surgery, which makes it less technically-challenging and helps save time; (d) the use of quartz glass micropipettes for cervical surgeries enables easy penetration of the cervical segments.
Three weeks following intraspinal delivery of adeno-associated viruses expressing fluorescent reporters, reporter expression should be seen in the side of the spinal cord that was injected and along the rostro-caudal axis of the spinal cord spanning the spinal segments that were injected. To determine the efficacy of viral injection, we harvested the spinal cord (Kennedy et al., 2011) from spinal-injected mice (following cardiac perfusion with 10 mL of PBS followed by 10 mL of 4% PFA), post-fixed cords by incubating cords in 4% PFA for one hour, cryo-protected cords by incubating in 30% sucrose overnight, embedded cords in OCT medium (cryo-embedding compound), cut transverse sections (50 μm thickness) of the spinal cord and assessed every 10th section (∼ at 500 μm intervals) for reporter expression. Typically, reporter expression should be confined to the side of the spinal cord that was injected (for unilateral injections) (Figures 7A and 7B) or be present on both sides (for bilateral expression) and gradually taper off in the spinal segments flanking the injection sites. (Troubleshooting 5)
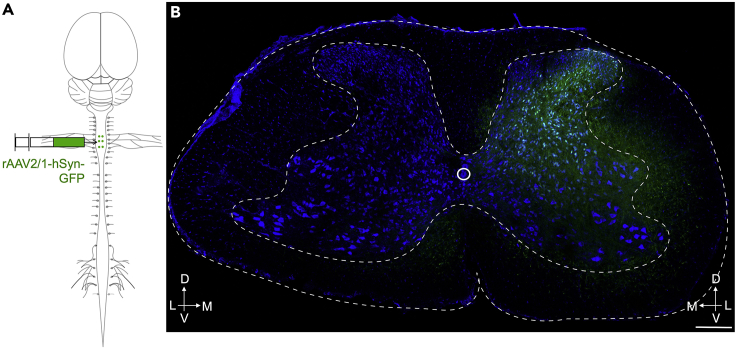
Figure 7.
Expected outcome
(A) Schematic showing unilateral injection of anterogradely transporting rAAV2/9-hSyn-GFP into the cervical cord.
(B) Transverse section showing unilateral distribution of GFP in the cervical spinal cord. A six-week-old mouse was injected as shown in A. Transverse sections were collected 3 weeks following injection and examined for the presence of GFP.
Scale bar – 200 μm.
Limitations
An inherent limitation of intraspinal injections is that muscles overlying the cervical and lumbar segments will need to be cut. Since this may affect behavior, ensure that control mice are similarly operated upon and injected. Since cuts will take time to heal, allow 3–4 weeks between surgery and behavior experiments. Another limitation of spinal injections is that while neurons in the dorsal and ventral sections of the spinal cord can be differentially targeted, it remains difficult to exclusively target neurons in a specific lamina.
Troubleshooting
Problem 1
The mouse suffers from respiratory distress during cervical surgery. (Part 1, Step 1, (c))
Potential solution
It is likely that the mouse is pressed too firmly against the plate. In this case, adjust the tapes so that there is enough slack.
Problem 2
Excessive hemorrhaging during cervical and lumbar surgeries. (Parts 2 & 3)
Potential solution
Bleeding should be minimal, if at all, during cervical injections since very few major blood vessels run through the muscles that overlie the cervical segments. Ensure that no muscles are cut apart from the ones mentioned in the steps. On the other hand, bleeding is common in lumbar surgeries. To minimize hemorrhaging, take care not to cut any blood vessels running along the midline of the spinal cord. Additionally, ensure that the retractors are not pulling hard on the tissue.
Problem 3
Bending of the glass micropipette while attempting to inject the spinal cord. (Part 3, Step 18)
Potential solution
The presence of meninges overlying the spinal cord may provide resistance to the insertion of the micropipette tip and may result in the bending or breaking of the micropipette tip. This typically happens more often during cervical than lumbar injections, because the dura and white matter in the dorsal surface is thicker in the cervical compared to lumbar segments (Yang et al., 2019; Kwon et al., 2018). Tip breaking incidents can be reduced by using capillary tubes made of quartz instead of borosilicate to make micropipettes, and by using a micropipette whose tip is beveled and sharp. Additionally, ensure that the meninges have been completely removed during surgery before proceeding to injections.
