Abstract
Nonalcoholic fatty liver disease (NALFD) is now a leading cause of chronic liver disease worldwide, in part as a consequence of rapidly rising levels of obesity and metabolic syndrome, and is a major risk factor for cirrhosis, hepatocellular carcinoma and liver-related mortality. NAFLD stems a myriad of clinical challenges related to both diagnosis and management. A growing body of evidence suggests an intricate linkage between the gut microbiome and the pathogenesis of NAFLD. We highlight how our current knowledge of the gut-liver axis in NAFLD may be leveraged to develop gut microbiome-based personalized approaches for disease management, including use as a non-invasive biomarker for diagnosis and staging, as a target for therapeutic modulation and as a marker of drug response. We will also discuss current limitations of these microbiome-based approaches. Ultimately, a better understanding of microbiota-host interactions in NAFLD will inform the development of novel preventative strategies and precise therapeutic targets.
Keywords: Microbiota, nonalcoholic steatohepatitis, biomarker, cirrhosis, fibrosis
In Brief (eTOC blurb)
Sharpton et al. review our current understanding of the gut-liver axis in nonalcoholic fatty liver disease (NAFLD). They highlight potential opportunities for the development of gut microbiome-based personalized approaches for NAFLD management, including their use as a non-invasive biomarker for diagnosis and staging, as targets for therapeutic modulation and as markers of drug response.
It is expected that the practice of medicine will shift in the coming decades with continued advances in our in-depth understanding of biomarkers and disease pathogenesis, paving the way for personalized medicine in which patients can be more precisely stratified based on prognosis, response to therapy and risk for development of adverse events with therapeutic intervention (Vinks, 2017). The gut microbiome, a diverse microbial community comprised of trillions of bacteria, fungi, viruses, archaea and protists that encodes for, by several orders of magnitude, more functional genes than the human genome, has the ability to modulate human health and will likely be an integral component of the personalized medicine movement (Turnbaugh et al., 2007). Although under normal circumstances the relationship between the human host and gut microbiome is mutually beneficial, perturbations of the gut microbiota have been associated with many chronic diseases (Lynch and Pedersen, 2016). Gut microbial species engage each other and the human host, exchanging signaling molecules and substrates, and these interactions can modulate host cellular physiology and immune response (Lynch and Pedersen, 2016). Technological advances in gut microbiome research have yielded methods for detailed characterization of not only gut microbial taxonomy, but also microbial gene content and metabolic output, expanding our understanding of functional attributes of the gut microbiome in disease pathogenesis (Gilbert et al., 2016).
Nonalcoholic fatty liver disease (NAFLD) refers to a range of disorders secondary to excessive fat accumulation in the liver among individuals who consume little or no alcohol and do not have a secondary cause for hepatic steatosis, such as viral hepatitis, lipodystrophy or exposure to medications known to induce this condition. Excessive fat deposition in the liver leads to progressive liver injury due to multiple mechanistic pathways. NAFLD represents a major public health concern, as it is now the leading cause of chronic liver disease worldwide with an estimated 80–100 million adults with NAFLD in the United States alone (Younossi et al., 2018a). Among persons with NAFLD in the United States, approximately 20% present with steatohepatitis (NASH), a severe form of NAFLD that may progress to cirrhosis, hepatocellular carcinoma and liver-related mortality (Younossi et al., 2016). Cardiovascular complications and malignancy are important life-threatening comorbidities that are commonly associated with NAFLD, with cardiovascular disease representing the leading cause of mortality in such subjects (Adams et al., 2017). Despite the overwhelming burden of NAFLD, major challenges remain in its clinical management, including the need for non-invasive diagnostic tools and the paucity of effective therapeutics.
There is a growing body of evidence supporting the role of the gut microbiome-liver axis in the pathogenesis of NAFLD progression (Sharpton et al., 2019a), and pre-clinical evidence suggests a causal link between the gut microbiota and the disease. For example, mice lacking gut microbiota are resistant to diet-induced obesity and hepatic steatosis, and germ-free mice that receive fecal microbiota transplantation from donor mice with metabolic syndrome develop liver steatosis independent of dietary intake, demonstrating that NAFLD is potentially a transmittable process (Backhed et al., 2007; Le Roy et al., 2013).
The prospect of incorporating the gut microbiome in the management of NAFLD, among other metabolic co-morbidities associated with it, such as obesity, diabetes and cardiovascular disease, offers an exciting and novel strategy for disease prevention and/or management. Here, we provide a perspective of the potential for personalized microbiome-based approaches in NAFLD, based on current knowledge of the gut microbiome in the disease development and progression and available strategies for therapeutic manipulation of the microbiota. Specifically, we will highlight potential clinical applications of the gut microbiome in NAFLD, including use as a non-invasive biomarker for diagnosis and staging, as a target for therapeutic modulation, and as a marker of drug response (Table 1). We will also address current challenges and limitations of these applications.
Table 1.
Potential gut microbiome-based clinical applications in nonalcoholic fatty liver disease
| Non-invasive biomarker for diagnosis and staging | ■ Identification of persons at risk of developing NAFLD ■ Determination of disease phenotype ○ Steatohepatitis ○ Liver fibrosis stage |
| Therapeutics | Gut barrier integrity ■ Toll-like receptor signaling |
| Modulation of intestinal dysbiosis ■ Antimicrobials (antibiotics, antifungals) ■ Prebiotics ■ Probiotics ■ Synbiotics ■ Fecal microbiota transplantation (FMT) ■ Bacteriophage therapy | |
| Targeting of gut microbial metabolism ■ Postbiotics ■ Small molecule inhibition ■ Genetically engineered microbes | |
| Personalized nutrition | |
| Pharmacomicrobiomics | Appropriate drug selection (maximize efficacy and/or minimize toxicity) |
Gut microbiota as a non-invasive biomarker for NAFLD
Non-invasive tools that reliably and accurately differentiate major histologic determinants of NAFLD are needed. Liver biopsy continues to be the gold standard for diagnosis and staging of liver disease in NAFLD, including not only for assessment of fibrosis but also for determining the grade of inflammation (i.e. steatohepatitis) (Younossi et al., 2018b). Adequate assessment of fibrosis is critical to the management of patients with NAFLD, as fibrosis is the key determinant of risk for development of cirrhosis and hepatocellular carcinoma (Dulai et al., 2017; Hagstrom et al., 2017). Liver biopsy carries procedural risks such as bleeding, penetration of abdominal viscera, pneumothorax, and even death, in addition to potential limitations of sampling error.
A number of cross-sectional human studies have demonstrated an association between perturbation in gut microbiota composition and clinical phenotypes of NAFLD severity (simple steatosis [NAFL], steatohepatitis [NASH], and NAFLD-related advanced fibrosis) in both pediatric and adult patient populations, recently reviewed in detail elsewhere (Sharpton et al., 2019a). The most striking results related to the correlation between dysbiosis and clinical phenotype of NAFLD have been in persons with NAFLD-related advanced fibrosis (i.e., stage 3–4). Advanced fibrosis is associated with an overall decrease in microbial diversity secondary to enrichment in gram-negative bacteria (likely a source of endotoxin), and multiple studies have identified associations between Bacteroides and Escherichia spp. and advanced fibrosis in NAFLD (Sharpton et al., 2019a).
