Abstract
The quantitative polymerase chain reaction (qPCR) method presented in this study allows the identification of pneumococcal capsular serotypes in cerebrospinal fluid without first performing DNA extraction. This testing approach, which saves time and resources, demonstrated similar sensitivity and a high level of agreement between cycle threshold values when it was compared side-by-side with the standard qPCR method with extracted DNA.
Keywords: Streptococcus pneumoniae, direct real-time PCR, pneumococcal serotype
Streptococcus pneumoniae is a leading bacterial etiology of a wide range of infections, including community-acquired pneumonia, meningitis, otitis media, and sepsis [1]. There are now 100 recognized S. pneumoniae serotypes whose distribution varies both temporally and geographically [2]. Quantitative polymerase chain reaction (qPCR) methods for the identification of S. pneumoniae serotypes using DNA extracted from clinical specimens or pneumococcal isolates have been described previously [3–5]. It is a laborious process, and the DNA extraction step can constitute a source of cross-contamination and a bottleneck in high-throughput laboratories. To alleviate some of the burden associated with the above-mentioned traditional qPCR, we validated a direct qPCR method that allows the identification of S. pneumoniae serotypes directly from cerebrospinal fluid (CSF) specimens positive for S. pneumoniae by lytA qPCR without first extracting DNA, thereby reducing processing time, cost, labor, and risk of cross-contamination.
MATERIALS AND METHODS
The triplex direct qPCR method was validated using the same schemes and the same oligonucleotides as described by Pimenta et al [3], with the exception that the final concentrations of the oligonucleotides were optimized in the direct qPCR (Table 1).It consists of 7 sequential triplex reactions (21 assays) that identify 37 pneumococcal capsular serotypes as 11 individual serotypes plus 10 small serogroups. Reaction mixtures were prepared in a final volume of 25 µL, including variable volumes of each primer and probe to reach the desired final concentration, 2 µL of CSF as DNA template, 12.5 µL of mastermix (PerfeCTa MultiPlex qPCR ToughMix, QuantaBio), and PCR-grade water (Supplementary Materials). The thermal profile condition for the qPCR runs was 1 cycle of 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Each run included appropriate positive control DNA and no template negative controls (NTCs). A reaction was considered valid if all NTCs were negative and control DNA was positive.
Table 1.
Primer and Probe Sequences and Concentrations Used in the Study
| Primer/Probe ID [3] | Sequence (5′ – 3′) [3] | Probe Dye [3] | Probe Special Chemistry [3] | Quencher (3′) [3] | Traditional PCR, nM [3] | Direct PCR, nM |
|---|---|---|---|---|---|---|
| 1-F | TTTCATCCCTATGTGTGGTATAG | 300 | 600 | |||
| 1-R | GCTTTAGAAGGTAGAGTTAACAAC | 300 | 600 | |||
| 1-Probe | TGCCAAAGCCAGCCAT | FAM | LNAa | BHQ1 | 100 | 200 |
| 2-F | TGTTATCCCATATAAGAACCGAGTGT | 300 | 600 | |||
| 2-R | AAAATTACCCCAAAAGCTATCCAA | 300 | 600 | |||
| 2-Probe | TTGCAATT“T”CAATTTTTTTGCCCCAATCTC | FAM | “T” b= BHQ1 | BHQ1 | 200 | 400 |
| 3-F | CCACTAAAGCTTTGGCAAAAGAAA | 300 | 600 | |||
| 3-R | CCCGAACGTAAAGCTTCTTCA | 300 | 600 | |||
| 3-Probe | TTGTAGACCGCCCCACAA“T”TCATTTTGT | HEX | “T” b= BHQ1 | BHQ1 | 200 | 400 |
| 4F | GCTTCTGCTGTAACTGTTGTGC | 300 | 600 | |||
