Abstract
Hepatitis C virus (HCV) is a chronic infection that can lead to severe liver damage if left untreated. With increased availability and affordability of curative treatments, screening for HCV has become an important first step in reducing morbidity and mortality. At a rural federally qualified health center (FQHC) in North Carolina, two quality improvement initiatives—an electronic health record (EHR) prompt and educational flyers—were implemented to improve HCV screening rates. We compared the proportion of eligible patients born from 1945 to 1965 who received HCV screening before, during, and after the initiatives. HCV screening rates were highest during the two initiatives (30% and 39%, respectively). Screening rates fell in the six-month period following the initiatives’ conclusion (12%) but remained higher than at baseline (6%). While HCV screening can increase with simple interventions, more durable solutions are needed to maintain screening coverage.
Keywords: hepatitis C, screening, quality improvement
Introduction
Over 2 million people in the United States are infected with hepatitis C virus (HCV).1 If untreated, chronic HCV infection increases the risk of hepatic cirrhosis, fibrosis, and hepatocellular carcinoma. HCV infection contributes to approximately 17,000 deaths per year, surpassing the top 60 other reportable infectious diseases combined.2,3 However, fewer than half of people with HCV are aware of their infection, and only a minority of these receive treatment.4,5
The release of direct acting antiviral (DAA) therapy revolutionized treatment options for HCV infections over the last decade. Several effective and well-tolerated therapy regimens are available, although they remain expensive in the United States. Despite a growing body of literature suggesting DAA therapy is more cost-effective than previous regimens, cost remains a significant barrier to care.6–8 Limited health care access and lack of screening also contribute to low treatment rates. By some estimates, only 7%–11% of people with HCV have received antiviral therapy.5 Treatment rates are particularly low among minority, low income, and Medicaid patients.
Given the under-diagnosis of HCV infection and the availability of effective treatments, adequate screening in the primary care setting is key to reducing HCV morbidity and mortality. Until 2019, the United States Preventive Services Task Force (USPSTF) recommended one-time screening for HCV among high-risk adults, including those born between 1945–1965 or who had another significant risk factor such as injection drug use.9 Coinciding with the opioid crisis and rising injection drug use, the number of acute HCV infections has risen by 3.5-fold over the past decade.10,11 In response, the USPSTF published a new guideline that recommends HCV screening for all adults aged 18 to 79 years.12 Unfortunately, many primary care centers fail to screen for HCV infection in the majority of eligible patients.13,14
For eligible institutions, the 340B Drug Pricing Program can provide a financially sustainable method of providing HCV treatment.15,16 A federal program created in 1992, 340B mandates that drug manufacturers provide medications at reduced costs to covered entities, including safety net hospitals and federally qualified health centers (FQHCs).17 In addition, the presence of a pharmacy at the clinic facilitated the use of the patient assistance program for patients without health insurance. Piedmont Health, a multicenter FQHC in North Carolina, started a monthly liver clinic at several of their locations to streamline HCV treatment for infected patients. However, in 2017, only 19% of eligible patients at Piedmont Health received HCV screening, limiting the number of patients identified who could benefit from the affordable treatment program.
To facilitate HCV screening, some health organizations have implemented a modification in the electronic health record (EHR) that automatically prompts screening in eligible patients. A form of clinical decision support system (CDSS), this method has been used to raise HCV screening rates in a variety of settings, including primary care clinics with underserved populations.18–25 In 2017, Piedmont Health developed an EHR modification, the view all protocols (VAP) prompt, to streamline identification of required screenings due for the patient. Subsequently, a quality improvement (QI) initiative was proposed at the Moncure Community Health Center, a rural Piedmont Health clinic. The aim of this study was to evaluate the effect of a QI initiative to increase HCV screening rates by promoting use of the VAP prompt and displaying educational flyers at the Moncure Community Health Center.
Methods
Setting
The study took place at the Moncure Community Health Center, a site of Piedmont Health Services, which is a group of FQHC clinics providing medical, dental, nutrition, and pharmacy care for underserved persons across 14 counties in central North Carolina. Moncure, a rural clinic, has a panel of 7,921 patients, who average 2.5 visits per year for a total of 19,815 annual visits (Figure 1). Over half (53%) of patients indicate Spanish as their preferred language, with 46% preferring English and 1% other language(s). Self-pay (49%), Medicaid (23%), and private insurance (16%) are the most common insurance statuses, with Medicare, Medicare Health Maintenance Organization (HMO), and North Carolina Health Choice insurance program for children or other for the remaining patients. Nearly one in five patients (19.3%, n=1,528) was born between 1945 and 1965.