Given the association between gut dysbiosis and NAFLD, a clinical consideration is whether the gut microbiota may serve as a non-invasive biomarker of disease phenotype and/or can provide prognostic value in terms of risk for progression to cirrhosis and hepatic decompensation (including risk for subsequent development of hepatocellular carcinoma). Fecal markers of disease have been noted in other disease states such as colorectal cancer (Thomas et al., 2019; Wirbel et al., 2019; Yu et al., 2017) and breast cancer subtypes (Banerjee et al., 2018). Fecal markers have also been shown to portend prognostic value and predict overall mortality risk in persons with severe alcoholic hepatitis.(Duan et al., 2019)
Loomba et al. examined whether a gut microbial signature exists for advanced fibrosis in NAFLD, and performed a prospective cohort study in which metagenomic sequencing was performed in 86 patients with biopsy-proven NAFLD, of which 72 had no or minimal fibrosis (i.e, stage 0–2) and 14 had advanced fibrosis (i.e, stage 3–4).(Loomba et al., 2017) It was noted that 37 bacterial species, including Escherichia coli and Bacteroides vulgatus, were differentially represented between minimal and advanced fibrosis phenotypes. When the differentially abundant species were incorporated into a prediction model that also incorporated patient age, body mass index, and a microbial diversity index, the model possessed an area under the receiver operating characteristic curve (AUROC) of 0.936 for detecting advanced fibrosis. A subsequent study identified seven bacterial species, including Bacteroides caccae, Escherichia coli, and Clostridium sporogenes, that strongly correlated with the presence of NAFLD-related advanced fibrosis on liver biopsy.(Caussy et al., 2018) More recently, a microbiome-derived signature of NAFLD-associated cirrhosis encompassing 27 discriminatory bacterial species was derived from a cohort of 98 probands and validated in a separate cohort of first degree relatives.(Caussy et al., 2019) A subsequent study examined the combination of the stool metagenome profile and patient age and serum albumin levels, reporting an AUROC of 0.91 in detecting cirrhosis in a multi-national cohort of 163 adults.(Loomba, 2020) Altogether, these results suggest that there is the potential for a fecal sample to yield a non-invasive assessment of advanced fibrosis in NAFLD, perhaps negating the need for more invasive assessments such as liver biopsy, although because of differences in biomarkers associated with technical methodology cross-validation across populations of a single assay is essential. Whether microbial markers may predict risk for disease progression and risk for hepatic decompensations before their occurrence in NAFLD has never been examined.
There are, however, a number of limitations in the use of the gut microbiota as a biomarker. The baseline gut microbiota is shaped by a number of factors including age, sex, geographic localization and lifestyle factors (e.g., alcohol, smoking, physical activity). Unlike the human genome, the gut microbiome is dynamic.(Uhr et al., 2019) Numerous studies have demonstrated that endogenous and exogenous factors influence short- and long-term temporal dynamics in the gut microbial community, including hormonal cycles, diet, diurnal variation, and xenobiotics (including antimicrobials).(Lynch and Pedersen, 2016) Moreover, exposure to such factors may leave long-lasting changes in the gut microbiome that modify subsequent responses to exogenous stimuli. While a single DNA-based measurement of the gut microbiome may yield reliable information regarding taxonomic composition and gene content, the transcriptome (and ensuing metabolome) show the most fluctuation, implying that multiple snapshots (i.e. longitudinal sampling) might be more representative of the gut microbiota.(Mehta et al., 2018) Moreover, the gut metagenome encodes redundancy among microorganisms resulting in incongruence between microbial species abundance and gene expression, and studies have demonstrated that lean and obese individuals are better differentiated based on their gut metagenome as opposed to their taxonomic profile.(Le Chatelier et al., 2013)
It is also plausible that there is not one unique microbial signature of disease phenotype in NAFLD and that several pathogenic microbiome states exist. This concept has been noted in heterogeneous conditions such as obesity (Duvallet et al., 2017; Finucane et al., 2018; Walters et al., 2014), but less so in other disease states such as inflammatory bowel disease (Walters et al., 2014). For example, while it was previously thought that obesity was associated with a unique gut microbial signature, an analysis of publicly available data found no such association between body mass index and gut microbial composition.(Finucane et al., 2018) Intriguingly, Duvallet et al. combined datasets from 28 microbiome-wide association studies and found an overlap of microbiome signatures among different diseases, implying that several previously reported host-microbiome links might represent a shared response to disease development or to generic features of inflammation.(Duvallet et al., 2017) Altogether, the predictive value of the gut microbiota for NAFLD disease phenotype is likely to be more robust when incorporating additional patient characteristics (e.g., age, body mass index), other non-invasive biomarkers, as well as known risk factors for NAFLD risk and progression such as comorbid metabolic conditions and genetic polymorphisms(Younossi et al., 2018a), but this is an area that requires further investigation.
Finally, a variety of methods exist for characterizing the gut microbiome, each of which has advantages and limitations.(Sharpton et al., 2019a) While advances in high throughput sequencing technologies have yielded methods to obtain a more comprehensive snapshot of the gut microbial community, these methods remain time-intensive, costly and subject to bias.(Lynch and Pedersen, 2016) Uniform sequencing standards are needed to ensure reproducibility of results across studies and reduce biases in their interpretation. High throughput sequencing methods generate immense amounts of multidimensional data, and integrating and analyzing multi-omic datasets creates a number of statistical challenges.(Badal et al., 2019) Multi-dimensional data not only makes drawing biological inferences difficult and subject to risk of false discovery (as significant correlations are expected by random chance), but also requires data storage capabilities and extensive computational resources.
Therapeutic manipulation of the gut microbiome in NAFLD
Therapeutic options in NAFLD are sorely needed. While there are multiple ongoing Phase 2 and 3 trials examining therapeutics in NAFLD, there are currently no established FDA-approved pharmacologic therapies.(Younossi et al., 2018c) Lifestyle modification to achieve weight loss, including dietary modifications and regular physical activity, remains the cornerstone of NAFLD management. However, even the optimal dietary regimen for persons with NAFLD remains controversial. Altogether, less than 50% of persons with NAFLD achieve the necessary weight loss goal (i.e., 5–10% of total body weight) to reduce hepatic inflammation and/or fibrosis, and even those that do achieve adequate weight loss may experience weight regain over time.(Romero-Gomez et al., 2017)
NAFLD is a heterogeneous and multi-factorial disorder with diverse pathogenic mechanisms including de novo hepatic lipogenesis, oxidative stress, insulin resistance, inflammasome activation and/or fibrogenesis.(Friedman et al., 2018) The prospect of manipulating the gut microbiome in the management of NAFLD offers an exciting and novel strategy for disease prevention and/or management (Figure 1). However, establishing causality in microbiome-host interactions remains a challenge. Human microbiome studies are at risk for bias related to confounding and multiple hypothesis testing. More importantly, cross-sectional human studies yield correlative data only, with findings from these studies requiring evaluation in pre-clinical models to confirm causality. This includes experiments such as gnotobiotic transfer of human microbiome samples into controlled animal models or colonizing mice with communities that differ via a single bacterial strain, in order to link bacteria with modulation of disease.(Donia and Fischbach, 2015) Nevertheless, once causative links between the gut microbiome and disease pathogenesis in NAFLD are well-established, a diverse array of potential therapeutic strategies exist, which we highlight here.