| 4-R | CACCACCATAGTAACCAAAGTTCC | 300 | 600 | |||
| 4-Probe | TTCCACAAAAGAAGAGCCTACAGGTAACCCCA | ROX | BHQ2 | 100 | 200 | |
| 5-F | CATGATTTATGCCCTCTTGCAA | 300 | 600 | |||
| 5-R | GACAGTATAAGAAAAAGCAAGGGCTAA | 300 | 600 | |||
| 5-Probe | TCTTCTTCTCA“T”CGTTTCCGCATGCTTTT | HEX | “T” b= BHQ1 | BHQ1 | 200 | 400 |
| 6A/6B/6C/6D-F | GTTTGCACTAGAGTATGGGAAGG | 200 | 400 | |||
| 6A/6B/6C/6D-R | TAGCCTTTCTGAAAACATTTAGCG | 200 | 400 | |||
| 6A/6B/6C/6D-Probe | TGTTCTGCCC“T”GAGCAACTGGTCTTGTATC | FAM | “T” b= BHQ1 | BHQ1 | 200 | 400 |
| 6C/6D-F | TTGGGATGATTGGTCGTATTAG | 200 | 400 | |||
| 6C/6D-R | CTCTTCAATTAGTTCTTCAGTTCG | 200 | 400 | |||
| 6C/6D-Probe | CCACGCAATTCGCCATC | FAM | LNAa | BHQ1 | 100 | 200 |
| 7F/7A-F | ATGAAGGCTTTGGTTTGACAGG | 200 | 400 | |||
| 7F/7A-R | ATTCTCGCCATCAATTGCATATTC | 200 | 400 | |||
| 7F/7A-Probe | ACACCACTATAGGCTGTTGAGACTAACGCACA | ROX | BHQ2 | 100 | 200 | |
| 9V/9A-F | AGGTATCCTATATACTGCTTTAGG | 300 | 600 | |||
| 9V/9A-R | CGAATCTGCCAATATCTGAAAG | 300 | 600 | |||
| 9V/9A-Probe | ACACATTGACAACCGCT | HEX | LNAa | BHQ1 | 100 | 200 |
| 11A/11D-F | AAATGGTTTGGATATGGTTTGTTTGG | 300 | 600 | |||
| 11A/11D-R | AAATGGTTTGGATATGGTTTGTTTGG | 300 | 600 | |||
| 11A/11D-Probe | ATTCCAACTTCTCCCAATTTCTGCCACGG | ROX | BHQ2 | 100 | 200 | |
| 12F/12A/12B/44/46-F | GCACCCACGGGTAAATATTCTAC | 300 | 600 | |||
| 12F/12A/12B/44/46-R | CAACTAAGAACCAAGGATCCACAG | 300 | 600 | |||
| 12F/12A/12B/44/46-Probe | TGCCCACCAACACCAGGTCCAGGT | ROX | BHQ2 | 200 | 400 | |
| 14-F | AGAGTGTATGAGGAATCC | 300 | 600 | |||
| 14-R | ATATATCTACTGTAGAGGGAAT | 300 | 600 | |||
| 14-Probe | CGCCAAGTAACA“T”TTCCATTCCATT | FAM | “T” b= BHQ1 | BHQ1 | 100 | 200 |
| 15A/15F-F | AATTGCCTATAAACTCATTGAGATAG | 200 | 400 | |||
| 15A/15F-R | CCATAGGAAGGAAATAGTATTTGTTC | 200 | 400 | |||
| 15A/15F-Probe | CCCGCAAACTCTGTCCT | FAM | LNAa | BHQ1 | 100 | 200 |
| 16F-F | TAATGTTATGACCTTGGTAATCTTCCC | 300 | 600 | |||
| 16F-R | TCCCAAAGGATAATCAATAACTTTTAGAAG | 300 | 600 | |||
| 16F-Probe | AGCCATAAGTCT“T”CCAAATGCTTAACCGCT | HEX | “T” b= BHQ1 | BHQ1 | 100 | 200 |
| 18C/18A/18B//18F-F | TCGATGGCTAGAACAGATTTATGG | 200 | 400 | |||
| 18C/18A/18B/18F-R | CCATTGTCCCTGTAAGACCATTG | 200 | 400 | |||
| 18C/18A/18B/18F-Probe | AGGGAGTTGAATCAACCTATAATTTCGCCCC | HEX | BHQ1 | 100 | 200 | |
| 19A-F | CGCCTAGTCTAAATACCA | 200 | 400 | |||
| 19A-R | GAGGTCAACTATAATAGTAAGAG | 200 | 400 | |||
| 19A-Probe | TATCAATGAGCCGATCCGTCACTT | FAM | BHQ1 | 100 | 200 | |
| 19F-F | TGAGGTTAAGATTGCTGATCG | 300 | 600 | |||
| 19F-R | CACGAATGAGAACTCGAATAAAAG | 300 | 600 | |||
| 19F-Probe | CGCACTGTCAATTCACCTTC | ROX | LNAa | BHQ2 | 100 | 200 |
| 22F/22A-F | TCTATTAAATAACCCATTGGAATTGAAACG | 200 | 400 | |||
| 22F/22A-R | TCGCAATTGAAGACCACATAAACTG | 200 | 400 | |||
| 22F/22A-Probe | TCCGTAAT“T”CGCTTATGGGCACATTCTCCA | HEX | “T” b= BHQ1 | BHQ1 | 200 | 400 |
| 23A-F | CTCCCCTCCATTACCCATTTGG | 200 | 400 | |||
| 23A-R | TGAAGAAAGTGCTGTTTGTGAACC | 200 | 400 | |||
| 23A-Probe | AGCTAGAAC“T”CCCACACTCCCTACTCCCA | ROX | “T” b= BHQ2 | BHQ2 | 100 | 200 |
| 23F-F | GACAGCAACGACAATAGTCATCTC | 300 | 600 | |||
| 23F-R | TCCATCCCAACCTAACACACTTC | 300 | 600 | |||
| 23F-Probe | ATTGTGTCCA“T”AACCCTTCGTCGTATTTCCAAAG | ROX | “T” b= BHQ2 | BHQ2 | 200 | 400 |
| 33F/33A/37-F | GGAACTGGTTCAGCAACTATACG | 200 | 400 | |||
| 33F/33A/37-R | GGTTCTAAGACCGTCTGAAATAC | 200 | 400 | |||
| 33F/33A/37-Probe | CCCCAAATAGGAC“T”TTTCTGCCATGCCAAA | HEX | “T” b= BHQ1 | BHQ1 | 200 | 400 |
aLocked nucleic acid nucleotides are underlined.