Figure 1.
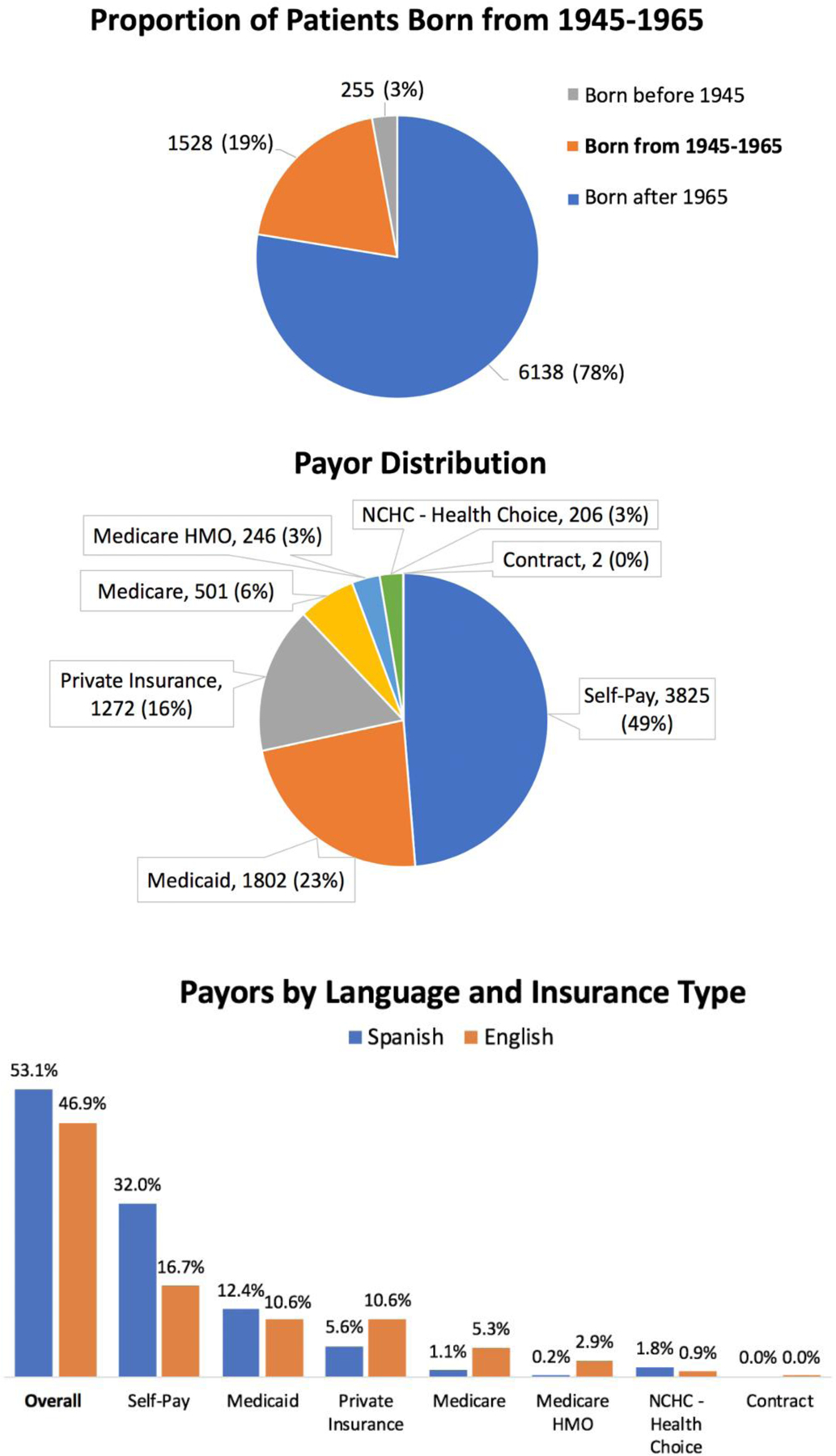
Patient Demographics
HCV screening
Medical assistants or providers could offer HCV screening to patients born between 1945–1965 according to 2018 USPSTF guidelines.9 Patients who agreed to screening received a venipuncture to collect blood for presence of HCV antibodies, which reflexed to HCV RNA if the person tested positive for antibodies. All laboratory testing was conducted via LabCorp. Patients were informed of their results, and those who screened positive were referred to the monthly liver clinic at Moncure for further care.