Problem 4
Solution does not flow through the micropipette. (Part 3, Step 18)
Potential solution
The presence of air bubbles or residual debris from previous injections could impede the flow of the contents of the syringe. Before performing the injections, ensure there are no air bubbles in the syringe. If there are air bubbles towards the tip of the micropipette, hold the syringe with the micropipette tip facing up and allow the bubbles to rise and then expel the bubbles. If there appears to be any debris, insert the needle in an Eppendorf tube containing saline and expel a few microliters into the saline. Alternatively, increase the flow rate of the syringe pump to 1000–2000 nl/min and stop as soon as a drop of solution pools at the micropipette tip and then run the program as usual (75–100 nl/min).
Problem 5
Expression of the virus after injection might be unsatisfactory. (expected outcomes)
Potential solution
Typically, we inject viruses at a concentration of 1–5 × 1012 genome copies/mL. If reporter expression is weak and/or is present in fewer cells than expected, test 3 different titers between 5–20 × 1012 genome copies/mL to determine the optimal titer. Since repeated freeze-thaw cycles might lower the transduction efficiency of the virus, aliquot viruses in small volumes (1 ul per PCR tube) and discard any virus solution that remains after injections. Another possibility is that in some cases, fluorescent reporter expression could be weak, and so, spinal cord sections may need to be immuno-stained with antibodies against the fluorescent protein to be able to visualize reporter expression.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Anupama Sathyamurthy (anupamasathy@iisc.ac.in).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
The authors gratefully acknowledge the technical support of Annappaswamy K and the staff at the central animal facility, IISC, for mouse husbandry; Dr. Narendra Ramanan at the Centre for Neuroscience, IISc, for access to the cryostat and fluorescence microscope; and the bioimaging facility at IISc for access to the confocal microscope. Addgene viral prep #50465-AAV1 was a gift from Bryan Roth (Addgene plasmid #50465; http://n2t.net/addgene:50465; RRID:Addgene_50465). This research was supported in part by funds from the International Brain Research Organization and the Department of Biotechnology, Government of India. A.S. and S.C. were supported by funds from the Ramalingaswamy fellowship awarded by the Department of Biotechnology, Government of India, and A.B. was supported by funds from the intermediate career fellowship awarded by the DBT/Wellcome Trust India Alliance.
Author contributions
Conceptualization, writing – original draft, and visualization, A.S. and S.C.; investigation, S.C. and A.B.; writing – review and editing, S.C., A.S., and A.B.; supervision and funding acquisition, A.S. and A.B.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100786.
Supplemental information
Data and code availability
The published article includes all data sets generated or analyzed during this study.
References
- Barik A., Sathyamurthy A., Thompson J., Seltzer M., Levine A., Chesler A. A spinoparabrachial circuit defined by Tacr1 expression drives pain. eLife. 2021;10 doi: 10.7554/eLife.61135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaldella E., Takeoka A., Sigrist M., Arber S. Multisensory signaling shapes vestibulo-motor circuit specificity. Cell. 2015;163:301–312. doi: 10.1016/j.cell.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Bourane S., Grossmann K.S., Britz O., Dalet A., Del Barrio M.G., Stam F.J., Garcia-Campmany L., Koch S., Goulding M. Identification of a spinal circuit for light touch and fine motor control. Cell. 2015;160:503–515. doi: 10.1016/j.cell.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Caggiano V., Leiras R., Caldeira V., Bellardita C., Balueva K., Fuchs A., Kiehn O. Descending command neurons in the brainstem that halt locomotion. Cell. 2015;163:1191–1203. doi: 10.1016/j.cell.2015.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Hachisuka J., Brett M.A., Magee A.R., Omori Y., Iqbal N.-U.-A., Zhang D., DeLisle M.M., Wolfson R.L., Bai L. Parallel ascending spinal pathways for affective touch and pain. Nature. 2020;587:258–263. doi: 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner J.M., Bohannon A., Igarashi M., Taniguchi J., Baltar N., Azim E. Modulation of tactile feedback for the execution of dexterous movement. 2021. [DOI] [PMC free article] [PubMed]
