Abstract
Objective:
Sleep disturbances, including insomnia (difficulty falling or staying asleep), are common nicotine withdrawal symptoms particularly during the initial stage of nicotine abstinence, and increase the likelihood of relapse within the first 4 weeks of quitting. Although clinically recognized as a key symptom of nicotine withdrawal, sleep disturbances are not addressed in the clinical guidelines for nicotine dependence treatment. Unfortunately, Nicotine Replacement Therapy (NRT) and other pharmacologic interventions do not attenuate withdrawal-provoked sleep disturbances, with several even exacerbating sleep disruption. The present study tested the impact of 30-minutes of daily moderate exercise, morning versus evening, on key polysomnographic indicators of sleep disturbances during initial three days (72 hours) of nicotine withdrawal.
Methods:
Forty-nine daily smokers (53% male) completed three separate abstinence periods, during which they completed either morning exercise, evening exercise, or a non-exercising magazine reading control condition. Order of condition was counterbalanced across subjects with a 1-week wash out in between each three-day abstinence period.
Results:
Exercise engagement mitigated several changes in sleep architecture associated with acute nicotine deprivation and other time-related effects on sleep, specifically frequency of arousals (B = −2.8, SE = .95; t(1271) = −3.0, p = .003) and reductions in sleep maintenance (B = .58, SE = .21; t(1270) = 2.8, p = .005). Additionally, smokers who reported greater perceived withdrawal severity had the longest latency to fall asleep, but experienced the greatest attenuation of this effect following PM exercise.
Conclusion:
Overall, results suggest a role for exercise as an adjunct smoking cessation treatment to specifically target sleep disturbances during early acute nicotine withdrawal.
Keywords: comorbid insomnia, nicotine withdrawal, exercise, cigarettes smoking, Sleep latency, sleep continuity
Introduction
Cigarette smoking remains the leading cause of preventable morbidity and mortality in the US, despite its declining prevalence over the past 5 decades (Samet, 2013). Nearly fourteen of the seventeen million Americans who try to quit each year relapse within the first month (CDCTobaccoFree, 2019). This cessation failure is often attributed to the onset of problematic acute nicotine withdrawal, a combination of unpleasant symptoms, including irritability, negative mood, intense cravings for smoking and sleep disturbances, which ensue within a few hours of smoking cessation. Poor sleep health as characterized by shorter sleep duration, difficulty falling asleep, difficulty staying asleep, early awakenings and night-time awakenings are more common in smokers than nonsmokers. Of particular relevance to smoking cessation efforts, sleep health deteriorates following cessation in many smokers, and this in turn is implicated in relapse (Patterson et al., 2019).
Sleep disturbances are especially common during the initial periods of abstinence, with diminished sleep continuity and increased number of arousals observed within the first 24-36h of abstinence, as well as changes in sleep quality reported by up to 42% of individuals (Okun et al., 2011), which may persist for several months (Moreno-Coutiño et al., 2007). Importantly, sleep disturbances, either predating or concurrent with a quit attempt, are associated with increased risk of relapse (Boutou et al., 2008; Peltier et al., 2017; Peters et al., 2011). Unfortunately, Nicotine Replacement Therapy (NRT) and other pharmacotherapies for smoking cessation, while effective at increasing the rates of abstinence, do not reverse sleep disturbances and, by some accounts, exacerbate them (Aubin, 2002; Thomas et al., 2015). For example, sleep disturbances are reported by up to 50% of smokers using NRT (Gourlay et al., 1999), while up to 46% of quitting smokers experience some degree of sleep disturbances on varenicline and bupropion (Jorenby et al., 1999; Ashare et al., 2017). Research incorporating sleep-specific interventions for smoking cessation is substantially lacking, however a small preliminary study suggests that adjunct cognitive behavioral therapy for insomnia (CBT-I) with smoking cessation counseling may increase time to relapse (Fucito et al., 2014).