Intervention planning:
The leadership of Piedmont Health Services identified HCV screening as a priority QI item in their monthly meetings. Medical students from the University of North Carolina (UNC) at Chapel Hill on their 4-month outpatient family medicine rotation met with clinic leadership, providers, and staff to discuss the HCV screening flow. Based on a driver diagram that outlined contributors to low screening rates (Figure 2), the team identified two leading intervention opportunities to increase screening rates: promotion of the “View All Protocols” (VAP) tool to remind providers to offer screening in eligible patients, and informational flyers and posters to improve patient knowledge about HCV screening.
Figure 2.
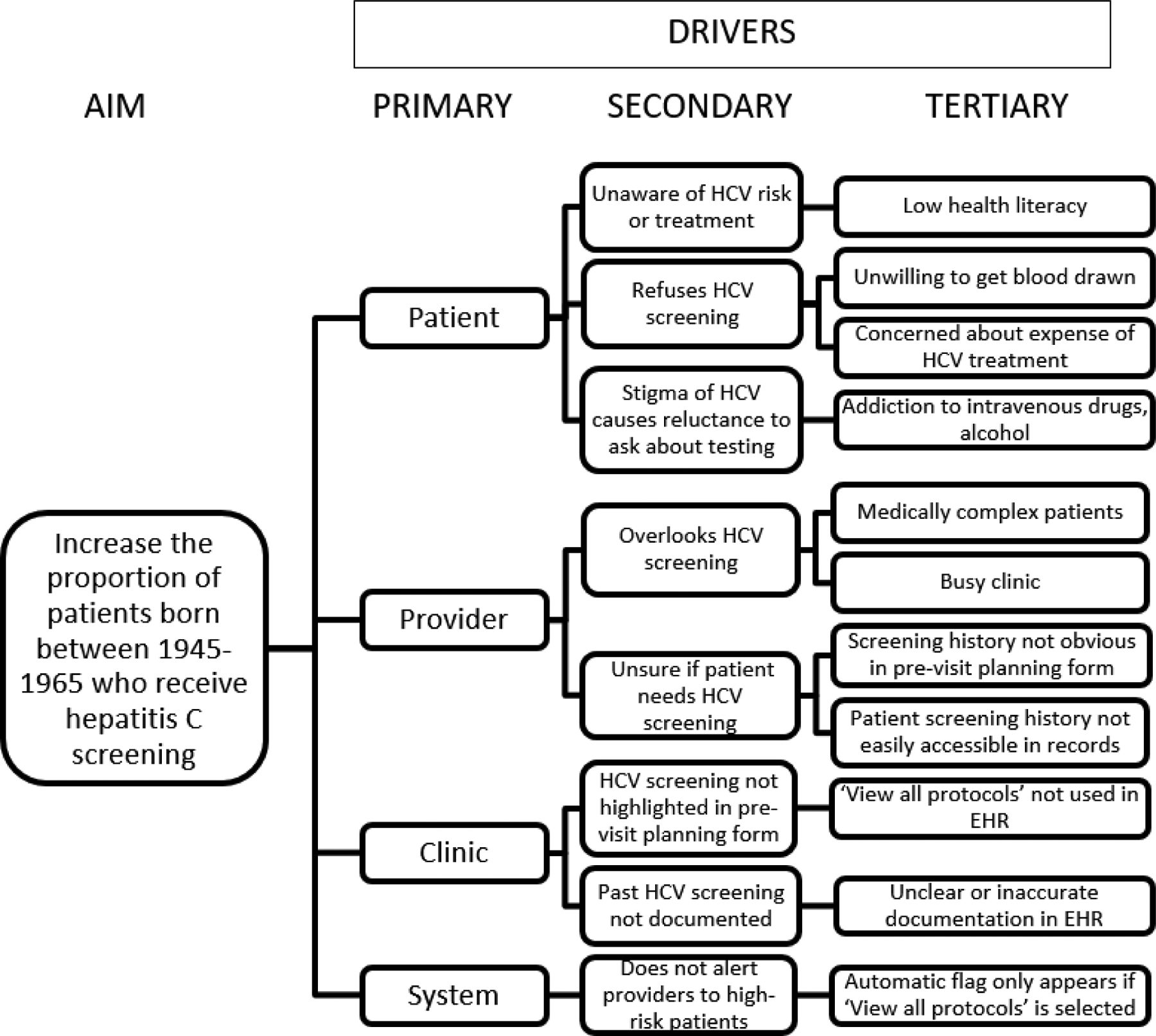
Driver Diagram. Description of primary, secondary, and tertiary drivers of low HCV screening rates at our institution. States the aim of each PDSA cycle of the QI initiative. HCV: hepatitis C virus
PDSA Cycles
Two Plan-Do-Study Act (PDSA) cycles were conducted over four months each. Each cycle was designed and promoted by the UNC medical student rotating at Moncure the time.
