Abstract
Background and Aims
In tundra systems, soil-borne lichens are often the dominant groundcover organisms, and act to buffer microclimate extremes within or at the surface of the soil. However, shrubs are currently expanding across tundra systems, potentially causing major shifts in the microclimate landscape.
Methods
Here, we compared soil temperature and moisture underneath the dwarf birch Betula nana and seven abundant lichen species in sub-alpine Norway. We also examined mixtures of lichens and dwarf birch – an intermediate phase of shrubification – and measured several functional traits relating to microclimate.
Key Results
We found that all lichen species strongly buffered the daily temperature range, on average reducing maximum temperatures by 6.9 °C (± 0.7 s.d.) and increasing minimum temperatures by 1.0 °C (± 0.2 s.d.) during summer. The dwarf birch had a much weaker effect (maximum reduced by 2.4 ± 5.0 °C and minimum raised by 0.2 ± 0.9 °C). In species mixtures, the lichen effect predominated, affecting temperature extremes by more than would be expected from their abundance. Lichens also tended to reduce soil moisture, which could be explained by their ability to intercept rainfall. Our trait measurements under laboratory conditions suggest that, on average, lichens can completely absorb a 4.09 mm (± 1.81 s.d.) rainfall event, which might be an underappreciated part of lichen–vascular plant competition in areas where summer rainfall events are small.
Conclusions
In the context of shrubification across tundra systems, our findings suggest that lichens will continue to have a large effect on microclimate until they are fully excluded, at which point microclimate extremes will increase greatly.
Keywords: Betula nana, Cladonia stellaris, dwarf birch, functional traits, lichen, lichen mat, microclimate, non-additive, shrubification, shrub expansion, Stereocaulon
INTRODUCTION
Climate change is occurring rapidly at regional scales, yet species-generated microclimates might serve to buffer its immediate impacts on the biosphere (De Frenne et al., 2013). As macroclimates begin to exceed species niche limits, there may still be suitable microclimate patches within species ranges. These patches can arise from geographical variation, such as topography and aspect (Dobrowski, 2011; Maclean et al., 2015; Graae et al., 2018), or the effects of biota, such as temperature buffering in tree hollows and under forest canopies (von Arx et al., 2012; O’Connell and Keppel, 2016). Where microclimate buffering is very strong, patches might be habitable for thousands of years (microrefugia) while others, often termed holdouts, can only delay the immediate effects of macroclimate change, leading to ‘climatic lags’ in species responses (De Frenne et al., 2013; Hannah et al., 2014).
Shrubs are currently expanding across arctic and alpine tundra systems (Martin et al., 2017; Shevtsova et al., 2020), largely due to elevated nitrogen and phosphorus mineralization rates with warmer temperatures (Hartley et al., 1999; Hill and Henry, 2011; Fraser et al., 2014). However, the expansion of shrubs has generally been matched by a decline in the abundance of lichen mats that grow on the soil surface (Van Wijk et al., 2004; Hudson and Henry, 2010; Elmendorf et al., 2012; Fraser et al., 2014), although this pattern is weaker in the high arctic (Cornelissen et al., 2001). This community shift towards darker, taller, less compact organisms represents a major change to microclimate dynamics in tundra systems (Chapin et al., 2005).
Lichen mats cover substantial areas of tundra and play an important role in modifying the microclimate at ground level (Kershaw, 1978; Crittenden, 2000; Fig. 1). Early studies in Canadian conifer woodlands established that lichen mats reduce the temperature range of the underlying soil surface and delay evaporative water loss from the soil (Kershaw and Rouse, 1971; Kershaw and Field, 1975; Bell and Bliss, 1980; Coxson and Lancaster, 1989). It was not until more recently that similar effects were reported for soil-borne lichens in Antarctic (Molina-Montenegro et al., 2013) and Fennoscandian tundra systems (Broll, 2000; Nystuen et al., 2019; van Zuijlen et al., 2020). It might be assumed that microclimate amelioration by lichens would enhance vascular plant recruitment in a similar manner to nurse plants or an insulating layer of leaf litter (Suding and Goldberg, 1999; Cavieres et al., 2007), but this advantage trades off against light competition and other inhibitive effects (reviewed by Favero-Longo and Piervittori, 2010). Mixed results from germination trials affirm that the relationship is complex, and the effect is more facilitative in more extreme climates (i.e. follows the stress gradient hypothesis sensuBertness and Callaway, 1994) in some instances (Hawkes and Menges, 2003; Sedia and Ehrenfeld, 2003; Nystuen et al., 2019).
Fig. 1.
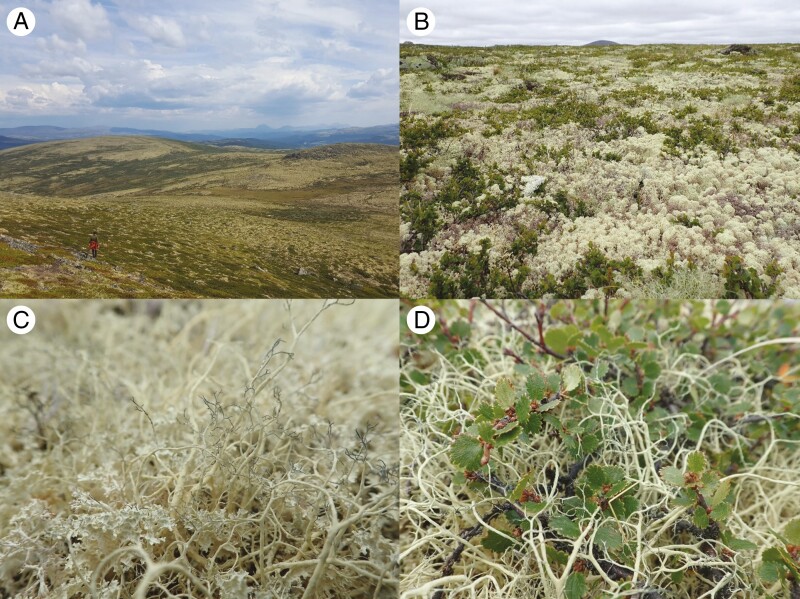
Photographs of lichen mats in the Hjerkinn Protected Landscape, illustrating (A) lichen mats (yellow) covering the exposed areas of the landscape, (B) a typical mid-slope community of Cladonia spp. and the dwarf birch Betula nana, (C) intertwining thalli of Alectoria ochroleuca and Flavocetraria nivalis, and (D) lichens filling gaps in the canopy of Betula nana (photograph credit: M. Mallen-Cooper).
Microclimate is mediated by water and energy balances (Weller and Holmgren, 1974; Davis et al., 2019). At small scales, these balances are affected by the arrangement and structure of the organisms themselves (Larson and Kershaw, 1976). In lichens, there is a high degree of variation in physiological and morphological traits, particularly among species (Cornelissen et al., 2007; Gauslaa and Coxson, 2011; Asplund and Wardle, 2014; Roos et al., 2019). However, past microclimate research has generally treated lichen mats as one entity rather than a mixture of species with different microclimate effects and sets of traits. In this study, we adopt the latter approach, and thus test the generalizability of microclimate findings at the mat level and explore the possibility of fine-scale microclimate heterogeneity within lichen mats, which could play an important role in creating ‘stepping stones’ for expanding vascular plants (Lembrechts et al., 2018).
In a mixture of species, the default approach is to assume that species have equal, or abundance-scaled, effects on an ecosystem property (Garnier et al., 2004). Yet this approach does not account for synergistic and antagonistic interactions between species that result in ‘non-additive’ effects on functioning (Hättenschwiler et al., 2005). For example, the fine leaf litter of one tree species can fill the spaces between the coarser litter of another, reducing ventilation in the litterbed and decreasing flammability more than can be expected from the average of species effects (Zhao et al., 2016). This type of morphological interaction, which could be described as a three-dimensional packing effect, is also likely to be present in lichen mats, where species often intertwine their thalli (Fig. 1C), possibly producing a denser mat than any species could produce alone. In a similar way, lichens tend to fill the spaces in the canopy of dwarf shrubs (Fig. 1D), which might also increase the density of biomass in a given area and thus lead to non-additive effects on microclimate and traits associated with microclimate.