b“T” indicates a black hole quencher placed internally on the thymidine base.
The lower limits of detection (LLD) for the traditional and direct PCR methods in this study were determined as described previously [3, 6]. In brief, bacterial suspensions of target serotypes were prepared in 0.85% saline buffer to a turbidity reading of approximately 0.5 McFarland standard. These suspensions were further serially 10-fold (10–1 to 10–7) diluted in 0.85% saline buffer and in CSF that had been previously tested and known to be PCR negative. DNA was extracted from 200 µL of serial dilution suspensions as previously described [7]. The DNA concentrations were measured using a NanoDrop instrument and plotted against cycle threshold (Ct) values. The concentration that yielded a Ct of 35 was considered the LLD.
RESULTS
The LLD of the individual serotypes for the direct qPCR, determined as described above, varied between 7 and 16 genome equivalents per reaction (3.5–8 genome equivalents/µL CSF), which were comparable to the LLD obtained for the traditional qPCR (between ~7.5 and ~15 cell genome equivalents per reaction [3]).
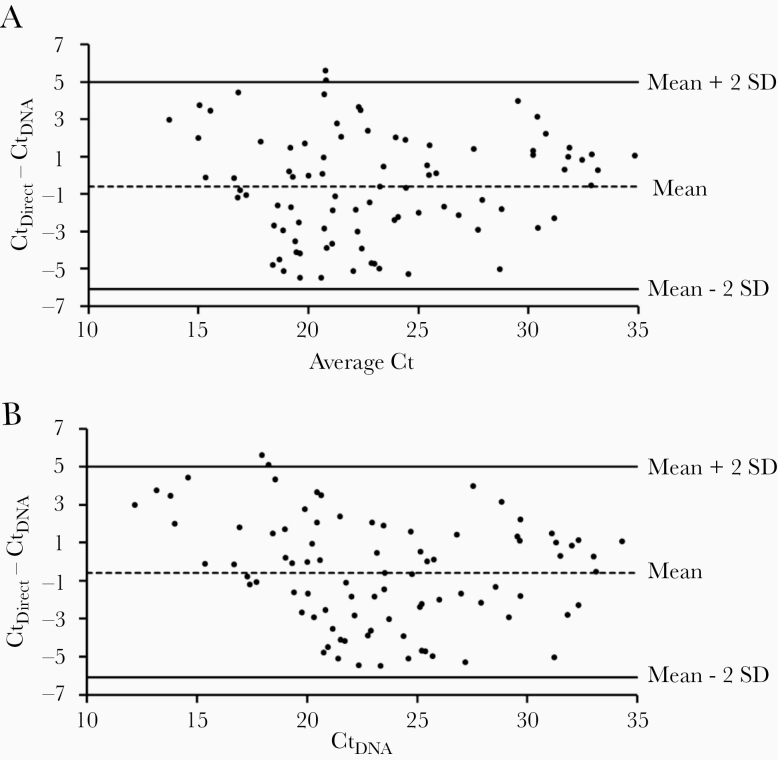
The direct qPCR and the qPCR method with extracted DNA were compared concurrently, using the same set of 120 CSF specimens that had tested positive for the S. pneumoniae gene target, lytA [6], at the national reference laboratories for meningitis surveillance in Burkina Faso (n = 85) and Vietnam (n = 35). For traditional qPCR, DNA was extracted from a 200-µL aliquot of each CSF specimen as described above. To measure the agreement between the 2 methods, CSF specimens and their corresponding extracted DNA were tested concomitantly on the same plate by the direct and the traditional methods, respectively. The Ct difference (ΔCt) between the direct and the traditional PCR (CtDirect – CtDNA) with extracted DNA was plotted as a function of the average Ct values of the 2 methods [8]. The upper and lower limits of agreement between the 2 methods were calculated as the mean ΔCt ± 2 standard deviations (Figure 1A). If the 2 methods agree, we expect at least 95% of the ΔCt to fall within the limits of agreement. In this plot, only 2 data points (~1.7 %) were outside the limits of agreement, demonstrating a very good strength of agreement between the direct and the traditional PCR. Specimens that were found negative for the 37 serotypes (n = 29) by direct PCR were also negative when the PCR was conducted on their extracted DNA. There was no discrepancy among the serotypes identified by the 2 methods. Because this was a prospective study (the serotypes were not known prior to the study) in 2 specific regions, serotypes/serogroups 4, 19A, 23A, 6C/6D, 7F/7A, 22F/22A, and 33F/33A/37 were not detected in the CSF tested.