PDSA cycle 1 (March – June 2018):
A staff meeting was held to introduce the HCV screening initiative and regular incorporation of the VAP prompt in the Centricity EHR. Weekly meetings with providers and medical assistants were held throughout the duration of the cycle to ensure understanding of the VAP prompt and to gather feedback. Provider teams were given feedback on past and current HCV screening rates on a monthly basis throughout the PDSA cycle to encourage continued improvement.
PDSA cycle 2 (July – October 2018):
After discussing the successes and challenges with implementing regular use of the VAP prompt, medical assistants and providers noted that some patients declined HCV screening because they were not aware of the virus, screening process, or treatment options. To help with planning, we asked some patients about their willingness and hesitancy to receive HCV screening and identified a desire for additional information about the condition. We adapted simple educational flyers from the “Know More Hepatitis” campaign by the Centers for Disease Control and Prevention (CDC), including translating them into Spanish given Moncure’s patient demographics.27 We originally planned to ask the front desk staff to distribute the flyers to patients upon check-in, but a provider recommended placing the flyers in the exam rooms to reduce the burden on front desk staff and paper waste. Flyers were displayed in both English and Spanish in two locations (quarter-sheet versions on neon colored paper in patient exam rooms, and digitally on the TV in the lobby waiting area). The medical student checked each exam room weekly to ensure that the flyers were still present and replenished copies as needed.
Statistical analysis
The proportion of patients screened for HCV was calculated as the number of patients who received HCV screening in a given month divided by the number of patients eligible for HCV screening who had visits at our institution in that same month (Table 1). Patients eligible for HCV screening were defined as patients born from 1945 to 1965, per 2018 USPSTF guidelines.
Table 1.
Summary of proportion of eligible patients who received HCV screening and use of ‘View All Protocols’ (VAP) feature by PDSA cycle.
| Pre-PDSA (Sep 2017 – Feb 2018) | 70 | 4 (6%) | 11% |
| Combined PDSAs (Mar – Oct 2018) | 175 | 63 (36%) | 24% |
| PDSA 1 (Mar – Jun 2018) | 61 | 18 (30%) | 22% |
| PDSA 2 (Jul – Oct 2018) | 114 | 45 (39%) | 26% |
| Post-PDSA (Nov 2018 – Apr 2019) | 220 | 27 (12%) | 24% |
Two sample tests of proportion with α of 0.05 were used to compare the proportion of eligible persons screened for the six months preceding both interventions (September 2017 – February 2018), during the eight months when students were in clinic leading QI projects (March – October 2018), and during the six months following student presence (November 2018 – April 2019).
VAP use was defined as the number of times the VAP protocol was used, as recorded by Centricity, per the total number of patients seen each month for any visit. A linear regression was performed to evaluate the relationship between HCV screening proportions and monthly VAP use from September 2017 to April 2019.
All analyses were conducted with R (R Core Team 2018).26
Ethics approval
This study was deemed nonhuman subjects research at UNC Chapel Hill (IRB 19–3446).
Results
Pre-PDSA 1:
In the six months preceding the PDSA cycles (September 2017 – February 2018), 6% of patients (n=4 of 70 eligible) received HCV screening (Table 1), with a monthly range of 0% to 8%. VAP usage was 11% during this time (Figure 3).
Figure 3.