Here we examine microclimate heterogeneity in a lichen- and dwarf shrub-dominated tundra system in central Norway. Building on recent species-explicit approaches to measuring microclimates in lichen mats (Nystuen et al., 2019; van Zuijlen et al., 2020), which involve measuring single lichen species, we additionally measure a dwarf shrub species, Betula nana, and several species mixtures. It should be noted that our field measurements were restricted to 3 d periods (soil temperature) and point measurements (soil moisture) during summer, and thus the purpose of our study is not to capture the complete temporal extent of microclimate variation but rather to compare a snapshot of microclimate amelioration among species. The dwarf birch, B. nana, is known to be expanding in many tundra systems (Van Wijk et al., 2004; Vanneste et al., 2017), and could reduce microclimate buffering due to its more open structure relative to lichens and its ability to reduce water loss via stomatal closure. In contrast, lichens lack mechanisms to regulate water loss and thus passively re-release any absorbed rainfall (Kershaw and Field, 1975). We then perform a correlative investigation of functional traits, such as light interception and water-holding capacity, that might be driving microclimate effects. Tall species that were densely growing (high light interception), absorbent, reflective and slow to desiccate were expected to provide the greatest microclimate amelioration. We also test for non-additivity in traits and microclimate effects using mixtures of lichen species, as well as some mixtures of lichen and dwarf birch. Finally, we conduct a vegetation survey to determine if species, or mixtures, with a high capacity to moderate microclimate support a higher abundance and richness of plants. In summary, we aim to address the following questions. (1) Are there in situ differences in the soil microclimate among the dwarf birch and species of lichen? (2) Are microclimate differences correlated with functional traits? (3) Are there non-additive effects in the traits and buffering capacities of species mixtures due to three-dimensional packing? (4) Does a more buffered microclimate coincide with a richer and more abundant plant community?
MATERIALS AND METHODS
Study site
The study was conducted in the alpine tundra of the Hjerkinn Protected Landscape (Hjerkinn landskapsvernområde; Fig. 1A) in Dovre, central Norway (62.24°N, 9.59°E). According to the Fokstugu weather station (930 m a.s.l.), located approx. 22 km south-west of the study site, mean annual precipitation in this area was 435 mm from 1961 to 1990. In 2019, the mean daily rainfall was 1.4 mm and daily rainfall rarely exceeded 10 mm (see Supplementary data Fig. S1). The mean annual air temperature for the 1961–1990 period was –0.1 °C, with mean temperatures in July and January of 9.8 °C and –8.8 °C, respectively (data available at http://www.yr.no/). The sampled regions of the study site range from 1140 to 1282 m a.s.l., and are likely to be 1.7 °C cooler, on average, than temperatures reported by the Fokstugu weather station, given a tropospheric lapse rate of 6 °C km–1 (Mokhov and Akperov, 2006). Our data suggest that the mean soil temperature underneath lichens and shrubs during the study period was 10.7 °C.
As is typical for alpine tundra systems, the distribution of plant communities in this area is strongly controlled by wind exposure and moisture availability (Holt et al., 2007; Graae et al., 2011). The well-drained, exposed ridgetops are characterized by patchy gravel and a community dominated by soil-borne lichens, such as Alectoria spp. and Flavocetraria nivalis, and sparse dwarf shrubs, including Betula nana and Kalmia procumbens (see Vanneste et al., 2017 for a survey of the region). Only 10–30 m downslope, the community typically transitions to a near-complete cover of Cladonia lichen species and B. nana, intermixed with graminoids such as Festuca ovina and other dwarf shrubs such as Vaccinium vitis-idaea and Empetrum nigrum (Fig. 1B). It is worth noting that the dwarf birch B. nana can be found growing among most lichen species but most commonly occurs within patches of Alectoria ochroleuca (e.g. Fig. 1D) and Cladonia stellaris. With increasing soil moisture and shelter from wind, communities are characterized by a prominence of taller shrubs such as Salix glauca and Juniperus communis, fewer lichens and greater abundances of graminoids and herbaceous plants.
Experiment and survey design
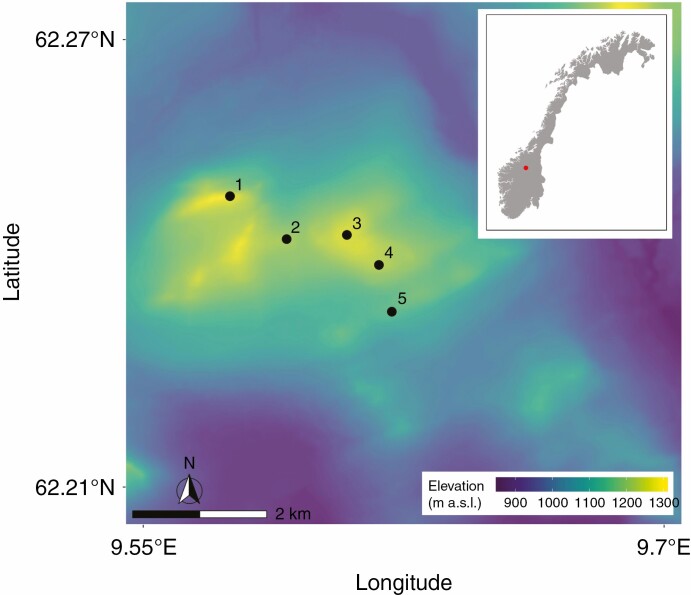
Our study is comprised of three components, corresponding to research questions (1), (2) and (4). The first was a microclimate monitoring experiment with paired control removal plots (Supplementary data Fig. S2), comparing soil temperature and moisture among lichens and the dwarf birch. We then conducted field- and laboratory-based measurements of functional traits relating to microclimate and, finally, a survey of bryophytes and vascular plants growing within lichen mats. The microclimate monitoring and vegetation survey were conducted on five ridges, spaced at least 0.5 km apart, within the Hjerkinn Protected Landscape (Fig. 2). The local elevation maxima on selected ridges ranged between 1157 and 1282 m a.s.l.
Fig. 2.
Map of selected ridges in the Hjerkinn Protected Landscape overlaid on a digital elevation model of the region (inlaid map of Norway with the study site indicated by a red circle).
For all components of this study, we measured the same 13 species or mixtures. This set included seven species of lichen which were abundant on exposed ridges: Alectoria ochroleuca (aloc), Bryocaulon divergens (brdi), Cladonia arbuscula (clar), Cladonia rangiferina (clra), Cladonia stellaris (clst), Flavocetraria nivalis (flni) and Stereocaulon paschale (stpa). We also included the dwarf birch, B. nana (bena), as a target species, i.e. a species in which we examined microclimate, trait and plant community relationships. To address the third research question relating to non-additive effects, we measured mixtures of species that commonly co-occur, namely A. ochroleuca + F. nivalis (Fig. 1C), S. paschale + C. stellaris, B. nana + C. stellaris and B. nana + A. ochroleuca (Fig. 1D), and a random trio of target lichen species. In summary, we measured seven lichen species, one shrub species, three lichen-only mixtures and two lichen–shrub mixtures.
Microclimate amelioration
We used an in situ paired control design to assess microclimate amelioration in target species and mixtures. On each ridge, we established four north–south transects that were 50 m in length and spaced approx. 50 m apart. Target species or mixtures were located by selecting a random point along the transect and searching radially outwards until a suitable patch was found. The criteria for a suitable patch was an area of at least 150 cm2 and on a substrate of soil. When a patch of B. nana could not be found in isolation, underlying lichens or plants had to be removed to create a pure patch. For every patch (n = 260), a paired removal control plot (n = 260) was created 0.5 m away in the direction that best matched the elevation, slope and aspect of the patch (Supplementary data Fig. S2). Control plots were created by removing all plants and lichens in a 40 × 40 cm square and then left for 24 h before microclimate data were collected, allowing time for microclimate conditions to stabilize (the remaining disconnected root mass would not have influenced soil moisture since there were no leaves to drive a water potential gradient). In this way, the cleared control plots were similar to target patches in all variables (e.g. aspect, slope, elevation and soil properties) except lichen, or shrub, cover, and it was thus possible to evaluate the effects of that cover on microclimate conditions.