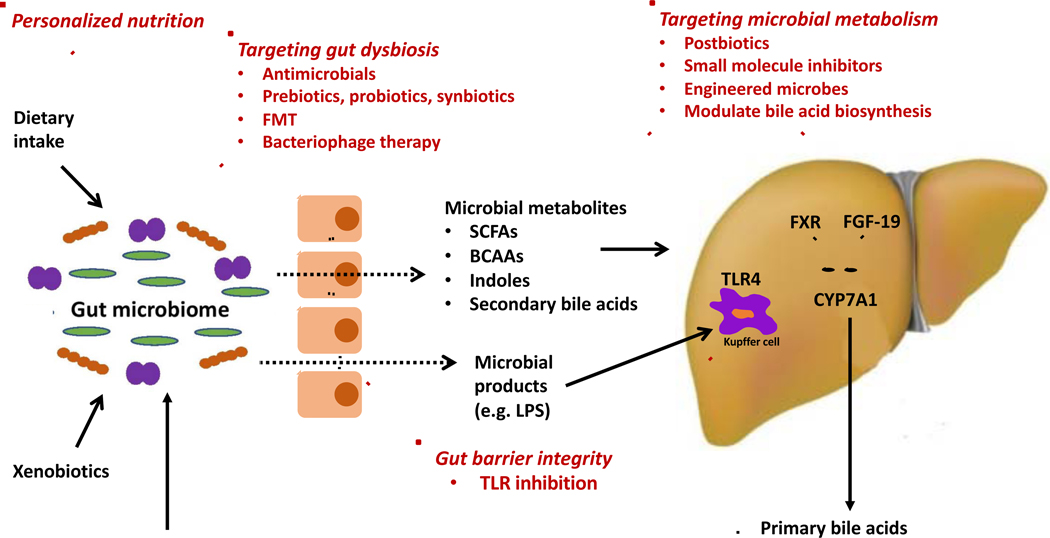
Figure 1. Potential microbiome-based therapeutic interventions in nonalcoholic fatty liver disease (NAFLD).
Intestinal barrier dysfunction, in part related to leaky tight junctions, allows microbial products from gut bacteria, such as lipopolysaccharide, to enter the portal circulation and stimulate toll-like receptors (TLRs), leading to activation of the innate immune system. NAFLD is associated with perturbations in gut microbiota composition, often referred to as intestinal dysbiosis. Modulation of the gut microbiota is feasible via a diverse array of strategies including antimicrobials, prebiotics, probiotics, synbiotics, fecal microbiota transplantation (FMT), and bacteriophage therapy. Gut microbiota contribute to disease pathogenesis through metabolism of dietary substrates, xenobiotics, as well as host molecules (such as primary bile acids), resulting in the production of bioactive small molecules that enter the portal circulation and modulate microbiota-host interactions. Interventions specifically targeted to microbial metabolism may include postbiotics and small molecule inhibition and genetically engineered microbes to precisely alter the production of bacterial metabolites. Metabolites of particular interest in NAFLD pathogenesis include short-chain fatty acids (SCFAs), branched-chain amino acids (BCAAs), indoles, and secondary bile acids (derived from microbial metabolism of primary bile acids). Personalized dietary approaches may modulate dietary-microbiome crosstalk, with ensuing effects on both microbiota composition and metabolic output.
Modulating gut barrier dysfunction and endotoxemia
The intestinal epithelial barrier represents the first line of defense against pathogens, and the gut microbiome plays a significant role in maintenance of gut barrier function. Impairment of the gut barrier, predominantly caused by disruption of intercellular tight junctions, can be present in adults with NAFLD and can even occur in healthy subjects transitioned to a high-fat diet.(Luther et al., 2015a; Miele et al., 2009; Pendyala et al., 2012) This disruption leads to microbiota-host interactions involving cell-associated ligands (e.g., lipopolysaccharides) that are released into the portal circulation and influence microbial sensing and response systems such as toll-like receptors, which function as immune gatekeepers.(Cani et al., 2007) Endotoxin translocation and induction of toll-like receptors (TLR) in the liver, specifically TLR4, leads to downstream activation of transcription factors inducing an inflammatory response, and TLR4 knockout may mitigate hepatic inflammation.(Henao-Mejia et al., 2012)
Hepatic TLR4 expression is upregulated in persons with NAFLD (Sharifnia et al., 2015), and preclinical data suggest that TLR4 inhibition could mitigate steatohepatitis through mechanisms including reduction of IL-6 and IL-12 and inhibition of hepatic stellate cell proliferation and collagen expression (Dattaroy et al., 2016; Du et al., 2016; Gan et al., 2014). However, despite robust pre-clinical data in support of TLR4 inhibition, a Phase 2 randomized, placebo controlled, double-blind, trial of a TLR4 antagonist (JKB-121) revealed no difference in liver fat content when compared to 24 weeks of placebo, albeit the placebo response rate in this study was unexpectedly high (Diehl et al., 2018). These negative results may also indicate that the pathogenesis of NAFLD is not equally driven by the gut microbiota in all patients, and that subsets of patients will derive more benefit from microbiota-targeted therapies than others. This is further corroborated by a meta-analysis that found that only 39.1% of persons with NAFLD had increased intestinal permeability, although individuals with NAFLD were significantly more likely to have increased intestinal permeability than controls (Luther et al., 2015b).
It has been demonstrated in multiple studies that the gut microbiota influence neutrophil migration and function, as well as promote the differentiation of T cell populations into various types of helper T cells and regulatory T cells (Fujimura et al., 2016; Owaga et al., 2015; Xu et al., 2018). A recent study found that mice with NASH had increased hepatic and intestinal α4β7+ CD4+ T cells relative to controls. This increase in CD4+ T cells was promoted by expression of mucosal addressin cell adhesion molecule 1 (MAdCAM-1) in the colonic mucosa, which was highly correlated with increased mucosa-associated Proteobacteria. These findings suggest that targeting dysbiosis-associated T cell recruitment to the gut and liver may hold therapeutic potential in NAFLD (Rai et al., 2020).
Targeting gut microbiota dysbiosis
1. Antibiotics, prebiotics, probiotics, and synbiotics
The majority of currently available strategies aim to alter microbial composition through exogenous administration of antimicrobials (e.g., antibiotics and antifungals), living microorganisms (e.g., probiotics), indigestible fermentable dietary fibers that stimulate the growth and survival of microbes (e.g., prebiotics), or a combination thereof (e.g., synbiotics). The most well-studied use of this category of microbiome-targeted therapies in chronic liver disease is in the management of symptomatic hyperammonemia resulting from cirrhosis (i.e., hepatic encephalopathy). Lactulose, a prebiotic and synthetic disaccharide of galactose and fructose utilized routinely as first-line management of hepatic encephalopathy, enhances growth of Bifidobacterium and Lactobacillus at clinically relevant dosages (Bouhnik et al., 2014), although effects on gut microbiota composition may be variable (Sarangi et al., 2017). In contrast, rifaximin, a minimally absorbable oral antibiotic also used in the management of hepatic encephalopathy, leads to minimal changes in microbial taxonomic composition but significant changes in microbial metabolic function are seen while on the therapy (Bajaj et al., 2013).
The benefit of similar microbiota-targeted therapies in NAFLD is not well delineated. A recent meta-analysis and meta-regression examined evidence from 21 randomized controlled trials (n = 1252 participants) that examined microbiome-targeted therapy in individuals with NAFLD and noted that the use of probiotics or synbiotics was associated with improvement in specific markers of hepatic inflammation, liver stiffness (via elastography), and steatosis (via ultrasound imaging). However, there was significant heterogeneity in included trials due to a number of factors including varying severity of liver disease phenotype, additional patient-level factors including population age, and intervention-level factors including selection and duration of microbiota-targeted therapy (Sharpton et al., 2019b). This may be in part due to the fact that probiotics have not been shown to lead to shifts in the gut microbial community. A meta-analysis that included 7 randomized clinical trials found no effects of probiotics on microbiota composition and no evidence for persistent probiotic engraftment (Kristensen et al., 2016). More recent evidence suggests that engraftment of bacterial strains can be predicted based on a person’s baseline gut microbiota, with autochthonous bacterial strains demonstrating higher ecological fitness than allochthonous strains (Maldonado-Gomez et al., 2016). Next-generation probiotics (e.g., those that are comprised of core microbial species that are underrepresented in dysbiosis) are also currently being developed (Olle, 2013).