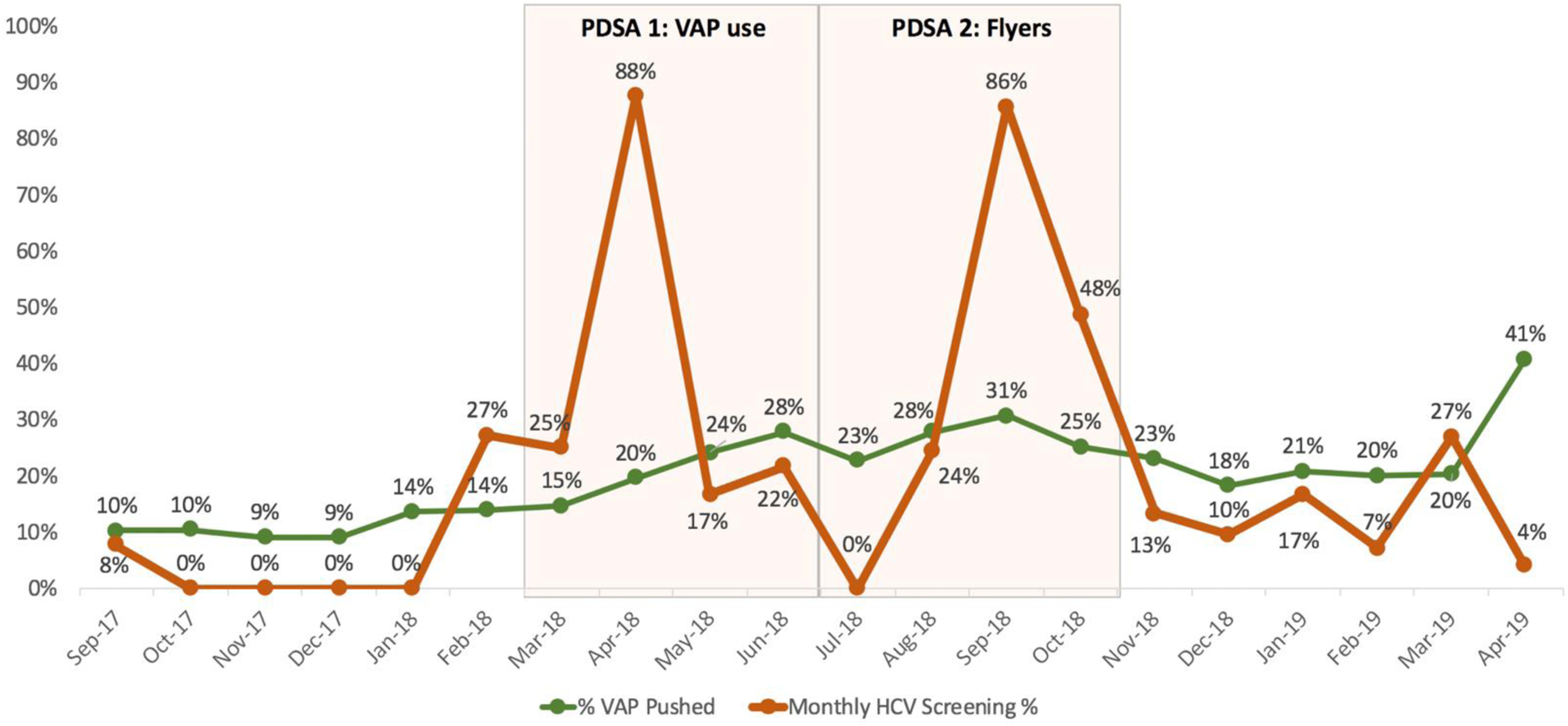
Monthly proportion of HCV screening and VAP use by PDSA cycle.
PDSA 1:
From March to June 2018, 30% of patients (n=18 of 61 eligible) received HCV screening. HCV screening rates during each of the four months ranged from 17% to 88% of eligible patients (Figure 3). Overall, HCV screening rates of eligible patients rose by 24% over the first PDSA cycle. VAP usage rose to 22% during this time.
PDSA 2:
From July to October 2018, 40% of patients (n=45 of 114 eligible) received HCV screening. HCV screening rates during each of the four months ranged from 0% to 86% of eligible patients. VAP usage rose to 26% during this time.