Exercise is one promising behavioral intervention that may aid smoking cessation. While meta-analyses casts some doubts on the long term efficacy of aerobic exercise as a smoking cessation intervention (Klinsophon et al., 2017;Ussher et al., 2014), more focused studies suggest that it may have a role in targeting specific problematic symptoms. Potential mechanisms for its impact include its effects on mood, abstinence induced cravings and cue reactivity. For example exercise may be one behavioral method to improve mood during abstinence as it has been shown to have a positive impact on negative mood experienced by female smokers upon quitting (Bock et al., 1999). Other smoking cessation studies have evidenced that exercise can attenuate both abstinence-induced craving and negative mood (Ussher et al., 2012), and as reactivity to smoking related cues and abstinence induced cravings (Conklin et al., 2017).
A large body of literature supports exercise to ameliorate sleep disturbances in individuals with insomnia disorder and insomnia symptoms (Kovacevic et al., 2018; Lowe et al., 2019), but no study, to date, has used exercise to specifically target sleep disturbances associated with acute nicotine withdrawal. Several potential mechanisms have been proposed for the beneficial effects of exercise on sleep, including reduction in inflammatory markers (Kredlow et al., 2015), alteration of core body temperature (Uchida et al., 2012) and regulation of circadian rhythms (Youngstedt et al., 2019)
Thus, the goal of the present study was to test the impact of daily moderate exercise on key sleep disturbances during three days of acute nicotine abstinence. Using a within-subject design, the impact of moderate in-session exercise (30 minutes of treadmill walking at 60% Age-Predicted Maximum Heart Rate-APMHR ) at two times of the day (either AM or PM), as well as non-exercising control activity (30 minutes of magazine reading) was assessed on several objective sleep parameters during 3 separate 72-hour bouts of abstinence. The duration and intensity of exercise were chosen in consideration of the Department of Public Health’s recommendation of moderate physical activity for at least 30 minutes several days a week as a general guideline for health maintenance and reduction of cardiovascular risk (Committee & others, 2008). Further, this decision was informed by several past studies demonstrating that low to moderate intensity exercise reduces cigarette craving for up to 15 min post-exercise during abstinence (Janse Van Rensburg et al., 2009), and that walking can attenuate cue-induced craving (Taylor & Katomeri, 2007).
We hypothesized that exercise overall, compared to control activity, would lead to reductions in sleep disturbance severity. Additionally, the study examined differences in the efficacy of exercise to relieve polysomnographic markers of sleep disturbances as a function of age, gender, and nicotine dependence, as well as exercise time of day (AM/PM). The latter time variable was included based on considerable recent evidence that evening exercise is not associated with worse sleep (Buman et al., 2014; Myllymäki et al., 2011; Myllymäki et al., 2012; Thomas et al., 2020), and to determine if that finding is upheld even during acute nicotine withdrawal
Method
Study design and General Procedures
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments and were approved by the Institutional Review Board of the University of Pittsburgh.
A within-subjects crossover design was used to examine sleep during baseline (ad lib smoking) and three smoking-abstinent exercise conditions (AM exercise, PM exercise, No exercise control) among non-treatment seeking non-exercising smokers. All participants abstained from smoking for three consecutive days (72-hours) for each of the three abstinence periods, or blocks. In between experimental blocks, participants were instructed to return to regular smoking for one week. Exercise condition was counterbalanced and stratified by gender such that all condition order combinations occurred an equal number of times across all subjects and equally across male and female participants.
Participants
Participants were male and female daily smokers, age 18 to 45, who met criteria for nicotine dependence (DSM-IV), had been smoking for at least one year, had smoked at least 10 cigarettes per day for the past six months, did not report an attempt to quit for longer than one week in the previous month and were not taking medication for smoking cessation (including NRT). An age cut off of 45 was set for inclusion in order to limit the number of medical co-morbidities (including sleep apnea) of participants. In addition, individuals were only eligible to enter the study if they were not regularly exercising (defined as exercising fewer than 3 times per week and for no more than 20 minutes each time, i.e. comfortably below the WHO guidelines for exercise in adults), were free of medical illness and were able to exercise (as determined by medical history, EKG, and blood tests), and had not met criteria for any psychiatric disorders in the past year, assessed via the Structured Clinical Interview (Non-patient Version) for DSM-IV (SCID-NP). Eligible participants could not have young children in the home (under 3 years of age) or do shift work. Potential participants were told that the study would examine the effects of exercise on nicotine withdrawal symptoms. Participants were recruited through various advertisement strategies, including flyers, ads in local publications and on main bus routes. Figure 1 shows participants flow through the study.