2. Fecal microbiota transplantation (FMT)
FMT represents a potential microbiota-targeted therapeutic strategy for NAFLD. The most well-known example of clinical use of FMT is in the management of Clostridium difficile-associated diarrhea, a disorder preceded by gut dysbiosis predominantly following exposure to antibiotics. In a pivotal randomized trial, FMT in C. difficile colitis demonstrated more success than antibiotic treatment and resulted in overall shift in microbial community composition away from a dysbiotic state (Van Nood et al., 2013). FMT into mice using isolates of high-alcohol-producing Klebsiella pneumoniae, a microbe shown to be strongly associated with NASH in a cohort of patients, induced NAFLD. (J et al., 2019) To date, FMT trials in human subjects with metabolic disease have been limited to obese adults with metabolic syndrome but without clearly defined NAFLD (Allegretti et al., 2020; Kootte et al., 2017; Vrieze et al., 2012), but at least one randomized trial examining FMT in adults with biopsy-confirmed NAFLD is actively recruiting subjects at this time. (ClinicalTrials.gov Identifier: NCT04465032)
It is worth noting that FMT can be associated with risks for the recipient, including the potential for inadvertent transplantation of pathobionts and/or detrimental interactions with the recipient’s existing gut microbiota. It was recently reported that two adults who had undergone FMT in the clinical trial setting developed extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli bacteremia, and one of these patients died as a result of bacteremia. Genomic sequencing linked both cases to the stool donor, highlighting the potential for adverse infectious events with FMT (DeFilipp et al., 2019). Furthermore, our mechanistic understanding of the gut microbiome’s response to FMT is limited. In trials including individuals with C. difficile-mediated colitis, recipients had an increase in alpha diversity and a shift in gut microbial composition toward donors (Smillie et al., 2018; Vermeire et al., 2016). However, in other FMT trials, particularly those that included persons with metabolic syndrome and inflammatory bowel disease, recipient microbial community post-FMT did not associate closely with the donor (Fuentes et al., 2017; Kootte et al., 2017), and recipient and donor strains were found to co-exist in recipients for months following FMT (Kumar et al., 2017; Lee et al., 2017; Moss et al., 2017). While discrepant findings on engraftment of donor microbiota amongst FMT studies can in part be attributed to study methodologic differences, a better understanding of the determinants of microbial engraftment with FMT is needed.
3. Phage-mediated modulation of the gut microbiota
A potential paradigm in microbial modulation is a shift away from relatively untargeted approaches for dysbiosis that may yield broader perturbations in the microbial community structure (including antimicrobials, prebiotics, probiotics, synbiotics and even FMT) and toward more targeted approaches. An example of a more targeted strategy includes the use of bacteriophages, viruses that are ubiquitous in the human gut and infect and replicate within bacteria often with species-level specificity. While this approach has not been evaluated in NAFLD, pre-clinical data supports this strategy in alcohol-related liver disease.
The therapeutic use of bacteriophages was initially described in the 1910s but has seen a recent renewal in interest, including in gastrointestinal diseases (Sabino et al., 2020). It was only recently discovered that phage therapy can be utilized to modulate the gut microbiota, not only affecting susceptible bacterial species within a timeframe of hours to days, but further regulating non-targeted bacteria through interbacterial interactions as well altering the gut metabolome (Hsu et al., 2019; Volkova et al., 2014). Bacteriophage therapy has been examined in a murine model of alcoholic hepatitis, demonstrating potential therapeutic promise. Humanized mice treated with bacteriophages that target cytolytic Enterococcus faecalis, previously found to be associated with increased risk for poor outcomes in persons with alcohol hepatitis, led to attenuation of ethanol-induced liver disease without changes in hepatic metabolism in ethanol or in overall composition of the gut microbiota (Duan et al., 2019). However, phage therapy to target the gut microbiota in liver disease has not been studied outside of murine models and requires further evaluation not only for efficacy but also for clinical safety in humans.
Modulating gut microbial metabolism
It is increasingly recognized that the gut microbiome contributes to disease via microbial metabolism and production of bioactive metabolites. Microbial metabolites are synthesized from the metabolism of dietary input and xenobiotics (among other substrates), as well as from the modulation of host-derived molecules, such as bile acids. These small molecules enter the portal circulation, facilitating a gut-liver crosstalk. This mechanism is of particular interest given that the gut microbiota produces tens of thousands of bioactive small molecules, some found at concentrations comparable to administration of clinical drugs (Donia and Fischbach, 2015). Altogether, the function and biochemical output of the gut microbiota is more important than the underlying composition. Gut microbial metabolites have now been linked with a diverse range of disease states, including cardiovascular disease, autism spectrum disorder, Parkinson’s disease, diabetes mellitus and colon cancer (Canfora et al., 2019; Kasahara and Rey, 2019; Martinez et al., 2017; Wang et al., 2017).
1. Postbiotics, small molecule inhibition, and engineered microbes
Targeting downstream signaling pathways and mitigating effects of either excess or deficient microbial-derived metabolites represents a promising source of potential drug targets (Brown and Hazen, 2017; Skelly et al., 2019). This may be achieved via diverse methods such as administration of bioactive compounds (more recently referred to as postbiotics), small molecule inhibition of bacterial enzymes, or genetically engineering bacteria to perform specific functions. One of the most well-delineated links between a microbial-derived metabolite and clinical phenotype in humans is the role of Trimethylamine-N-oxide (TMAO) in atherosclerotic cardiovascular disease. A number of human studies have not only found associations between serum levels of TMAO and coronary atherosclerosis but also noted that this metabolite carries prognostic value in prediction of major adverse cardiovascular events (Yang et al., 2019). Subsequent pre-clinical studies have demonstrated that inhibition of a distinct microbial enzyme responsible for generation of TMA (the first step in TMAO production) reduces atherosclerotic lesion development in murine models, yielding a proof-of-concept for targeting of microbial metabolism (Pathak et al., 2020; Wang et al., 2015).
Kurtz et al. recently developed an engineered Escherichia coli strain that converted systemic ammonia to L-arginine in a murine model, which was subsequently well tolerated in 52 healthy volunteers and resulted in a dose-dependent increase in urinary nitrate (Kurtz et al., 2019). Similar reductions in systemic ammonia were achieved with inoculation of a consortium of 8 engineered bacteria with minimal urease gene content in a murine model (Shen et al., 2015). These findings exemplify the potential for more precise microbiota-based therapeutics for symptomatic hyperammonemia in persons with chronic liver disease (and other hyperammonemia disorders), particularly when compared to non-precision approaches using lactulose and/or rifaximin, as described previously. Genetically modified bacteria have been examined in other applications (Mimee et al., 2016), although there are many practical challenges to overcome including prevention of horizontal gene transfer amongst microbes (Wegmann et al., 2017).
Potential mechanisms of NAFLD disease pathogenesis related to microbial metabolism have been recently reviewed (Sharpton et al., 2018). Emerging evidence suggests that microbial metabolites derived from methylamine and branched chain amino acid metabolism and carbohydrate fermentation may be of significance in the pathogenesis of NAFLD. Of note, bile acid metabolism by the gut microbiome is also suspected to play a role in NAFLD pathogenesis, which we discuss separately. Hoyles et al. performed an integrated analysis of the gut metagenome, hepatic transcriptome, and serum metabolome in a cohort of obese women without diabetes, which yielded discovery of a microbial-derived metabolite (phenylacetic acid) that was enriched in those with NAFLD (Hoyles et al., 2018). Phenylacetic acid was subsequently found to induce hepatic steatosis both in vitro (in a primary culture of human hepatocytes) and in vivo (in a murine model). Ma et al. found that indole, a microbiota-derived metabolite found in lower concentrations in persons with obesity, alleviates diet-induced hepatic steatosis in a PFKFB3-dependent manner in mice (Ma et al., 2020). These are two key examples of microbial-derived small molecules – one that is deleterious and one that is beneficial – that may have implications in NAFLD therapeutics, but these have not been studied in humans.