- Dougherty K.J., Zagoraiou L., Satoh D., Rozani I., Doobar S., Arber S., Jessell T.M., Kiehn O. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron. 2013;80:920–933. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Esposito M.S., Capelli P., Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Fink A.J.P., Croce K.R., Huang Z.J., Abbott L.F., Jessell T.M., Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature. 2014;509:43–48. doi: 10.1038/nature13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E., Wildner H., Tudeau L., Haueter S., Ralvenius W.T., Jegen M., Johannssen H., Hösli L., Haenraets K., Ghanem A. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François A., Low S.A., Sypek E.I., Christensen A.J., Sotoudeh C., Beier K.T., Ramakrishnan C., Ritola K.D., Sharif-Naeini R., Deisseroth K. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron. 2017;93:822–839.e6. doi: 10.1016/j.neuron.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fripont S., Marneffe C., Marino M., Rincon M.Y., Holt M.G. Production, Purification, and Quality Control for Adeno-associated Virus-based Vectors. JoVE (Journal of Visualized Experiments) 2019 doi: 10.3791/58960. e58960. [DOI] [PubMed] [Google Scholar]
- Gatto G., Bourane S., Ren X., Di Costanzo S., Fenton P.K., Halder P., Seal R.P., Goulding M.D. A functional topographic map for spinal sensorimotor reflexes. Neuron. 2021;109:91–104.e5. doi: 10.1016/j.neuron.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M., O’Brien A., Adams L., Cowin G., Ruitenberg M.J., Sengul G., Watson C. Vertebral landmarks for the identification of spinal cord segments in the mouse. NeuroImage. 2013;68:22–29. doi: 10.1016/j.neuroimage.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Kennedy H.S., Puth F., Hoy M.V., Pichon C.L. A method for removing the brain and spinal cord as one unit from adult mice and rats. Lab. Anim. 2011;40:53–57. doi: 10.1038/laban0211-53. [DOI] [PubMed] [Google Scholar]
- Kwon S., Suh S.-W., Kim D., Rhyu I.J., Yu H., Han S.W., Hong J.-Y. Analysis of dural sac thickness in the human cervical spine. Anat. Sci. Int. 2018;93:284–290. doi: 10.1007/s12565-017-0412-z. [DOI] [PubMed] [Google Scholar]
- Li C., Jude Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev, Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- Murray A.J., Croce K., Belton T., Akay T., Jessell T.M. Balance control mediated by vestibular circuits directing limb extension or antagonist muscle co-activation. Cell Rep. 2018;22:1325–1338. doi: 10.1016/j.celrep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Naso M.F., Tomkowicz B., Perry W.L., I.I.I., Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder L., Takeoka A., Arber S. Long-distance descending spinal neurons ensure quadrupedal locomotor stability. Neuron. 2016;92:1063–1078. doi: 10.1016/j.neuron.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Samulski R.J., Muzyczka N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu Rev Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- Sathyamurthy A., Barik A., Dobrott C.I., Matson K.J.E., Stoica S., Pursley R., Chesler A.T., Levine A.J. Cerebellospinal neurons regulate motor performance and motor learning. Cell Rep. 2020;31:107595. doi: 10.1016/j.celrep.2020.107595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.D., Warwick C.A., Fanien L.G., Ross S.E. The neurokinin-1 receptor is expressed with gastrin-releasing peptide receptor in spinal interneurons and modulates itch. J. Neurosci. 2020;40:8816–8830. doi: 10.1523/JNEUROSCI.1832-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo D.G.R., Hwang B.-Y., Viswanathan S., Gaj T., Lavzin M., Ritola K.D., Lindo S., Michael S., Kuleshova E., Ojala D. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usseglio G., Gatier E., Heuzé A., Hérent C., Bouvier J. Control of orienting movements and locomotion by projection-defined subsets of brainstem V2a neurons. Curr. Biol. 2020;30:4665–4681.e6. doi: 10.1016/j.cub.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Wang D., Tai P., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nature reviews. Drug discovery. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Yang X., Lan X., Zhang H., Wang M., Zhang Y., Xu Y., Zhen P. [Structure and mechanical characteristics of spinal dura mater in different segments of sheep’s spine] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019;33 doi: 10.7507/1002-1892.201807085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data sets generated or analyzed during this study.