Figure 1:
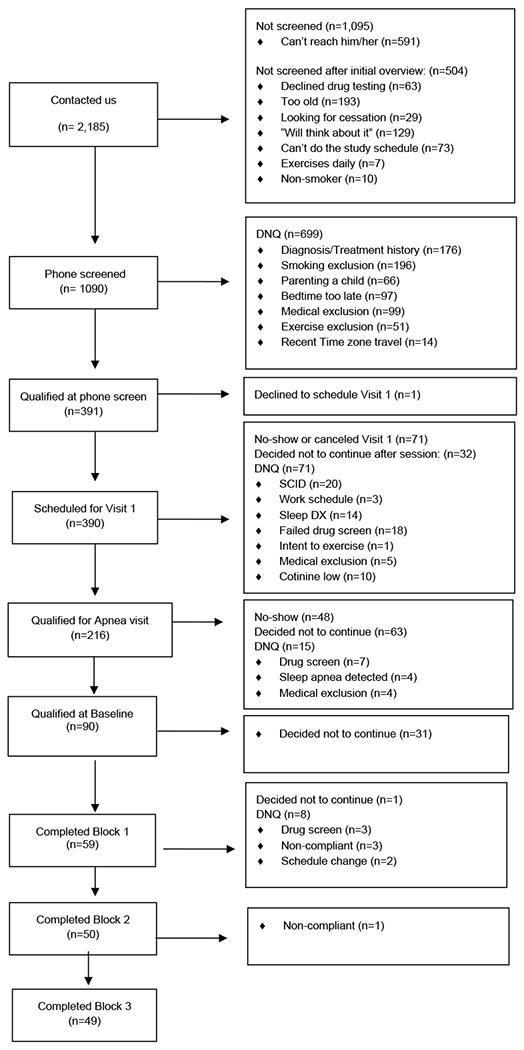
Recruitment and Participant flow through the study
Initial screening:
After meeting initial telephone screening eligibility criteria (self-reported age, height, weight, smoking history, Fagerstrom test of nicotine dependence (Heatherton et al., 1991) sleep patterns, non-exercising criteria, recent travel across time zones, absence of young children in the home, shift work, no current medical or psychiatric disorder) participants were invited to complete an in-person screening to determine further eligibility. This included signing informed consent, providing a CO breath sample (>10ppm), urine cotinine (greater than 100ng/ml) and drug screening, and getting weighed and measured (BMI < 40). To confirm regular smoking, participants had to give an expired-air carbon monoxide breath sample >10ppm (Vitalograph Inc., Lenexa, KS), and a urine cotinine level > 100ng/ml, measured as a 3 or greater on the NicAlert urine test, a cutoff set by the company to indicate that an individual is a smoker (Nymox Corporation, Hasbrouck Heights, NJ). These cutoffs adhere to recommended bioverification cut-points and are widely used across smoking studies (“Biochemical Verification of Tobacco Use and Cessation,” 2002). After meeting biological criteria, participants who were still eligible completed a SCID interview (First & Gibbon, 2004) (no history of mood, anxiety, psychotic disorders, chemical dependencies other than nicotine in the past year) and Sleep SCID (no current sleep disorder) with a study experimenter. Those who continued to be eligible were sent to the lab for an EKG and blood test. Participants who met all eligibility criteria, were called within the week. They were asked to determine their habitual bedtime for the past two weeks by averaging their recalled nightly bedtime and were scheduled for their sleep apnea screening night.