Further exploratory studies in well-characterized NAFLD cohorts are needed to identify microbial metabolites of interest. However, our understanding of the microbial metabolome is in its infancy. While analytical chemistry techniques yield information on vast number of small molecules, our characterization of the microbial metabolome is incomplete due to the fact that only a small fraction of data generated by untargeted metabolomics can be annotated. There are ongoing efforts to expand current databases to facilitate metabolite identification. An additional challenge in characterizing the metabolome is that microbial metabolism is dynamic and substrate driven, with metabolite composition often changing in the post-prandial state, indicative that metabolomics should not only be applied to fasting samples (Li-Gao et al., 2018; Schugar et al., 2018). Finally, microbial metabolites may have pleiotropic effects in the human host. For example, short-chain fatty acids (SCFAs) may exert differential effects on T-cell mediated immune responses, dependent on both SCFA concentration and immunological mileu (Kespohl et al., 2017). Despite these current limitations, metabolite-based therapeutics offer significant therapeutic promise.
2. Bile acid-microbiota crosstalk
Primary bile acids are secreted by the liver and metabolized by the gut microbiota into secondary bile acids. Bile acids serve as ligands for farnesoid X receptor (FXR) and G-protein coupled receptors (TGR5) with effects on lipid, glucose and energy metabolism. In addition to their host effects, bile acids also exhibit feedback to the gut microbiota through membrane-damaging effects and indirectly through induction of antimicrobial protein expression. Serum concentrations of primary and secondary bile acids are increased in individuals with NAFLD (Jiao et al., 2018). Bile acids prevent intestinal bacterial overgrowth, both directly (via membrane damaging effects) and indirectly (via production of antimicrobial proteins). As such, bile acids modulate the composition of the gut microbiota. On the other hand, the gut microbiota deconjugates and converts primary bile acids to secondary bile acids, thus regulating the bile acid pool (Ridlon et al., 2016).
An engineered analogue of fibroblast growth factor 19 (FGF19), a hormone that dampens bile acid synthesis through effects on cholesterol 7alpha hydroxylase (CYP7A1), improves histologic features in subjects with biopsy-proven NAFLD (Harrison et al., 2020). Although the link between supraphysiologic bile acid concentrations and the development of NAFLD is not well understood, it is likely precipitated by the gut microbiome. Yao et al. demonstrated that deletion of a single bacterial gene (from a strain within the Bacteroides genus) in a gnotobiotic model specifically modulated the in vivo bile acid pool with ensuing effects on weight gain and lipid levels, suggesting mechanistic link between the gut microbiota and bile acid metabolism in metabolic disease (Yao et al., 2018).
Leveraging gut microbiota-diet interactions to guide personalized nutrition
Dietary-microbiota crosstalk may affect development and progression of metabolic diseases, including NAFLD, which has led to the emergence of the concept of “personalized nutrition”. Daily microbial fluctuations are strongly influenced by the timing and type of food intake (Thaiss et al., 2016; Tognini et al., 2017). Personalized nutrition approaches aim to identify key microbiota features that predict response to diet, which subsequently informs individual tailoring of dietary interventions for disease prevention and/or management (Kolodziejczyk et al., 2019).
Optimizing nutrition has been a longstanding recommendation for all patients in the management of NAFLD (Saeed et al., 2019). Epidemiological studies and randomized trials show that a Western diet, associated with increased intake of simple sugars, is associated with NAFLD severity (Abdelmalek et al., 2010; Ma et al., 2015). Ouyang et al. compared dietary history between individuals with biopsy-proven NAFLD and controls (matched for age, sex and body mass index) and found that individuals with NAFLD had a two- to three-fold higher consumption of fructose, which was associated with an increased enzymatic activity for lipogenesis (Ouyang et al., 2008). A cross-sectional analysis of liver steatosis on computed tomography in 2,634 participants in the Framingham Heart Study cohort showed a dose-response association between consumption of refined sugars and NAFLD (Ma et al., 2015). A subsequent randomized trial found that administering sucrose-sweetened beverages to overweight adults for six months resulted in hepatic fat accumulation on magnetic resonance spectroscopy, as well as concomitant increases in visceral and skeletal fat mass (Maersk et al., 2012). Conversely, fructose restriction in children results in both a reduction in hepatic steatosis and visceral adipose tissue when compared to controls fed an isocaloric diet, irrespective of baseline hepatic steatosis (Schwarz et al., 2017).
A Mediterranean diet, in part characterized by reduced carbohydrate intake but also by an emphasis on plant-based foods, has also been suggested to be effective in NAFLD management. Cohort studies have noted inverse associations of NAFLD severity with adherence to a Mediterranean diet (Baratta et al., 2017; Kontogianni et al., 2014). Moreover, the Mediterranean diet has been shown to reduce the risk of cardiovascular disease and diabetes, co-morbidities highly relevant in persons with NAFLD (Estruch et al., 2013). More recently, Markova et al. found that diets high in animal and/or plant protein significantly reduced hepatic steatosis and markers of hepatic inflammation, independent of changes in body weight, in persons with type 2 diabetes and NAFLD (Markova et al., 2017). Altogether, evidence to date suggests that dietary intake is an important contributor in the development of NAFLD.
Nevertheless, recent evidence suggests that a “one size fits all” approach to dietary interventions in the management of metabolic disease may be inadequate, with inter-individuality in processing key nutrients driven by the gut microbiome. Johnson et al. performed shotgun metagenomics on daily fecal samples in 34 healthy adults over a period of 17 days and found that microbial responses were correlated with food choices but were highly personalized (Johnson et al., 2019). Zeevi et al. examined glucose levels in an 800-person cohort and also found high variability among subjects in response to identical meals. A machine learning algorithm that included the gut microbiota, among other measurements such as anthropometrics and physical activity, predicted personalized postprandial glycemic response, and this algorithm was subsequently validated an external cohort (Zeevi et al., 2015).
In addition to dietary choices, dietary timing – influenced by circadian rhythmicity and intermittent fasting – affects metabolic risk via effects on gut microbial composition and function. It is known that circadian rhythms and the gut microbiota are intricately linked and exhibit bidirectional crosstalk, with studies in murine models demonstrating that disruption of circadian rhythm and feeding patterns can lead to dysbiotic states, and conversely antibiotic-induced microbiota changes result in circadian gene dysregulation (Mukherji et al., 2013; Zarrinpar et al., 2014). Alterations in circadian rhythms and feeding patterns have ensuing metabolic effects. The transfer of gut microbiota from jet-lagged humans into germ-free mice results in the development of insulin resistance and obesity (Thaiss et al., 2014), whereas intermittent fasting restores glucose tolerance (Leone et al., 2015; Li et al., 2017).