In July 2018, we measured soil temperature and moisture under all patches and in all control plots. Triplicate measurements of soil moisture at 5 cm depth were recorded on one dry day (no rainfall in the preceding 72 h and lichens in their brittle desiccated state) and one wet day (rainfall within the preceding 12 h and lichens in their soft hydrated state) using a hand-held Trime-Pico soil moisture probe (IMKO GmBh, Ettlingen, Germany). The triplicate measurements, spaced apart by 5–10 cm, were averaged for each patch or control plot, and were conducted after the 24 h stabilization period. Soil temperature was recorded hourly across a 72 h period in July 2018 using HOBO® Pendant UA-002-64 temperature loggers. The loggers were buried under 2 cm of soil to avoid direct sunlight heating the temperature sensor. Moisture measurements were conducted separately from temperature measurements in order to specifically capture the comparison of dry and wet days.
Functional traits
In all species and mixtures, including Betula, we measured the following functional traits: albedo, maximum height, ground-level light interception (proportion of the soil surface shaded by thallus or vegetative matter), mid-level light interception (proportional shading at 3 cm below the thallus or vegetative boundary), specific thallus (or vegetative) mass (Gauslaa and Coxson, 2011), time to 50 % evaporative water loss (T50; Michel et al., 2012) and water-holding capacity (Gauslaa and Coxson, 2011). All traits except albedo and height were measured in controlled laboratory conditions and all were averaged at the species level to examine their associations with microclimate effects (n = 5 per species for all traits except albedo, n = 10 per species, and height, n = 55 per species). A replicate represented one dwarf birch plant, or a collection of lichen thalli constituting a target species or mixture.
To measure the rate of water loss, we conducted a protocol similar to that of Zotz et al. (2000). We collected five samples of each species or mixture from Ridge 2, resulting in a total sample size of n = 65. Ridge 2 was selected for detailed trait measurements because it was representative of other ridgetop communities in the region and simple to access on foot. Betula samples were cut at soil level because our study only concerns the effects of above-ground biomass on soil microclimate. Each sample was submerged in water for 30 min in order to reach maximum water-holding capacity. Samples were then held aloft for 30 s, allowing large drips to fall off, and weighed to the nearest milligram. We chose not to blot the saturated samples because surface water is a valid component of total water-holding capacity (Gauslaa and Coxson, 2011). Samples were placed on flat trays in a room that had a stable mean temperature of 20.6 °C (± 0.6 s.d.), and reweighed every 30 min for 12 h, and every 12 h thereafter until a minimum mass was attained. Any pooled water under the samples was dried during each weighing. Since Betula is not poikilohydric, samples containing Betula steadily lost mass over the measuring period as the wood slowly dried (Simpson, 1983). We therefore stopped measuring Betula samples at 8 d and used this value as a minimum mass, by which time we assumed all external water had evaporated. We derived the maximum water-holding capacity of each sample by dividing the difference between wet and dry masses by the area of the sample (Gauslaa and Coxson, 2011). We also calculated the potential rainfall interception of a lichen as its water-holding capacity (g cm–2) multiplied by 10, given that 1 mm of rainfall corresponds to 0.1 g of water per cm2. Specific thallus mass (or vegetative mass for Betula) was simply the dry mass divided by the area of the sample, as per Gauslaa and Coxson (2011). The area of the lichen thallus or vegetative biomass was calculated in ImageJ (Abràmoff et al., 2004) using a photo taken directly above the intact sample.
Previously, light interception by lichen mats was determined by setting a specimen in resin and placing it between a light source and a photometric sensor (Kershaw and Harris, 1971). However, this method is impractical in a field setting and is complicated by the absorption of light by the resin block. We therefore developed a novel method to rapidly estimate light interception in the field, by measuring the proportion of the soil surface that was shaded by thallus or vegetative matter [often termed ‘ground cover’ (Chakwizira et al., 2015) but, to avoid confusion with other cover variables, we will refer to this trait as light interception]. We did not include photosynthetically active radiation (PAR) in our measurements because our aim was to examine the effects of light interception on the soil microclimate rather than on carbon cycling. First, lichen samples were separated from the soil, and shrub samples were cut at ground level. Most soil-borne tundra lichens die off at their base and so are easily separated (Crittenden, 2000), while others, such as C. stellaris, have a long necromass that gradually transitions into soil, in which case we removed the lower layer of necromass containing soil particles. Lichen or shrub samples were then placed on a single sheet of white paper suspended at half the height of an enclosed box (Supplementary data Fig. S3). A torch from 10 cm above the sample was then shone directly downwards onto the sample, casting a shadow onto the paper, which was then photographed from below. We observed that when a patch of fruticose lichen is removed from the continuous mat, there are often edge branches that would have been intertwined in adjacent mat organisms, and therefore do not truly represent the vertical shading of that species. To avoid this source of error, we took a circular sub-sample of 4 cm diameter from the centre of each shadow, and calculated light interception as the proportion of this area that was shaded from torchlight. In practical terms, the calculation of light interception involved setting a brightness threshold (120) in ImageJ, which was constant for all species and mixtures, and deriving the shaded area from a binary mask. Different thresholds were used for different species because species with fine wispy thalli (e.g. A. ochroleuca) produced a more diffuse shading than those with thicker or wider thalli (e.g. F. nivalis). Using the same method but cutting the specimens at a different height, we also measured light interception at 3 cm below the upper thallus or vegetative boundary (hereafter, mid-level light interception). The 3 cm value was chosen because this was the average height of those plant species that grew underneath a higher thallus or vegetative boundary, according to our survey, and therefore represents the height at which PAR is typically absorbed by underlying plants.
We measured a simple proxy of albedo using a smartphone camera and the Albedo app (available at https://play.google.com/store/apps/details?id=com.h2optics.albedo). The app normalizes the light intensity values in the red, green and blue colour channels using a grey balance and then calculates broad-band albedo as the mean of the three channels (similar to Leeuw and Boss, 2018). Averaging reflectance across the visible spectrum in this manner has been used in the long-term monitoring of snow albedo with reasonable success (Garvelmann et al., 2012).
Maximum height was simply the perpendicular distance from the mineral soil surface to the uppermost living tissue of the lichen or plant.
Vegetation survey
The aim of the survey was to record the plant communities that co-occur within areas dominated by the 13 target species and mixtures, and thus investigate correlations between microclimate amelioration and plant community properties. On each ridge in July 2019, we established 50 m long north–south transects (not those used in the microclimate experiment) with a 10 m spacing. A 20 × 20 cm quadrat was placed every metre and if it was dominated by a target species or mixture (i.e. contained >75 % of a single species, >40 % of each species in a two-species mixture or >30% of each species in a three-species mixture), we recorded the identity, abundance and maximum height of all plant species. We also recorded the maximum height of the target lichen or shrub. Quadrat sampling continued until there were five replicates of all species or mixtures on each ridge (except for Ridge 2, where there were ten replicates per species, thus total n = 390).
Statistical analysis
All microclimate results are presented as arithmetic means. For the water retention trait T50, we first fitted three different models (negative exponential, Weibull and discrete parallel) for each replicate using the ‘litterfitter’ R package (Cornwell and Weedon, 2014). From the model with the lowest Akaike information criterion (AIC), in most cases the discrete parallel model, we extracted T50 as the time (h) at 50 % mass loss (Supplementary data Fig. S4).
If an effect is additive, a mixture of species will exhibit the average effect of the component species in monoculture. The average can be weighted by the abundance of the component species (Michel et al., 2012), but abundance is difficult to measure in complex interwoven lichen mats and thus we calculated the unweighted average as the expected additive value. For example, in a mixture of three lichen species, we used the average of the three trait, or microclimate, values as the expected value, which assumes the component species were equally abundant within the mixture. When the observed effect, or trait, of a mixture of species is different from the expected value, it is considered non-additive. Paired t-tests were used to compare observed and expected trait values in species mixtures, and the traits were considered significantly non-additive if the confidence interval (CI) did not include 0.