Combined PDSA 1 and 2:
Overall, during the eight months of PDSA cycles, 36% of patients (n=63 of 175 eligible) received HCV screening. The proportion of eligible patients who received HCV screening was 30% higher (95% CI: 21–39%) during the two PDSA cycles than in the six-month period prior to the PDSA cycles (p<0.001). Average VAP use was 24%, a 13% increase from the pre-PDSA time period.
Feedback on flyers from administration and providers was positive. Providers were enthusiastic about providing patient education materials in a digestible format. The neon color of the flyers visually prompted some providers to ask patients about screening for HCV. However, one medical assistant said she threw away many of the flyers because patients (usually children) were drawing on them, and that she did not know why the flyers were in the room. We did not systematically obtain feedback from patients regarding the flyers, but providers and medical assistants stated that they received multiple positive comments about the flyers. VAP use from July to October 2018 remained higher than pre-PDSA 1.
Post-PDSA 2:
HCV screening rates declined in the six-month period after the two PDSA cycles (November 2018 – April 2019), coinciding with the medical students departing the clinic. The HCV screening during each of the six months following PDSA 2 ranged from 4% to 27%. Overall, 12% (n=27 of 220 eligible) received HCV screening in this period. The proportion of eligible patients screened for HCV post-PDSA cycles was 24% lower (95% CI: −14 - −30%) than during active PDSA months (April – October 2018) (p <0.001). The proportion of eligible patients screened for HCV post-PDSA cycles was 7% higher (95% CI: 0.4–13.5%) than prior to the interventions, but the difference did not reach statistical significance (p=0.12).
Monthly VAP usage remained at 24%, consistent with the usage during the active PDSA months. Although multiple additional copies of the flyers were left with the clinic staff, no data were collected to show if and for how long flyers were adequately stocked in the exam rooms.
Association of VAP use with HCV screening:
The results of the linear regression indicated that the monthly percent of VAP use was not significantly associated with the number of monthly HCV screenings (β=1.01, 95% CI: −0.47–2.48; p=0.17).
Limitations
Our study has several important limitations. First, our results are limited to the percent of eligible patients who actually received HCV screening. These numbers do not reflect patients who were offered HCV screening but declined it, as “declined testing” is not currently recorded in the Centricity EHR. Therefore, our data do not directly capture whether the QI initiative led to an increase in offered HCV screening by providers. In addition, we were unable to account for possible duplications from patients who had previously screening HCV screening because we did not have access to individual patient data.
Importantly, the study occurred at a 340B-covered clinic with a newly opened liver clinic to provide a financially affordable and accessible source of HCV treatment. HCV screening was therefore more practical and a higher priority than in clinics without affordable treatment access.
This study was also limited in scope and time scale. The CDSS was implemented in the EHR Centricity, and further studies are needed to examine this study’s applicability to other EHRs and settings. In addition, restrictions on the percent of walls that could be covered with paper due to fire hazards limited the visibility of educational flyers. Finally, this intervention did not target eligible patients based on additional risk factors such as IV drug use or incarceration. More advanced EHR screening prompts could be developed to better identify high-risk patients.
Discussion
A QI initiative based on an EHR prompt and educational flyers was effective at increasing HCV screening rates in a primary care clinic. HCV screening rates of eligible patients rose significantly during the PDSA cycles, and post-PDSA screening rates remained higher than baseline rates. Costs were minimal and limited to buying paper and photocopies for flyers.
A key finding of our study is that the presence of an advocate for the QI initiative appeared to meaningfully impact the degree and sustainability of increased HCV screening rates. For our study, two medical students rotating in the clinic served to champion the initiative during each PDSA cycle. The observed drop in HCV screening rates following their rotations suggests that their presence was key to promoting and maintaining the interventions. Notably, through their role as clinical learners, the medical students may also have identified additional patients eligible for HCV screening, inadvertently contributing to the rise in screening rates.
Our results did not find a significant relationship between VAP use and HCV screening rates. This could be explained by several factors. First, the VAP prompt alerts providers to many pertinent screening tests and is not specific to HCV screening. In addition, our intervention may have shifted how providers use the VAP prompt rather than how often, as it may have encouraged providers to focus on the HCV screening prompt specifically. Finally, the low sample size during our PDSA cycles limits our power and ability to find a significant relationship.