These findings have led to interest in the role of altering feeding patterns with intermittent fasting as a management strategy of obesity and co-related metabolic diseases. Intermittent fasting refers to a low calorie period lasting less than 24 hours followed by a normal feeding period, and includes variations such as time-restricted feeding (specific time windows for food consumption within a day) and alternate-day fasting (alternation between normal caloric consumption days and caloric restriction days) (Hatori et al., 2012; Longo and Mattson, 2014). Intermittent fasting interventions had acceptable adherence and lead to weight loss and improvement in a number of metabolic parameters in several recent randomized trials of modest sample sizes.(Cai et al., 2019; Cienfuegos et al., 2020; Johari et al., 2019)
Altogether, while it remains unknown how the interface between dietary intake – including both “what to eat” and “when to eat” – and the gut microbiota can be manipulated in the management of NAFLD, there is mounting evidence for the potential for personalized dietary approaches.
Individualized drug selection in NAFLD
Individual responses to pharmacologic therapy vary in terms of both efficacy and risk for adverse drug reactions. While inherited genome variations may lead to differences in xenobiotic metabolism, it has long been recognized that the discrepancy in drug efficacy and toxicity is not explained by genetics alone (Kalow et al., 1998). The role of gut microbial metabolism in drug response was noted as early as the 1930s, at which time sulfasalazine was developed, requiring cleavage by the gut microbiota for in vivo activity. It has been more recently appreciated that interactions between the gut microbiota and xenobiotics are in fact bidirectional. The gut microbiota may contribute to the metabolism of xenobiotics, in a manner that is different than hepatic metabolism, but conversely the microbiota composition can be altered by xenobiotics (Koppel et al., 2017). In fact, interindividual variation in gut microbiota may be in part related to drug usage (Zhernakova et al., 2016).
Pharmacomicrobiomics, an emerging field that encompasses the study of the effect of microbiome variations on drug disposition, action and toxicity, may expand the scope of personalized medicine in terms of individualized drug selection. Microbiota interactions have been described with a number of pharmacologic agents including metformin (Wu et al., 2017), digoxin (Haiser et al., 2013), and anti-integrin therapy (Ananthakrishnan et al., 2017). For example, in a type 1 adult diabetic cohort, it was shown that metformin increased abundance of Akkermansia mucinophilia, a gut bacterial known to produce short-chain fatty acids and known to contribute to intestinal barrier integrity, thus mediating changes in glucose metabolism (de la Cuesta-Zuluaga et al., 2017). In a recent proof-of-concept study, administration of Akkermansia mucinophilia to overweight or obese volunteers with insulin resistance yielded improvement in several metabolic parameters including insulin sensitivity. (Depommier et al., 2019) Another key example of microbiota-xenobiotic interaction is the differential response to immunotherapy, in part attributable to an individual’s baseline gut microbiota, in a number of malignancies including melanoma, non-small cell lung cancer, renal cell carcinoma, urothelial carcinoma, and hepatocellular carcinoma (Zitvogel et al., 2016). For example, in a cohort study of 112 adults with melanoma, the gut microbiota in responders to therapy demonstrated higher alpha diversity and enrichment in Ruminococcaceae, which correlated with improved effected T cell function, highlighting a “favorable” microbiota for those receiving immunotherapy (Routy et al., 2018). Moreover, in a prospective multicenter cohort study of 196 persons undergoing immune checkpoint inhibitor therapy, prior exposure to antibiotics was associated with worse overall survival and higher likelihood of refractory disease (Pinato et al., 2019).
A number of Phase 2 and 3 studies are ongoing for NAFLD, evaluating drugs targeting diverse pathways including hepatic fat accumulation, insulin sensitization, oxidative stress and apoptosis, and hepatic fibrosis (Sumida and Yoneda, 2018). Whether the gut microbiota may serve as a marker for drug response, and can specifically lead to the ability to individually select a therapy with a higher likelihood for treatment success (and simultaneously mitigate risks for toxicity), is unknown.
Conclusion
A small but growing number of studies are expanding our understanding of the mechanisms by which NAFLD phenotypes are impacted by the gut microbiome. Further well-designed prospective human studies, combined with pre-clinical models, are needed to establish causal microbiota-host interactions in NAFLD pathogenesis. Nonetheless, we anticipate that the gut microbiome will emerge as an integral component of personalized medicine, specifically in multi-factorial chronic metabolic disorders such as NAFLD, in the coming years. Fecal microbial signatures could serve as non-invasive diagnostic tools or provide prognostic value. Precise manipulation of the gut microbiota may serve as a therapeutic option, either as monotherapy or in combination with other therapeutic targets. Finally, a broader understanding of microbial metabolism may lead to personalized drug selection.
Acknowledgements and grant support:
S.S. receives grant support from the Advanced/Transplant Hepatology Award and the Alan Hofmann Clinical, Translational, and Outcomes Research Award from the American Association for the Study of Liver Diseases (AASLD) Foundation. R.L. receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (R01DK106419, P30DK120515), and DOD PRCRP (CA170674P2). This review was supported by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Abbreviations:
- (FMT)
fecal microbiota transplantation
- (HCC)
hepatocellular carcinoma
- (NAFLD)
nonalcoholic fatty liver disease
- (NASH)
nonalcoholic steatohepatitis
- (SCFAs)
short chain fatty acids
- (TLRs)
toll-like receptors
Footnotes
Declarations of interest:
S.S. declares no competing interests. B.S. has served as a consultant for Ferring Research Institute, Intercept Pharmaceuticals, HOST Therabiomics and Patara Pharmaceuticals. B.S.’s institution University of California San Diego has received grant support from BiomX, NGM Biopharmaceuticals, CymaBay Therapeutics, Synlogic Operating Company and Axial Biotherapeutics. R.K. serves as a consultant or advisory board member for Biota Technology, CoreBiome, GenCirq, Micronoma, DayTwo, Cybele Microbiome, CommenSe, and BiomeSense. R.L. serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, NGM Biopharmaceuticals, Pfizer, pH Pharma, Prometheus, and Siemens. R.L. is also co-founder of Liponexus, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson, and Diehl AM (2010). Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LA, Anstee QM, Tilg H, and Targher G. (2017). Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. [DOI] [PubMed] [Google Scholar]
- Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, et al. (2020). Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, Cleland T, and Xavier RJ (2017). Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK S, Clay F, Semenkovich CF G, Jeffrey I, and Gordon JI (2007). Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badal VD, Wright D, Katsis Y, Kim HC, Swafford AD, Knight R, and Hsu CN (2019). Challenges in the construction of knowledge bases for human microbiome-disease associations. Microbiome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Heuman D, Sanyal A, Hylemon P, Sterling R, Stravitz R, Fuchs M, Ridlon J, Daita K, Monteith P, et al. (2013). Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, DeMichele AM, Alwine JC, and Robertson ES (2018). Distinct Microbial Signatures Associated With Different Breast Cancer Types. Front Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta F, Pastori D, Polimeni L, Bucci T, Ceci F, Calabrese C, Ernesti I, Pannitteri G, Violi F, Angelico F, et al. (2017). Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am J Gastroenterol. [DOI] [PubMed] [Google Scholar]
- Bouhnik YA,A, Joly FA, Riottot M, Dyard F, and Flourie B. (2014). Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur J. Clin Nutr [DOI] [PubMed] [Google Scholar]
- Brown JM, and Hazen SL (2017). Targeting of microbe-derived metabolites to improve human health: The next frontier for drug discovery. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, Chen RQ, and Chen ZY (2019). Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E, Meex R, Venema K, and Blaak E. (2019). Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. [DOI] [PubMed] [Google Scholar]
- Cani P, Amar J, Iglesias M, Poggi M, Knauf C, Bastelica A, Fava F, Tuohy K, Chabo C, Waget A, et al. (2007). Metabolic Endotoxemia Initates. Obesity. and Insulin Resistance. Diabetes. [DOI] [PubMed] [Google Scholar]