RESULTS
Microclimate amelioration
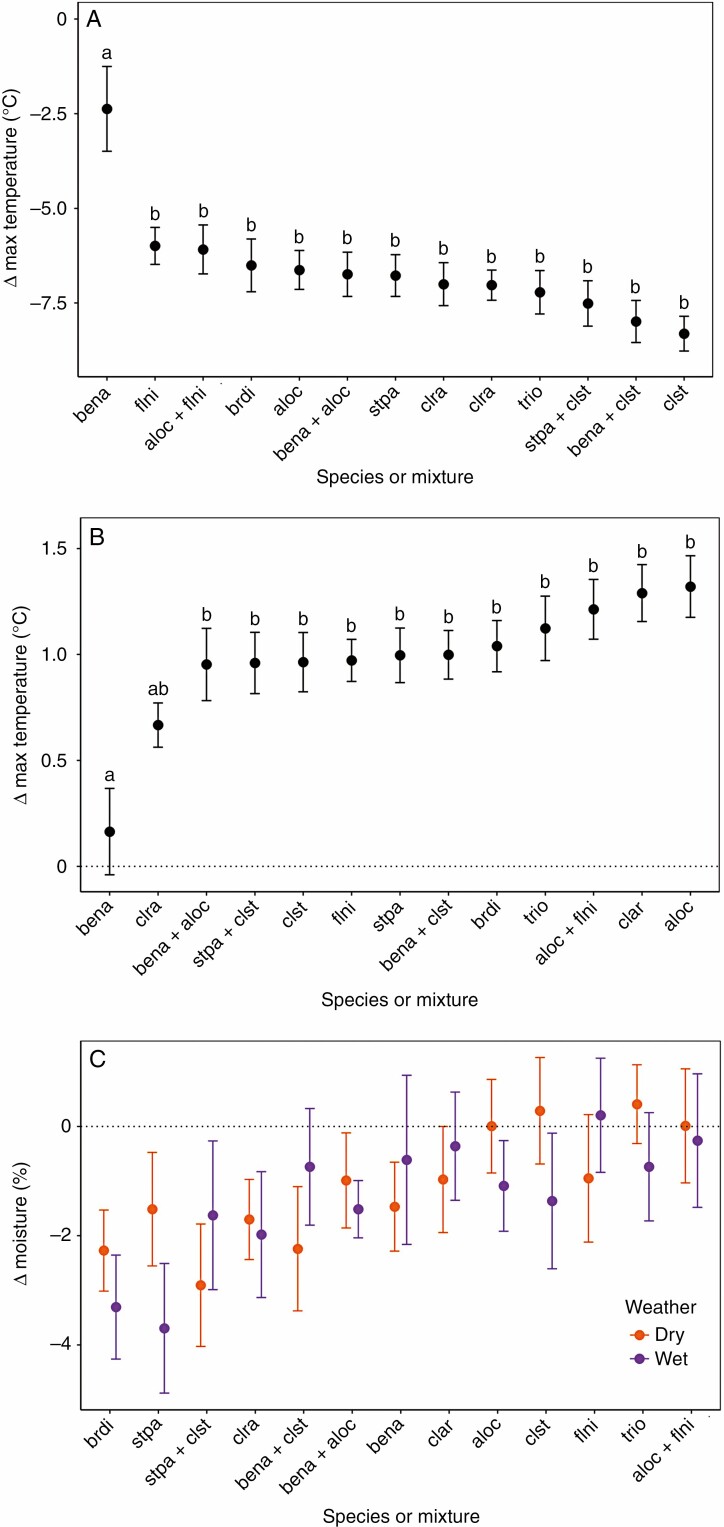
All species and mixtures, except the dwarf birch alone, significantly buffered temperature extremes in the uppermost soil layer (Fig. 3; Supplementary data Fig. S5). The dwarf birch significantly reduced maximum temperature, by a mean of 3.0 °C, but did not affect minimum temperature (mean 0.2 °C, 95% CI: –0.2 to 0.5). The lichen C. stellaris, even when intermixed with birch, had an outstanding ability to reduce maximum temperature. Temperature buffering in mixtures of dwarf birch and A. ochroleuca was also dominated by the lichen component, which represents a non-additive effect (Supplementary data Fig. S6). In lichen-only mixtures, temperature buffering tended to align with the average value of the component species, i.e. the effect was additive (Supplementary data Fig. S6). However, other than the dwarf birch, which had a particularly weak buffering effect, species and mixtures were largely indistinguishable in their buffering capacities (Fig. 3).
Fig. 3.
Means and standard errors for (A) minimum temperature, (B) maximum temperature and (C) soil moisture effects among species and mixtures (Δ = target patch – control; n = 20 per species or mixture). Species or mixtures that do not share a lowercase letter were significantly different (there were no significant differences in soil moisture effects). For species abbreviations, see the Materials and Methods.
We found no evidence of moisture amelioration by any species or mixture (Fig. 3C). On the contrary, many lichen species and Betula significantly reduced soil moisture. Given that the average soil moisture under lichens and shrubs was 11.6 %, soil moisture was reduced, at most, by a factor of 0.21 relative to the control (Supplementary data Fig. S7). There was a strong correlation between maximum temperature buffering and light interception (Pearson’s r = –0.74; Supplementary data Fig. S8), but no strong correlations between other traits and temperature variables (r < 0.51), suggesting that shading was the main driver of temperature buffering.
Functional traits and non-additivity
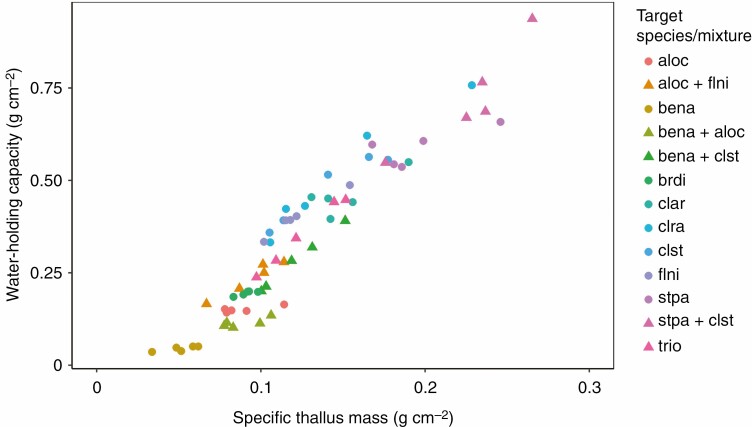
There were strong linear relationships between water-holding capacity and specific thallus mass (Pearson’s r = 0.95; Fig. 4) and between T50 and specific thallus mass (Pearson’s r = 0.84). It is worth noting that the mass per area trait – specific thallus mass – should be considered as vegetative mass for B. nana. Water-holding capacity, T50 and specific thallus mass were typically highest in S. paschale and species of Cladonia. Given their water-holding capacities at saturation, the average lichen (i.e. not including the dwarf birch and its mixtures) could theoretically fully absorb a 4.09 mm (± 1.81 s.d.) rainfall event, based on laboratory conditions and starting completely dry, which corresponds to the 82nd percentile of rain events measured in 2019 at the Fokstugu weather station (Supplementary data Fig. S1). The least absorbent lichen that we measured, A. ochroleuca, was capable of completely absorbing a 1.51 mm (± 0.08 s.d.) rainfall event, while the most absorbent lichen, S. paschale, was able to fully absorb a 5.88 mm (± 0.50 s.d.) event, which correspond to the 63rd and 90th percentile of 2019 rainfall events, respectively. Although it would vary with fluctuating temperature and humidity in the field, possibly taking a shorter time in sun-exposed microsites with high evaporation, the average time for lichens in laboratory conditions to completely dry after saturation was 75.6 h (± 35.7 s.d.), while 50 % drying was attained in 7.2 h (± 3.5 s.d.; Table 1).