It is important to note that this QI initiative focused on two key drivers of low HCV screening rates: lack of automation at the systems level and lack of awareness at the patient level. Future studies may consider other drivers at the patient, provider, clinic or system level as potential strategies for improvement. In addition, further research is warranted to examine applicability of these results to other settings and EHRs.
This study was conducted in accordance with 2018 USPSTF guidelines, which recommended one-time HCV screening in all patients born from 1945 to 1965.9 Notably, in 2020, the USPSTF published a new guideline to recommend HCV screening in all adults aged 18 to 79.12 If this change is finalized, EHR modification such as the VAP prompt could be updated accordingly. This could actually increase the number of patients who would potentially benefit from an initiative to increase HCV screening rates.
Conclusions
These results indicate that QI interventions may be an effective and low-cost strategy to improve HCV screening rates in the primary care setting. Our results are consistent with existing literature that demonstrates a rise in HCV screening rates following implementation of an EHR modification, particularly in primary care and underserved populations.18–23 It also adds to a growing body of literature on the potential of a combined approach with both EHR modification and patient awareness initiatives, such as educational flyers.24,25 These results could be applied to other primary care settings, particularly those with 340B funding to ensure a financially affordable source of HCV treatment for patients who screen positive.
Implications
Clinical decision support systems in the EHR and educational flyers may serve as effective and low-cost strategies to increase HCV screening. Given the availability of effective HCV treatment, QI initiatives to improve HCV screening may be an important target to reduce HCV morbidity and mortality. The presence of a QI advocate, however, appears essential to maintaining increased screening over time.
Acknowledgements:
We would like to acknowledge Gary Beck Dallaghan, PhD, Director of Educational Scholarship and Research Associate Professor of Pediatrics, UNC School of Medicine.
We also wish to thank the staff and providers at our institution for their efforts to increase HCV screening and for helping make this study become a reality.
Conflicts of Interest and Source of Funding:
The authors gratefully acknowledge salary support from Piedmont Health Services for Drs. East and Santos as well as the UNC Medical Scientist Training Program [grant T32GM008719] and the NIH individual fellowship [F30MH111370]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the study design, data collection and analysis, interpretation of results, or preparation of the manuscript for publication. The authors declare that there is no conflict of interest.
Biographical Sketches:
Melissa D. Klein, BS is an MD/MPH student at the University of North Carolina School of Medicine and Gillings School of Global Public Health in Chapel Hill, North Carolina.
Bryna J. Harrington, PhD is an MD/PhD student at the University of North Carolina at Chapel Hill, North Carolina.
Joan East, MD is a family medicine physician at the Moncure Community Health Center in Moncure, North Carolina. She is also an adjunct assistant professor at the Family Medicine Department of the University of North Carolina at Chapel Hill, North Carolina.
Jennifer Cunningham, BA, is a data analyst at Piedmont Health Services in Moncure, North Carolina.
Nicole Ifill, MBA, CPHQ is a Quality and Regulatory Officer at Piedmont Health Services in Moncure, North Carolina.
Jan Lee Santos, MD, MHA, MA is a Clinical Services Trainer at Piedmont Health Services in Chapel Hill, North Carolina. He is also an adjunct assistant professor at the Family Medicine Department of the University of North Carolina at Chapel Hill, North Carolina.
References:
- 1.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–1031. doi: 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance for Viral Hepatitis – United States, 2017.; 2019. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm.AccessedNovember 25, 2019.
- 3.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising Mortality Associated With Hepatitis C Virus in the United States, 2003–2013. Clin Infect Dis. 2016;62(10):1287–1288. doi: 10.1093/cid/ciw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spradling PR, Xing J, Rupp LB, et al. Uptake of and Factors Associated with Direct-acting Antiviral Therapy among Patients in the Chronic Hepatitis Cohort Study, 2014 to 2015. J Clin Gastroenterol. 2018;52(7):641–647. doi: 10.1097/MCG.0000000000000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–419. doi: 10.7326/M14-1152 [DOI] [PubMed] [Google Scholar]
- 7.Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The Cost-effectiveness, Health Benefits, and Financial Costs of New Antiviral Treatments for Hepatitis C Virus. Clin Infect Dis. 2015;61(2):157–168. doi: 10.1093/cid/civ220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chahal HS, Marseille EA, Tice JA, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med. 2016;176(1):65–73. doi: 10.1001/jamainternmed.2015.6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Final Update Summary: Hepatitis C: Screening - US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening.AccessedNovember 25, 2019.