- Caussy C, Hsu C, Lo M-T, Liu A, Bettencourt R, Ajmera VH, Bassirian S, Hooker J, Sy E, Richards L, et al. (2018). Novel link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology (Baltimore, Md.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ, et al. (2019). A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, and Varady KA (2020). Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattaroy D, Seth RK, Das S, Alhasson F, Chandrashekaran V, Michelotti G, Fan D, Nagarkatti M, Nagarkatti P, Diehl AM, et al. (2016). Sparstolonin B attenuates early liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation. Am J Physiol Gastrointest Liver Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velasquez-Mejia EP, Carmona JA, Abad JM, and Escobar J. (2017). Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care. [DOI] [PubMed] [Google Scholar]
- DeFilipp Z, Bloom P, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, and Hohmann EL (2019). Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. (2019). Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Harrison S, Caldwell S, Rinella M, Paredes A, Moylan C, Guy C, Bashir M, Wang Y, Miller L, et al. (2018). A Randomized Double-Blind, Placebo Controlled, Parallel-Group, Phase II Trial of JKB-121 for the Treatment of Nonalcoholic Steatohepatitis. [Google Scholar]
- Donia MS, and Fischbach MA (2015). HUMAN MICROBIOTA. Small molecules from the human microbiota. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Niu X, Wang R, Zhao S, Kong L, Zhang Y, and Nan Y. (2016). TLR4-dependent signaling pathway modulation: A novel mechanism by which pioglitazone protects against nutritional fibrotic steatohepatitis in mice. Mol Med Rep. [DOI] [PubMed] [Google Scholar]
- Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba M, et al. (2019). Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, et al. (2017). Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvallet C, Gibbons SM, Gurry T, Irizarry RA, and Alm EJ (2017). Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. (2013). Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Sharpton TJ, Laurent TJ, and Pollard KS (2018). A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Neuschwander-Tetri BA, Rinella M, and Sanyal AJ (2018). Mechanisms of NAFLD development and therapeutic strategies. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S, Rossen NG, van der Spek MJ, Hartman JH, Huuskonen L, Korpela K, Salojarvi J, Aalvink S, de Vos WM, D’Haens GR, et al. (2017). Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, et al. (2016). Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 22, 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, and Farrell GC (2014). Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, and Knight R. (2016). Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. [DOI] [PubMed] [Google Scholar]
- Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, and Kechagias S. (2017). Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 67, 1265–1273. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Gootenberg D, Chatman K, Sirasani G, Balskus E, and Turnbaugh PJ (2013). Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, Banerjee R, Jaros MJ, Owers S, Baxter BA, et al. (2020). NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. . Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong E, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick J, et al. (2012). Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. (2012). Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyles L, Fernandez-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, et al. (2018). Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nature medicine 24, 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, Silver PA, and Gerber GK (2019). Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Y, C C, J C, J L, C Y, X W, Zhao NL, S L, G X, et al. (2019). Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metabolism. [DOI] [PubMed] [Google Scholar]
- Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, et al. (2018). Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut [DOI] [PubMed] [Google Scholar]
- Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, Hafidz MIA, Chua BE, Hamid N, Arifin WN, et al. (2019). A Randomised Controlled Trial on the Effectiveness and Adherence of Modified Alternate-day Calorie Restriction in Improving Activity of Non-Alcoholic Fatty Liver Disease. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Vangay P, Al-Ghalith GA, Hillmann BM, Ward TL, Shields-Cutler RR, Kim AD, Shmagel AK, Syed AN, Walter J, et al. (2019). Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe. [DOI] [PubMed] [Google Scholar]
- Kalow W, Tang Bk Fau - Endrenyi L, and Endrenyi L. (1998). Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. [DOI] [PubMed] [Google Scholar]
- Kasahara K, and Rey FE (2019). The emerging role of gut microbial metabolism on cardiovascular disease. Curr Opin Microbiol. [DOI] [PubMed] [Google Scholar]
- Kespohl M, Vachharajani N, Luu M, Harb H, Pautz S, Wolff S, Sillner N, Walker A, Schmitt-Kopplin P, Boettger T, et al. (2017). The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4(+) T Cells. Front Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk AA, Zheng D, and Elinav E.A.-O.h.o.o (2019). Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. [DOI] [PubMed] [Google Scholar]
- Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, Fragopoulou E, Zafiropoulou R, Manios Y, and Papatheodoridis G. (2014). Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. [DOI] [PubMed] [Google Scholar]
- Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. (2017). Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metabolism. [DOI] [PubMed] [Google Scholar]
- Koppel N, Maini Rekdal V, and Balskus E. (2017). Chemical transformation of xenobiotics by the human gut microbiota. . Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, and Pedersen O. (2016). Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Yi N, Zhi D, Eipers P, Goldsmith KT, Dixon P, Crossman DK, Crowley MR, Lefkowitz EJ, Rodriguez JM, et al. (2017). Identification of donor microbe species that colonize and persist long term in the recipient after fecal transplant for recurrent Clostridium difficile. NPJ Biofilms Microbiomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz C, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, Isabella V, Kotula JW, Antipov E, Dagon Y, Denney W, et al. (2019). An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. . Sci Transl Med. [DOI] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T Fau - Qin J, Qin J Fau - Prifti E, Prifti E Fau - Hildebrand F, Hildebrand F Fau - Falony G, Falony G Fau - Almeida M, Almeida M Fau - Arumugam M, Arumugam M Fau - Batto J-M, Batto Jm Fau - Kennedy S, Kennedy S Fau - Leonard P, et al. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature. [DOI] [PubMed] [Google Scholar]
- Le Roy T, Llopis M L, Patricia Lepage P B., Aurelia Bruneau A, R., Sylvie Rabot S, B., Claudia Bevilacqua C, M., Patrice Martin P, P., Catherine Philippe C, W., Francine Walker F, B., Andre Bado A, P., Gabriel, et al. (2013). Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. [DOI] [PubMed] [Google Scholar]
- Lee STM, Kahn SA, Delmont TO, Shaiber A, Esen OC, Hubert NA, Morrison HG, Antonopoulos DA, Rubin DT, and Eren AM (2017). Tracking microbial colonization in fecal microbiota transplantation experiments via genome-resolved metagenomics. Microbiome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, et al. (2015). Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, et al. (2017). Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Gao R, de Mutsert R, Rensen PCN, van Klinken JB, Prehn C, Adamski J, van Hylckama Vlieg A, den Heijer M, le Cessie S, Rosendaal FR, et al. (2018). Postprandial metabolite profiles associated with type 2 diabetes clearly stratify individuals with impaired fasting glucose. Metabolomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, and Mattson MP (2014). Fasting: molecular mechanisms and clinical applications. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Oh T, Kim S, Caussy C, Evans R. (2020). A Universal Gut Microbiome-Derived Signature Predicts Cirrhosis. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, et al. (2017). Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell metabolism 25, 1054–1062.e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, et al. (2015a). Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cellular and molecular gastroenterology and hepatology 1, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, et al. (2015b). Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SV, and Pedersen O. (2016). The Human Intestinal Microbiome in Health and Disease. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, Saltzman E, and McKeown NM (2015). Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li H, Hu J, Zheng J, Zhou J, Botchlett R, Matthews D, Zeng T, Chen L, Xiao X, et al. (2020). Indole Alleviates Diet-induced Hepatic Steatosis and Inflammation in a Manner Involving Myeloid Cell PFKFB3. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maersk M, Belza A S-J, Stodkilde-Jorgensen H R, Steffen Ringgaard S, C., Elizaveta Chabanova E, T., Henrik Thomsen H, P., Steen B., Pedersen S A, Arne, Astrup A,R, Bjorn, and Richelsen B. (2012). Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. [DOI] [PubMed] [Google Scholar]