Fig. 4.
Specific thallus mass (g dry matter cm–2) and water-holding capacity (g water cm–2) in all species (circles) and mixtures (triangles), with Pearson’s r = 0.95. For species abbreviations, see the Materials and Methods.
Table 1.
Trait means (± s.d.) by species
| Trait | T 50 | WHC | Specific thallus mass | Light interception (ground level) | Light interception (mid level) | Height | Albedo | |
|---|---|---|---|---|---|---|---|---|
| Units | h | g cm–2 | g cm–2 | Unitless | Unitless | cm | Unitless | |
| Total n | 65 | 65 | 65 | 65 | 65 | 715 | 130 | |
| Species or mixture | aloc | 2.4 ± 0.6 | 0.15 ± 0.01 | 0.09 ± 0.02 | 0.73 ± 0.18 | 0.23 ± 0.12 | 9.5 ± 2.6 | 0.17 ± 0.01 |
| aloc + flni | 3.8 ± 1.2 | 0.24 ± 0.05 | 0.09 ± 0.02 | 0.97 ± 0.03 | 0.67 ± 0.11 | 7.9 ± 1.6 | 0.17 ± 0.02 | |
| bena | 2.6 ± 0.8 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.75 ± 0.12 | 0.35 ± 0.14 | 17.6 ± 7.6 | 0.03 ± 0.01 | |
| bena + aloc | 1.9 ± 0.2 | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.87 ± 0.05 | 0.66 ± 0.13 | 11.3 ± 3.2 | 0.08 ± 0.02 | |
| bena + clst | 5.7 ± 0.8 | 0.28 ± 0.08 | 0.12 ± 0.02 | 0.96 ± 0.06 | 0.92 ± 0.04 | 16.4 ± 5.1 | 0.13 ± 0.03 | |
| brdi | 5.9 ± 1.0 | 0.19 ± 0.01 | 0.09 ± 0.01 | 0.99 ± 0.01 | 0.73 ± 0.16 | 7.1 ± 2.3 | 0.01 ± 0.00 | |
| clar | 10.3 ± 2.2 | 0.46 ± 0.06 | 0.15 ± 0.02 | 0.97 ± 0.02 | 0.90 ± 0.08 | 8.2 ± 2.0 | 0.24 ± 0.03 | |
| clra | 7.1 ± 1.1 | 0.51 ± 0.17 | 0.15 ± 0.05 | 1.00 ± 0.00 | 0.81 ± 0.06 | 9.2 ± 3.4 | 0.09 ± 0.01 | |
| clst | 6.8 ± 2.5 | 0.48 ± 0.10 | 0.14 ± 0.03 | 1.00 ± 0.01 | 0.60 ± 0.17 | 9.5 ± 2.4 | 0.28 ± 0.05 | |
| flni | 5.1 ± 1.0 | 0.40 ± 0.05 | 0.12 ± 0.02 | 0.98 ± 0.02 | 0.94 ± 0.05 | 5.7 ± 1.4 | 0.26 ± 0.04 | |
| stpa | 11.9 ± 2.2 | 0.59 ± 0.05 | 0.20 ± 0.03 | 0.96 ± 0.04 | 0.94 ± 0.02 | 5.9 ± 1.1 | 0.16 ± 0.03 | |
| stpa + clst | 12.2 ± 2.5 | 0.72 ± 0.14 | 0.23 ± 0.03 | 0.95 ± 0.06 | 0.88 ± 0.04 | 7.7 ± 1.9 | 0.19 ± 0.03 | |
| trio | 6.4 ± 2.1 | 0.35 ± 0.09 | 0.12 ± 0.02 | 0.98 ± 0.05 | 0.81 ± 0.04 | 7.9 ± 2.4 | 0.14 ± 0.04 |
Evaporation T50, water-holding capacity (WHC), specific thallus (or vegetative) mass, ground- and mid-level light interception, height above ground and albedo (for each of the 13 species and mixtures, n = 5 except height, where n = 55, and albedo, where n = 10).
For species abbreviations, see the Materials and Methods.
Lichens provided near-complete interception of light at the soil surface, with the exception of A. ochroleuca, which has a more open, wispy structure (Table 1). In the mid-level region, mean interception for lichens (not including the dwarf birch and its mixtures) was 72.6 ± 22.5 % SD. There was a moderate association between mid-level light interception and specific thallus mass (Pearson’s r = 0.70). The dwarf birch was characterized by low values of albedo, light interception and water-holding capacity.
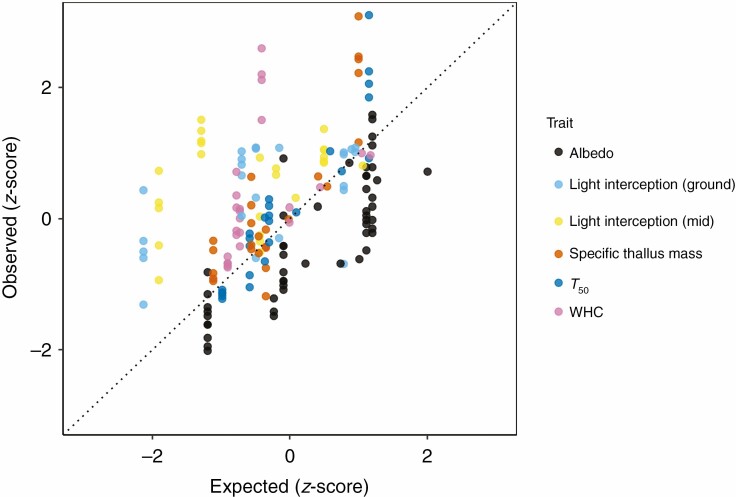
Species mixtures exhibited significant non-additivity in all traits except T50 (Fig. 5; Supplementary data Table S1), i.e. community functioning tended to deviate from the average trait value of the component species. For example, albedo was generally lower than expected from average trait values, while light interception and water retention traits were typically higher. Height was not assessed for non-additivity because it always represented the tallest species in the mixture and so was intrinsically non-additive.
Fig. 5.
Observed trait values (WHC = water-holding capacity) in species mixtures plotted against expected values based on the average trait value of the component species, with a dotted line to represent a perfectly additive effect (all values were z-standardized for graphical reasons).
Co-occurring plant communities in lichen-dominated mats
We recorded 22 species of vascular plant, other than dwarf birch, living within ridgetop lichen mat communities (Supplementary data Fig. S9). The mean cover of vascular plants, excluding dwarf birch, within lichen mats was 8.8 % (± 10.7 s.d.), with the highest mean cover recorded within quadrats dominated by dwarf birch (17.2 ± 19.0 % s.d.) and A. ochroleuca (14.7 ± 12.2 % s.d.; Supplementary data Table S2). The lowest vascular plant cover (3.6 ± 3.2 % s.d.) and richness (0.64 ± 0.64 s.d.) were recorded within mats where C, stellaris was intermixed with B, nana, although this mixture had the highest mean bryophyte cover (10.8 ± 18.7 % s.d.). The mean richness of vascular plants within lichen mats was 1.42 (± 0.49 s.d.), with the highest recorded within patches of S. paschale (2.17 ± 1.36 s.d.). The overall mean cover of bryophytes was 6.8 % (± 8.1 s.d.). Most plants tended to grow close to the thallus or vegetative canopy boundary, with only 5 % protruding >5 cm, most of which were graminoids (Supplementary data Fig. S9).
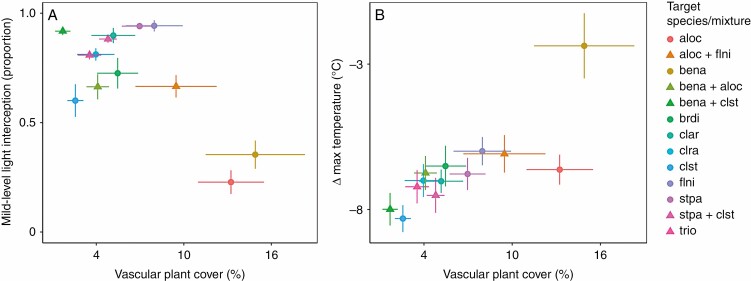
There was a moderately strong negative relationship between trait values of mid-level light interception and mean vascular plant cover (Pearson’s r = –0.85; Fig. 6A). However, there was no strong relationship between mid-level interception and mean vascular plant richness (Pearson’s r = –0.13). Species with poorer abilities to reduce maximum temperature were associated with higher vascular plant cover (Pearson’s r = 0.86; Fig. 6B).