- 10.Increase in hepatitis C infections linked to worsening opioid epidemic. https://www.cdc.gov/nchhstp/newsroom/2017/hepatitis-c-and-opioid-injection-press-release.html.Published 2017. AccessedNovember 25, 2019.
- 11.Surveillance for Viral Hepatitis - United States, 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/commentary.htm.AccessedDecember 18, 2019.
- 12.Final Recommendation Statement: Hepatitis C Virus Infection in Adolescents and Adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening1. Published March 2020. AccessedMarch 12, 2020.
- 13.Bourgi K, Brar I, Baker-Genaw K. Health Disparities in Hepatitis C Screening and Linkage to Care at an Integrated Health System in Southeast Michigan. Schanzer DL, ed. PLoS One. 2016;11(8):e0161241. doi: 10.1371/journal.pone.0161241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linas BP, Hu H, Barter DM, Horberg M. Hepatitis C screening trends in a large integrated health system. Am J Med. 2014;127(5):398–405. doi: 10.1016/j.amjmed.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones EA, Linas BP, Truong V, Burgess JF, Lasser Id KE. Budgetary impact analysis of a primary care-based hepatitis C treatment program: Effects of 340B Drug Pricing Program. 2019. doi: 10.1371/journal.pone.0213745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasser KE, Heinz A, Battisti L, et al. A hepatitis C treatment program based in a safety-net hospital patient-centered medical home. Ann Fam Med. 2017;15(3):258–261. doi: 10.1370/afm.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.340B Drug Pricing Program. https://www.hrsa.gov/opa/.AccessedDecember 14, 2019.
- 18.Armstrong H, Gonzalez-Drigo M, Norels G, et al. Electronic Clinical Decision Support Intervention to Increase Hepatitis C Screening and Linkage to Care Among Baby Boomers in Urban Safety Net Health Systems. Popul Health Manag. October2019:pop.2019.0105. doi: 10.1089/pop.2019.0105 [DOI] [PubMed] [Google Scholar]
- 19.Geboy AG, Nichols WL, Fernandez SJ, Desale S, Basch P, Fishbein DA. Leveraging the electronic health record to eliminate hepatitis C: Screening in a large integrated healthcare system. PLoS One. 2019;14(5). doi: 10.1371/journal.pone.0216459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel A, Sanchez J, Paulino L, et al. A systematic model improves hepatitis C virus birth cohort screening in hospital-based primary care. J Viral Hepat. 2017;24(6):477–485. doi: 10.1111/jvh.12669 [DOI] [PubMed] [Google Scholar]
- 21.Al-hihi E, Shankweiler C, Stricklen D, Gibson C, Dunn W. Electronic medical record alert improves HCV testing for baby boomers in primary care setting: adults born during 1945–1965. BMJ Open Qual. 2017;6(2):e000084. doi: 10.1136/bmjoq-2017-000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coyle C, Kwakwa H, Viner K. Integrating routine HCV testing in primary care: Lessons learned from five federally qualified health centers in Philadelphia, Pennsylvania, 2012–2014. Public Health Rep. 2016;131:65–73. doi: 10.1177/00333549161310S211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller LS, Rollin F, Fluker SA, et al. High-yield birth-cohort hepatitis C virus screening and linkage to care among underserved African Americans, Atlanta, Georgia, 2012–2013. Public Health Rep. 2016;131:84–90. doi: 10.1177/00333549161310S213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain MK, Rich NE, Ahn C, et al. Evaluation of a Multifaceted Intervention to Reduce Health Disparities in Hepatitis C Screening: A Pre-Post Analysis. Hepatology. 2019;70(1):40–50. doi: 10.1002/hep.30638 [DOI] [PubMed] [Google Scholar]
- 25.Konerman MA, Thomson M, Gray K, et al. Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers. Hepatology. 2017;66(6):1805–1813. doi: 10.1002/hep.29362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R: The R Project for Statistical Computing. https://www.r-project.org/.AccessedDecember 16, 2019. [Google Scholar]
- 27.CDC. Know More Hepatitis. https://www.cdc.gov/knowmorehepatitis/.Published April 3, 2020. AccessedJune 27, 2020.