Figure 1.
Bland–Altman plot (A) and Krouwer plot (B) of the cycle threshold differences (ΔCt) between the direct real-time polymerase chain reaction (PCR) method and the traditional real-time PCR method. The upper and lower limits of agreement are indicated by the upper and lower solid lines (SD = standard deviation of ΔCt). The dashed line indicates the mean ΔCt and the dots represent ΔCt values. These data were generated using 120 clinical cerebrospinal fluid samples.
A corrected Bland–Altman plot (Krouwer plot) suggests that if a new method is being compared to a reference method, one should plot the difference against the values of the reference method rather than the average values of both methods [9]. When the traditional qPCR was considered as the reference method and ΔCt was plotted as a function of CtDNA, the observed agreement between the 2 methods was unchanged (Figure 1B). In addition to the high level of agreement, there was a strong positive correlation between Ct values generated by the 2 methods (Pearson correlation coefficient: r = 0.86; P < .00001), which indicates that high Ct values of one assay correspond to high Ct values of the other and vice versa.
DISCUSSION
Identification of pneumococcal capsular serotypes is very important for monitoring the temporal and geographical distribution of disease-causing serotypes and for measuring pneumococcal conjugate vaccine impact. Molecular methods such as qPCR that allow the identification of serotypes most frequently associated with disease in humans are widely used and are perpetually being optimized. Recently, an expanded sequential PCR scheme consisting of 14 quadriplex reactions that identify 64 individual serotypes/serogroups, antibiotic resistance, and pili genes has been published [10]. The direct qPCR described here contributes to that optimization effort by considerably reducing labor and processing time and, most importantly, saving valuable specimen volumes. In addition, the sensitivity and the specificity of the direct qPCR are comparable to those of traditional qPCR for the identification of S. pneumoniae serotypes. With the recent validation of a triplex direct qPCR for the detection of bacterial species in CSF, including S. pneumoniae [6], it is now possible to rapidly and accurately detect this important pathogen and its capsular serotypes directly in CSF without first performing DNA extraction. We are planning to expand the direct qPCR to the new quadruplex assay that includes a larger number of serotypes.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention (CDC).
Supplement sponsorship. This supplement is sponsored by the World Health Organization and the U. S. Centers for Disease Control and Prevention.
Financial support. This work was supported by the CDC.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 2018; 16:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. mBio 2020; 11:e00937-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimenta FC, Roundtree A, Soysal A, et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol 2013; 51:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai F, Chochua S, Satzke C, et al. Single-plex quantitative assays for the detection and quantification of most pneumococcal serotypes. PLoS One 2015; 10:e0121064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarragó D, Fenoll A, Sánchez-Tatay D, et al. Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin Microbiol Infect 2008; 14:828–34. [DOI] [PubMed] [Google Scholar]
- 6.Ouattara M, Whaley MJ, Jenkins LT, et al. Triplex real-time PCR assay for the detection of Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae directly from clinical specimens without extraction of DNA. Diagn Microbiol Infect Dis 2019; 93:188–90. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol 2007; 45:2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–10. [PubMed] [Google Scholar]
- 9.Krouwer JS. Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Stat Med 2008; 27:778–80. [DOI] [PubMed] [Google Scholar]
- 10.Velusamy S, Tran T, Mongkolrattanothai T, Walker H, McGee L, Beall B. Expanded sequential quadriplex real-time polymerase chain reaction (PCR) for identifying pneumococcal serotypes, penicillin susceptibility, and resistance markers. Diagn Microbiol Infect Dis 2020; 97:115037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.