Fig. 6.
Correlation plots, for all species (circles) and mixtures (triangles), showing the relationships between co-occurring vascular plant cover (± s.e.) and (A) mid-level light interception (± s.e.) and (B) maximum temperature buffering (target patch – control, ± s.e.). For species abbreviations, see the Materials and Methods.
Discussion
We found strong evidence that lichens play a major role in buffering the soil temperature during summer temperature extremes, while the influence of the dwarf birch Betula nana on soil temperature was far weaker. In mixtures of lichens and dwarf birch, soil temperature was predominantly controlled by the lichens (Supplementary data Fig. S6). Contrary to expectations, most species reduced soil moisture (Fig. 3C), which could relate to both the strong water-holding capacity of many of the lichen species and the distribution of rain event sizes that would allow lichens to absorb rainfall before it reaches the soil. Mixed communities tended to intercept more light, absorb more water and have a lower albedo than the trait values of their component species would predict. Finally, we found that species with high light interception tended to have the greatest capacity to buffer temperature yet supported the lowest vascular plant cover.
Role of lichens in regulating local water and energy balance
Temperature buffering underneath the dwarf birch was small in comparison with lichens, which probably relates to the relatively poor shading capacity of the birch. Two previous studies also reported high insulating effects in lichens, particularly in Cladonia species (Nystuen et al., 2019; van Zuijlen et al., 2020). Unlike van Zuijlen et al. (2020), we found no clear correlation between temperature effects and water-holding capacity, although this may be a consequence of the limited temporal extent of our study. In the Siberian Arctic tundra, it has been shown that removing B. nana, while leaving the lichen mat intact, significantly enhances permafrost thaw, indicating that the shrub buffers maximum temperatures during summer (Blok et al., 2010). Our results comparing dwarf birch and lichens indicate that lichens may play an even greater role than the dwarf birch in reducing summer permafrost thaw in Arctic tundra and thus offsetting permafrost degradation with climate change (Yang et al., 2010). It is interesting to note that all lichen species reduced maximum temperature considerably more than they raised minimum temperature, suggesting that, at least in summer, lichens play a greater role in ameliorating afternoon heat stress than in avoiding summer frost events (Tolvanen, 1997; Marchand et al., 2005). While the study is contained within one season, summer represents an important time for soil microbial activity and plant growth and establishment (Billings and Mooney, 1968; Sistla and Schimel, 2013). In addition, maximum temperatures are highest in summer and most likely to damage or kill new seedlings and regenerating growth, and thus maximum temperature buffering is most critical in this season.
A surprising result was that some lichen species significantly reduced soil moisture relative to paired controls (Fig. 3C). Our trait measurements suggest that the average lichen, under laboratory conditions and starting completely dry, can fully absorb 4.09 mm (± 1.81 s.d.) of rainfall, which was only exceeded by 18 % of rainfall events in 2019 in the study region (Supplementary data Fig. S1). It is therefore possible that rainfall is largely absorbed by some species and then evaporates from their thalli during hot parts of the day without having reached the soil (i.e. no throughfall). Although soil moisture effects were not clearly greater on rainy days across all species, the rainfall interception signal is likely to be complicated by soil hydrological lags and horizontal water movement within the topsoil profile. It has been shown in a previous study that lichen mats can completely intercept a small rainfall event (Crittenden, 1989), which could provide a competitive advantage over vascular plants in climates where most summer rainfall events are small. In tundra systems, this advantage is unlikely to be important at snowmelt and in poorly drained areas where soil water is never limiting, but pre-emptive uptake of water by lichens during small- to-medium sized rainfall events could be an underappreciated part of lichen–vascular plant competition in sites with frequent, small rainfall events during summer. However, there is also a possibility that lichens reducing soil moisture by at most a factor of 0.21 does not represent a biologically significant effect, particularly given low evaporation rates in tundra systems (Miralles et al., 2011). Further studies would be required to determine the threshold of biological significance.
Based on studies of epiphytic lichens, traits are thought to affect water and energy balances primarily through changes to water-holding capacity, which is in turn driven by thallus mass per area (Gauslaa and Solhaug, 1998; Gauslaa and Coxson, 2011; Phinney et al., 2018). Our trait results suggest that the relationship between water-holding capacity and thallus mass also applies for soil-borne lichens in a tundra system (Fig. 4). In addition, our results demonstrated that lichens with a higher specific thallus mass are able to retain water for longer (higher T50) and tend to intercept more light than less compact lichens. Water retention has two potential effects on the soil microclimate: first, greater water mass per ground area increases the thermal inertia of the microsite and thus its capacity to buffer daily temperature extremes; and second, greater total water capture allows for more evaporative cooling over a longer time frame post-rainfall event, especially during the hottest parts of the day. However, our data suggest that temperature buffering was most closely associated with shading, not water-holding capacity, which implies that the influence of evaporative cooling and enhanced thermal inertia on the soil microclimate is weak. There remains a possibility that water retention plays a role in buffering the near-surface air microclimate.
Non-additive effects of lichens in multispecies mixtures
Non-additivity in the traits of species mixtures supports our hypothesis that the intertwining of species produces a denser, and thus more water-absorbent, mat. A study on bryophytes showed that mixed-species patches often had a greater than expected capacity to retain water because the component species produced a more compact cushion than when they were growing alone (Michel et al., 2012). Michel et al. (2012) argued that because bryophytes, like lichens, lack mechanisms to regulate their water loss, it is beneficial for them to facilitate community water retention rather than compete for soil water as vascular plants do. In a similar manner, lichens can increase their water uptake and water-holding capacity by clumping into a compact multispecies mat, enabling a longer period of photosynthesis that probably outweighs the negative effects of internal shading. Interestingly, it is likely to have been internal shading that produced the lower than expected values of albedo in species mixtures (Holmgren and Thuresson, 1998). The finding that temperature buffering in mixtures of lichen species was additive may be useful for fine-scale predictive models of the soil surface, since temperature buffering in lichen communities can be predicted solely using species means, rather than having to account for non-additivity.
Trade-off between temperature buffering and light competition
We found that co-occurring (non-Betula) vascular plants growing within lichen- and Betula-dominated patches were less abundant when the dominant species was more densely growing and strongly insulating (Fig. 6). This finding provides correlative evidence that, for plants growing within lichen mats, competition for light and water might outweigh the benefits of temperature buffering. A similar result has been observed for young juniper (Juniperus communis subsp. nana) bushes, whereby the benefits of microclimate buffering to underlying plants are outweighed by competition until the junipers age and develop a thinner canopy (Allegrezza et al., 2016). On one hand, our results imply that densely growing lichens may be resistant to expanding woody plants. Yet, on the other hand, it may be that already established dwarf shrubs with relatively open canopies, such as B. nana, will facilitate the expansion of other shrub species (i.e. a local-scale ‘invasional meltdown’, sensuSimberloff and Von Holle, 1999).