Sleep Apnea screening:
Each participant completed one sleep apnea screening night in the sleep laboratory. Polysomnographic (PSG) recordings included bilateral central and occipital EEG channels (C3, C4, O1, O2), referenced to A1 + A2, submentalis EMG, and V2 EKG. Sleep disordered breathing was assessed with an oral-nasal thermistor and a nasal pressure device to measure airflow, inductance plethysmography to measure chest and abdominal movements, and fingertip oximeter. Bilateral anterior tibialis EMG was also included to assess periodic leg movements. Respiratory disturbances were identified according to the American Academy of Sleep Medicine (AASM) criteria ( AASM 2007.). Participants were excluded for apnea-hypopnea index (AHI) >10 (i.e., 10 or more sleep apnea-hypopneas per hour of sleep) (Buysse et al., 2007). If no sleep apnea was present, participants were scheduled for their first experimental session.
Polysomnography measurement during Overnight Sessions:
Participants spent three consecutive overnight stays, on three different occasions 7-17 days apart, at the Neuroscience, Clinical and Translational Research Center (N-CTRC) sleep laboratory at Western Psychiatric Hospital, University of Pittsburgh. Each of the nine experimental nights (three 3-night sessions) at the N-CTRC involved polysomnograhic sleep recordings. Each night, EEG (C3 or C4 referenced to A1 + A2), two channels of electrooculogram (EOG; asymmetric lateral canthus from each eye, referenced to A1 + A2), and one channel of electromyogram (EMG); (bipolar submentalis) were recorded. Sleep studies were scored by trained polysomnographic technologists according to the AASM guidelines (AASM 2007) . The following sleep variables were recorded: sleep latency, number of arousals, time spent asleep, sleep efficiency, sleep maintenance, percent time in stage 1, percent time in stage 2, percent time in delta sleep, percent time in REM, and number or REM periods.
Experimental Blocks:
During each 3-day experimental block, the subject slept in the lab and continued to engage in their usual activities during the day (e.g., work, school). They were required to completely refrain from smoking during that 72-hour period, starting 6-hours before the they arrived for the first day of a block. All participants gave a CO sample each day and completed a urine drug screen on the first night of each of the three 3-day periods. On each sleep laboratory night, participants reported to the lab 5 hours prior to established bedtime, completed study measures, were required to go to bed at their established bedtime and remain in bed (except for bathroom use) until their habitual rise-time. Upon each arrival to the N-CTRC, they gave a CO breath sample. For the first day, after 6 hours of abstinence, participants were expected to show at least 50% reduction in CO from their baseline level (acquired during the screening process), as done in our previous studies involving smoking deprivation (Conklin et al., 2017). Participants were instructed that they were not allowed to use nicotine replacement products (such as the nicotine patch or gum) to aid in abstinence. Abstinence during the three-day experimental sessions continued to be monitored by collecting CO and testing urine cotinine each time the participant reported to the lab. Although urine cotinine was not necessarily expected to reduce during the three-day period, anything other than no change or a reduction in cotinine over a three-day abstinence block during that time was recorded as having smoked. If a subject failed CO the first day of a block, they were given one chance to reschedule their block. Participants who failed to remain abstinent once they started a 3-day block (N=5) were excused from the study. In between the three 3-day experimental blocks, participants were told to resume regular smoking. Those individuals who, at any point in the study, expressed the desire to permanently quit smoking were given educational material on smoking cessation, referred to specialty counseling, and were excluded from the study (N=1).