- Maldonado-Gomez MX, Martinez I, Bottacini F, O’Callaghan A, Ventura M, van Sinderen D, Hillmann B, Vangay P, Knights D, Hutkins RW, et al. (2016). Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe. [DOI] [PubMed] [Google Scholar]
- Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, Machann J, Petzke KJ, Hierholzer J, Lichtinghagen R, et al. (2017). Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology. [DOI] [PubMed] [Google Scholar]
- Martinez KB, Leone V, and Chang EB (2017). Microbial metabolites in health and disease: Navigating the unknown in search of function. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RS, Abu-Ali GS, Drew DA, Lloyd-Price J, Subramanian A, Lochhead P, Joshi AD, Ivey KL, Khalili H, Brown GT, et al. (2018). Stability of the human faecal microbiome in a cohort of adult men. Nat Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. (2009). Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 49, 1877–1887. [DOI] [PubMed] [Google Scholar]
- Mimee M, Tucker AC, Voigt CA, and Lu TK (2016). Programming a Human Commensal Bacterium, Bacteroides thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Syst. [DOI] [PubMed] [Google Scholar]
- Moss EL, Falconer SB, Tkachenko E, Wang M, Systrom H, Mahabamunuge J, Relman DA, Hohmann EL, and Bhatt AS (2017). Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A, Kobiita A, Ye T, and Chambon P. (2013). Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. [DOI] [PubMed] [Google Scholar]
- Olle B. (2013). Medicines from microbiota. Nat Biotechnol. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl A, Johnson RJ, and Abdelmalek M. (2008). Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaga E, Hsieh RH, Mugendi B, Masuku S, Shih CK, and Chang JS (2015). Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. Int J Mol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Helsley RN, Brown AL, Buffa JA, Choucair I, Nemet I, Baleanu Gogonea C, Gogonea V, Wang Z, Garcia-Garcia JC, et al. (2020). Small Molecule Inhibition of Gut Microbial Choline Trimethylamine Lyase Activity Alters Host Cholesterol and Bile Acid Metabolism. . Am J Physiol Heart Circ Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Walker JM, and Holt PR (2012). A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142, 1100–1101.e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al. (2019). Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. . JAMA Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, Kumar P, Smith T, Singhi AD, Nusrat A, et al. (2020). Blocking integrin α(4)β(7)-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. . J Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlon JM, Harris SC, Bhowmik S, Kang DJ, and Hylemon PB (2016). Consequences of bile salt biotransformations by intestinal bacteria. Gut microbes 7, 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Gomez M, Zelber-Sagi S, and Trenell M. (2017). Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. [DOI] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. [DOI] [PubMed] [Google Scholar]
- Sabino J, Hirten R, and Colombel J. (2020). Review article: bacteriophages in gastroenterology-from biology to clinical applications. Aliment Pharmacol Ther. [DOI] [PubMed] [Google Scholar]
- Saeed N, Nadeau B, Shannon C, and Tincopa M. (2019). Evaluation of Dietary Approaches for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review. . Nutrients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi AN, Goel A, Singh A, Sasi A, and Aggarwal R. (2017). Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schugar RC, Willard B, Wang Z, and Brown JM (2018). Postprandial gut microbiota-driven choline metabolism links dietary cues to adipose tissue dysfunction. Adipocyte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, Jones GM, Palii SP, Velasco-Alin M, Pan K, et al. (2017). Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM Jr., Abumrad NN, and Flynn CR (2015). Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpton SR, Ajmera V, and Loomba R. (2019a). Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin Gastroenterol Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, and Terrault NA (2019b). Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpton SR, Yong GJM, Terrault NA, and Lynch SV (2018). Gut Microbial Metabolism and Nonalcoholic Fatty Liver Disease. Hepatol Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Albenberg L.,, Bittinger K, Chehoud C, Chen Y, Judge C, Chau L, Ni J, Sheng M, Lin A, et al. (2015). Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly AN, Sato Y, Kearney S, and Honda K. (2019). Mining the microbiota for microbial and metabolite-based immunotherapies. Nat Rev Immunol. [DOI] [PubMed] [Google Scholar]
- Smillie CS, Sauk J, Gevers D, Friedman J, Sung J, Youngster I, Hohmann EL, Staley C, Khoruts A, Sadowsky MJ, et al. (2018). Strain Tracking Reveals the Determinants of Bacterial Engraftment in the Human Gut Following Fecal Microbiota Transplantation. Cell Host Microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida Y, and Yoneda M. (2018). Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, et al. (2016). Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, et al. (2014). Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, Beghini F, Manara S, Karcher N, Pozzi C, et al. (2019). Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognini P, Thaiss CA, Elinav E, and Sassone-Corsi P. (2017). Circadian Coordination of Antimicrobial Responses. Cell Host Microbe. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, R L, M H, C. M., F.-L. R K, and Gordon JI (2007). The human microbiome project. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr GT, Dohnalova L, and Thaiss CA (2019). The Dimension of Time in Host-Microbiome Interactions. . mSystems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal E, de Vos W, Visser C, Kuijper E, Bartelsman J, Tijssen J, et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Joossens M Fau - Verbeke K, Verbeke K, Wang J, Machiels K, Sabino J, Ferrante M, Van Assche G, Rutgeerts P, and Raes J. (2016). Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohns Colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinks AA (2017). Precision Medicine-Nobody Is Average. Clin Pharmacol Ther. [DOI] [PubMed] [Google Scholar]
- Volkova VV, Lu Z, Besser T, and Grohn YT (2014). Modeling the infection dynamics of bacteriophages in enteric Escherichia coli: estimating the contribution of transduction to antimicrobial gene spread. Appl Environ Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte R, Bartelsman J, Dallinga-Thie G, Ackermans M, Serlie M, Oozeer R, et al. (2012). Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. [DOI] [PubMed] [Google Scholar]
- Walters WA, Xu Z, and Knight R. (2014). Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wan J, Rong H, He F, Wang H, Zhou J, Cai C, Wang Y, Xu R, Yin Z, et al. (2017). Alterations in Gut Glutamate Metabolism Associated with Changes in Gut Microbiota Composition in Children with Autism Spectrum Disorder. . Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, et al. (2015). Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann U, Carvalho AL, Stocks M, and Carding SR (2017). Use of genetically modified bacteria for drug delivery in humans: Revisiting the safety aspect. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R, et al. (2019). Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Stahlman M, Olsson LM, Serino M, Planas-Felix M, et al. (2017). Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. [DOI] [PubMed] [Google Scholar]
- Xu W, Luo Z, Alekseyenko AV, Martin L, Wan Z, Ling B, Qin Z, Heath SL, Maas K, Cong X, et al. (2018). Distinct systemic microbiome and microbial translocation are associated with plasma level of anti-CD4 autoantibody in HIV infection. Sci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, Tian L, Li X, Liu L, Xing Y, et al. (2019). Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Seaton SC, Ndousse-Fetter S, Adhikari AA, DiBenedetto N, Mina AI, Banks AS, Bry L, and Devlin AS (2018). A selective gut bacterial bile salt hydrolase alters host metabolism. . Elife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, and Bugianesi E. (2018a). Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. [DOI] [PubMed] [Google Scholar]
- Younossi Z, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, et al. (2018b). Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, et al. (2018c). Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, and Wymer M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. [DOI] [PubMed] [Google Scholar]
- Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, et al. (2017). Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, and Panda S. (2014). Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. (2015). Personalized Nutrition by Prediction of Glycemic Responses. Cell. [DOI] [PubMed] [Google Scholar]
- Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, et al. (2016). Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Ma Y, Raoult D, Kroemer G, and Gajewski T. (2016). The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science. [DOI] [PubMed] [Google Scholar]