Implications for shrub expansion in tundra
The lichen–shrub patches in this study may represent an intermediate stage in the global trend towards increased woody plant dominance in tundra systems (Myers-Smith et al., 2011). It may be that many expanding plant species are already present in lichen mats and are poised to overgrow lichens when conditions become more favourable (e.g. Bret-Harte et al., 2001). While there may be some climatic changes that impair shrubs, such as early snowmelt (Wipf et al., 2009), most factors, including soil mineralization rates and disturbance by grazing and wildfire, are aligned in favour of shrub expansion and lichen exclusion (Sturm et al., 2005; Joly et al., 2009). Our results suggest that lichens will continue to have a large effect on microclimate until they are fully excluded by woody plant shading. That point – full lichen exclusion – may represent a tipping point in the system when microclimate extremes increase greatly.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: daily precipitation values in 2019 at Fokstugu weather station for days with >0 mm precipitation. Figure S2: a target patch of lichen and its accompanying paired removal control plot. Figure S3: diagram of the lightbox set-up for measuring light interception traits, and an example photograph of the shadow cast by a specimen of Bryocaulon divergens. Figure S4: average fitted evaporation curves for all species and mixtures. Figure S5: buffering effect of each target species and mixture. Figure S6: means and standard errors for maximum and minimum temperature buffering in each two-species mixture and their component species growing alone. Figure S7: moisture difference as a proportion of the cleared control plot. Figure S8: correlation plot showing the relationship between maximum temperature buffering and light interception at ground level for mixtures and single species. Figure S9: height of co-occurring plants relative to the height of the dominant lichen. Table S1: results of paired t-tests in species mixtures. Table S2: mean vascular plant and bryophyte cover among species and mixtures.
ACKNOWLEDGEMENTS
We thank Matteo Tolosano, Jessie Foest and Floor Marsman for fieldwork assistance, and the Norsk Villreinsenter for their hospitality. M.M. conceived the project and led the data collection in consultation with B.J.G. M.M. and W.K.C. contributed to data analysis. All authors contributed to interpretation of results. M.M. wrote the first draft of the manuscript and all authors contributed critically to edits therein. All data and the analysis code will be publicly archived on the Open Science Framework.
LITERATURE CITED
- Abràmoff MD, Magalhães PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42. [Google Scholar]
- Allegrezza M, Corti G, Cocco S, et al. 2016. Microclimate buffering and fertility island formation during Juniperus communis ontogenesis modulate competition–facilitation balance. Journal of Vegetation Science 27: 616–627. [Google Scholar]
- Asplund J, Wardle DA. 2014. Within-species variability is the main driver of community-level responses of traits of epiphytes across a long-term chronosequence. Functional Ecology 28: 1513–1522. [Google Scholar]
- Bell KL, Bliss L. 1980. Plant reproduction in a high arctic environment. Arctic and Alpine Research 12: 1–10. [Google Scholar]
- Bertness MD, Callaway R. 1994. Positive interactions in communities. Trends in Ecology & Evolution 9: 191–193. [DOI] [PubMed] [Google Scholar]
- Billings WD, Mooney HA. 1968. The ecology of arctic and alpine plants. Biological Reviews 43: 481–529. [Google Scholar]
- Blok D, Heijmans MM, Schaepman-Strub G, Kononov AV, Maximov TC, Berendse F. 2010. Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Global Change Biology 16: 1296–1305. [Google Scholar]
- Bret-Harte MS, Shaver GR, Zoerner JP, et al. 2001. Developmental plasticity allows Betula nana to dominate tundra subjected to an altered environment. Ecology 82: 18–32. [Google Scholar]
- Broll G. 2000. Influence of overgrazing by reindeer on soil organic matter and soil microclimate of well-drained soils in the Finnish Subarctic. In: Lal R, Kimble JM, Steward BA, eds. Global climate change and cold regions ecosystems. Boca Raton, FL: CRC Press, 163–172. [Google Scholar]
- Cavieres LA, Badano EI, Sierra-Almeida A, Molina-Montenegro MA. 2007. Microclimatic modifications of cushion plants and their consequences for seedling survival of native and non-native herbaceous species in the high Andes of central Chile. Arctic, Antarctic, and Alpine Research 39: 229–236. [Google Scholar]
- Chakwizira E, Meenken ED, George MJ, Fletcher AL. 2015. Can we use photography to estimate radiation interception by a crop canopy? Plant Biology (Stuttgart, Germany) 17: 574–582. [DOI] [PubMed] [Google Scholar]
- Chapin FS 3rd, Sturm M, Serreze MC, et al. 2005. Role of land-surface changes in arctic summer warming. Science 310: 657–660. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Callaghan TV, Alatalo J, et al. 2001. Global change and arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? Journal of Ecology 89: 984–994. [Google Scholar]
- Cornelissen JH, Lang SI, Soudzilovskaia NA, During HJ. 2007. Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Annals of Botany 99: 987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell WK, Weedon JT. 2014. Decomposition trajectories of diverse litter types: a model selection analysis. Methods in Ecology and Evolution 5: 173–182. [Google Scholar]
- Coxson D, Lancaster J. 1989. The morphological basis of variation in net photosynthetic and respiratory responses of the mat-forming lichen species Stereocaulon virgatum and S. tomentosum. Canadian Journal of Botany 67: 167–176. [Google Scholar]
- Crittenden P. 1989. Nitrogen relations of mat-forming lichens. In: Boddy L, Marchant R, Read DJ, eds. Nitrogen, phosphorus and sulphur utilization by fungi. Cambridge: Cambridge University Press, 243–268. [Google Scholar]
- Crittenden P. 2000. Aspects of the ecology of mat-forming lichens. Rangifer 20: 127–139. [Google Scholar]
- Davis KT, Dobrowski SZ, Holden ZA, Higuera PE, Abatzoglou JT. 2019. Microclimatic buffering in forests of the future: the role of local water balance. Ecography 42: 1–11. [Google Scholar]
- De Frenne P, Rodríguez-Sánchez F, Coomes DA, et al. 2013. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences, USA 110: 18561–18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowski SZ. 2011. A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology 17: 1022–1035. [Google Scholar]
- Elmendorf SC, Henry GH, Hollister RD, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecology Letters 15: 164–175. [DOI] [PubMed] [Google Scholar]
- Favero-Longo SE, Piervittori R. 2010. Lichen–plant interactions. Journal of Plant Interactions 5: 163–177. [Google Scholar]
- Fraser RH, Lantz TC, Olthof I, Kokelj SV, Sims RA. 2014. Warming-induced shrub expansion and lichen decline in the Western Canadian Arctic. Ecosystems 17: 1151–1168. [Google Scholar]
- Garnier E, Cortez J, Billès G, Navas M-L, et al. 2004. Plant functional markers capture ecosystem properties during secondary succession. Ecology 85: 2630–2637. [Google Scholar]
- Garvelmann J, Pohl S, Weiler M. 2012. Applying a time-lapse camera network to observe snow processes in mountainous catchments. Hydrology & Earth System Sciences Discussions 9: 10687–10717. [Google Scholar]
- Gauslaa Y, Coxson D. 2011. Interspecific and intraspecific variations in water storage in epiphytic old forest foliose lichens. Botany 89: 787–798. [Google Scholar]
- Gauslaa Y, Solhaug KA. 1998. The significance of thallus size for the water economy of the cyanobacterial old-forest lichen Degelia plumbea. Oecologia 116: 76–84. [DOI] [PubMed] [Google Scholar]
- Graae BJ, Ejrnæs R, Lang SI, Meineri E, Ibarra PT, Bruun HH. 2011. Strong microsite control of seedling recruitment in tundra. Oecologia 166: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graae BJ, Vandvik V, Armbruster WS, et al. 2018. Stay or go – how topographic complexity influences alpine plant population and community responses to climate change. Perspectives in Plant Ecology, Evolution and Systematics 30: 41–50. [Google Scholar]
- Hannah L, Flint L, Syphard AD, Moritz MA, Buckley LB, McCullough IM. 2014. Fine-grain modeling of species’ response to climate change: holdouts, stepping-stones, and microrefugia. Trends in Ecology & Evolution 29: 390–397. [DOI] [PubMed] [Google Scholar]
- Hartley AE, Neill C, Melillo JM, Crabtree R, Bowles FP. 1999. Plant performance and soil nitrogen mineralization in response to simulated climate change in subarctic dwarf shrub heath. Oikos 86: 331–343. [Google Scholar]
- Hättenschwiler S, Tiunov AV, Scheu S. 2005. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics 36: 191–218. [Google Scholar]
- Hawkes CV, Menges ES. 2003. Effects of lichens on seedling emergence in a xeric Florida shrubland. Southeastern Naturalist 2: 223–234. [Google Scholar]
- Hill GB, Henry GH. 2011. Responses of high Arctic wet sedge tundra to climate warming since 1980. Global Change Biology 17: 276–287. [Google Scholar]
- Holmgren P, Thuresson T. 1998. Satellite remote sensing for forestry planning – a review. Scandinavian Journal of Forest Research 13: 90–110. [Google Scholar]
- Holt EA, McCune B, Neitlich P. 2007. Succession and community gradients of arctic macrolichens and their relation to substrate, topography, and rockiness. North American Fungi 2: 1–21. [Google Scholar]
- Hudson JM, Henry GH. 2010. High Arctic plant community resists 15 years of experimental warming. Journal of Ecology 98: 1035–1041. [Google Scholar]
- Joly K, Jandt RR, Klein DR. 2009. Decrease of lichens in Arctic ecosystems: the role of wildfire, caribou, reindeer, competition and climate in north-western Alaska. Polar Research 28: 433–442. [Google Scholar]
- Kershaw K. 1978. The role of lichens in boreal tundra transition areas. Bryologist 81: 294–306. [Google Scholar]
- Kershaw K, Field G. 1975. Studies on lichen-dominated systems. XV. The temperature and humidity profiles in a Cladina alpestris mat. Canadian Journal of Botany 53: 2614–2620. [Google Scholar]
- Kershaw K, Harris G. 1971. A technique for measuring the light profile in a lichen canopy. Canadian Journal of Botany 49: 609–611. [Google Scholar]
- Kershaw K, Rouse W. 1971. Studies on lichen-dominated systems. I. The water relations of Cladonia alpestris in spruce–lichen woodland in northern Ontario. Canadian Journal of Botany 49: 1389–1399. [Google Scholar]
- Larson D, Kershaw K. 1976. Studies on lichen-dominated systems. XVIII. Morphological control of evaporation in lichens. Canadian Journal of Botany 54: 2061–2073. [Google Scholar]
- Leeuw T, Boss E. 2018. The hydrocolor app: above water measurements of remote sensing reflectance and turbidity using a smartphone camera. Sensors 18: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembrechts JJ, Lenoir J, Nuñez MA, et al. 2018. Microclimate variability in alpine ecosystems as stepping stones for non-native plant establishment above their current elevational limit. Ecography 41: 900–909. [Google Scholar]
- Maclean IM, Hopkins JJ, Bennie J, Lawson CR, Wilson RJ. 2015. Microclimates buffer the responses of plant communities to climate change. Global Ecology and Biogeography 24: 1340–1350. [Google Scholar]
- Marchand FL, Mertens S, Kockelbergh F, Beyens L, Nijs I. 2005. Performance of High Arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Global Change Biology 11: 2078–89. [DOI] [PubMed] [Google Scholar]
- Martin AC, Jeffers ES, Petrokofsky G, Myers-Smith I, Macias-Fauria M. 2017. Shrub growth and expansion in the Arctic tundra: an assessment of controlling factors using an evidence-based approach. Environmental Research Letters 12: 085007. [Google Scholar]
- Michel P, Lee WG, During HJ, Cornelissen JH. 2012. Species traits and their non-additive interactions control the water economy of bryophyte cushions. Journal of Ecology 100: 222–231. [Google Scholar]
- Miralles DG, Holmes TRH, De Jeu RAM, Gash JH, Meesters AGCA, Dolman AJ. 2011. Global land-surface evaporation estimated from satellite-based observations. Hydrology and Earth System Sciences 15: 453–469. [Google Scholar]
- Mokhov I, Akperov M. 2006. Tropospheric lapse rate and its relation to surface temperature from reanalysis data. Izvestiya, Atmospheric and Oceanic Physics 42: 430–438. [Google Scholar]
- Molina-Montenegro MA, Ricote-Martínez N, Muñoz-Ramírez C, et al. 2013. Positive interactions between the lichen Usnea antarctica (Parmeliaceae) and the native flora in Maritime Antarctica. Journal of Vegetation Science 24: 463–472. [Google Scholar]
- Myers-Smith I, Forbes B, Wilmking M, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters 6: 045509. [Google Scholar]
- Nystuen KO, Sundsdal K, Opedal OH, et al. 2019. Lichens facilitate seedling recruitment in alpine heath. Journal of Vegetation Science 30: 868–880. [Google Scholar]
- O’Connell C, Keppel G. 2016. Deep tree hollows: important refuges from extreme temperatures. Wildlife Biology 22: 305–311. [Google Scholar]
- Phinney NH, Solhaug KA, Gauslaa Y. 2018. Rapid resurrection of chlorolichens in humid air: specific thallus mass drives rehydration and reactivation kinetics. Environmental and Experimental Botany 148: 184–191. [Google Scholar]
- Roos RE, van Zuijlen K, Birkemoe T, et al. 2019. Contrasting drivers of community-level trait variation for vascular plants, lichens and bryophytes across an elevational gradient. Functional Ecology 33: 2430–2446. [Google Scholar]
- Sedia EG, Ehrenfeld JG. 2003. Lichens and mosses promote alternate stable plant communities in the New Jersey Pinelands. Oikos 100: 447–458. [Google Scholar]
- Shevtsova I, Heim B, Kruse S, et al. 2020. Strong shrub expansion in tundra–taiga, tree infilling in taiga and stable tundra in central Chukotka (north-eastern Siberia) between 2000 and 2017. Environmental Research Letters 15: 085006. [Google Scholar]
- Simberloff D, Von Holle B. 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1: 21–32. [Google Scholar]
- Simpson WT. 1983. Drying wood: a review – Part I. Drying Technology 2: 235–264. [Google Scholar]
- Sistla SA, Schimel JP. 2013. Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: identifying direct and indirect effects of long-term summer warming. Soil Biology and Biochemistry 66: 119–129. [Google Scholar]
- Sturm M, Schimel J, Michaelson G, et al. 2005. Winter biological processes could help convert arctic tundra to shrubland. Bioscience 55: 17–26. [Google Scholar]
- Suding KN, Goldberg DE. 1999. Variation in the effects of vegetation and litter on recruitment across productivity gradients. Journal of Ecology 87: 436–449. [Google Scholar]
- Tolvanen A. 1997. Recovery of the bilberry (Vaccinium myrtillus L.) from artificial spring and summer frost. Plant Ecology 130: 35–39. [Google Scholar]
- Van Wijk M, Clemmensen KE, Shaver G, et al. 2004. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Global Change Biology 10: 105–123. [Google Scholar]
- Van Zuijlen K, Roos RE, Klanderud K, Lang SI, Asplund J. 2020. Mat-forming lichens affect microclimate and litter decomposition by different mechanisms. Fungal Ecology 44: 100905. [Google Scholar]
- Vanneste T, Michelsen O, Graae BJ, et al. 2017. Impact of climate change on alpine vegetation of mountain summits in Norway. Ecological Research 32: 579–593. [Google Scholar]
- Von Arx G, Dobbertin M, Rebetez M. 2012. Spatio-temporal effects of forest canopy on understory microclimate in a long-term experiment in Switzerland. Agricultural and Forest Meteorology 166: 144–155. [Google Scholar]
- Weller G, Holmgren B. 1974. The microclimates of the arctic tundra. Journal of Applied Meteorology 13: 854–862. [Google Scholar]
- Wipf S, Stoeckli V, Bebi P. 2009. Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Climatic Change 94: 105–121. [Google Scholar]
- Yang ZP, Ou YH, Xu XL, Zhao L, Song MH, Zhou CP. 2010. Effects of permafrost degradation on ecosystems. Acta Ecologica Sinica 30: 33–39. [Google Scholar]
- Zhao W, Cornwell W, van Pomeren M, van Logtestijn R, Cornelissen J. 2016. Species mixture effects on flammability across plant phylogeny: the importance of litter particle size and the special role for non-Pinus Pinaceae. Ecology & Evolution 6: 8223–8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Schweikert A, Jetz W, Westerman H. 2000. Water relations and carbon gain are closely related to cushion size in the moss Grimmia pulvinata. New Phytologist 148: 59–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.