Participants who met CO deprivation criteria completed the following initial ratings within 30 minutes of arrival: Minnesota Nicotine Withdrawal Scale (MNWS)(Hughes & Hatsukami, 1986), Mood Form (Diener & Emmons, 1984), Brief Questionnaire of Smoking Urges (QSU-B) (Carter & Tiffany, 2001), as well as a brief computer-automated cue reactivity (CR) trial, used in past studies (Conklin et al., 2017), during which they viewed and rated their subjective craving in response to smoking and neutral pictorial stimuli. The events that followed differed as a function of block condition as follows:
PM Exercise:
Following completion of initial ratings and cue reactivity, participants completed exercise (as described below) followed by a post-exercise CR session, MNWS, Mood Form and QSU-B. They were then free to eat dinner, watch TV, read, until they were prepared for the night. Exercise timing was determined based on subjects’ habitual bedtime and occurred, for all participants, between 5 and 8pm. The following morning, they repeated the ratings and CR within 30 minutes form they wake up time and were then free to leave the lab and go about their day. Physical activity outside of sessions was recorded with actigraphy watches and evaluated for compliance with non-exercising criteria.
AM Exercise:
Participants arrived at the lab 5 hours prior to their habitual bedtime and completed initial ratings and CR. They were then free to eat dinner, watch TV, read, until they were prepared for the night. The following morning, they completed the pre-exercise self-report assessments and cue-reactivity within 30 minutes from their wake-up time. Participants then completed the exercise session, followed by a post-exercise CR session, MNWS, Mood Form and QSU-B and were then free to leave the lab.
Magazine Control:
Participants followed the same protocol described for the exercise sessions, but they were instructed to sit quietly and read magazines in place of exercise. Magazines were provided by the research team and had been screen carefully for absence of nicotine related content and smoking cues. Half of the participants were randomized to AM reading and half to PM reading.
Exercise protocol
Exercise consisted of 30 minutes of brisk walking at 60% of age-predicted maximal heart rate (APMHR) on a treadmill, preceded by 5 minutes of warm up (exercise at 25% APMHR), followed by 5 minutes of cool down. Participants wore a heart rate monitor during the entire session and research staff assured that participant’s heart rate stayed in the target range throughout the session, by adjusting the treadmill incline.
Statistical Analyses
The current study examined the main effect and interactive influences of experimental aerobic exercise and non-exercising control conditions of magazine reading, alongside severity of nicotine withdrawal, on a variety of polysomnographic sleep variables amid a sample of adult smokers undergoing 72-hour blocks of cessation. The primary analyses reported in the current study were evaluated via mixed model regressions using SAS PROC MIXED (v. 9.4, SAS Institute, Inc, 2015) with Bonferroni post-hoc comparisons to ascertain significant differences in time since smoking abstinence (night 1, 2, and 3) and exercise condition (exercise vs. non-exercising control) on sleep, while controlling for Type 1 Error rates. Mixed model regression features advantages over traditional forms of regression, due to the ability to handle repeated assessments of both predictor variables (e.g., night of deprivation within a given exercise condition) and outcome variables (e.g., sleep latency and sleep efficiency) in combination with between subject predictors (e.g., age, gender, and nicotine dependence).
Analyses of mixed model regressions tested the main effects of exercise condition (1 = AM/PM magazine reading non-exercising control; 2 = AM exercise; and 3 = PM exercise), perceived nicotine withdrawal severity, and time since abstinence (night 1, 2, and 3) on sleep variables. Separate models were evaluated for the following outcome variables: sleep latency, number of arousals, time spent asleep, sleep efficiency, sleep maintenance, percent time in stage 1, percent time in stage 2, percent time in delta sleep, percent time in REM, and number or REM periods. For each statistical model evaluated, baseline (non-deprived) levels of the respective dependent variable were entered as a covariate in addition to the ordering of experimental treatment (e.g., magazine control-AM exercise-PM exercise), to control for its predictive influence. Secondary analyses examined the following two-way interaction effects on sleep variables: exercise condition X time, exercise condition X nicotine withdrawal, and nicotine withdrawal X time. Simple slopes analyses were performed to probe significant interaction effects, in accord with recommendation (Aiken & West, 1991).
We based our initial power calculation on a study by Grove et. al (Grove et al., 2006) who found a significant effect of moderate 30-minute exercise on “time to sleep onset” among smokers during the first week of acute nicotine withdrawal. Comparing two group of smokers, the authors found a significant decrease in difficulty falling asleep during the first post-quit week among those in the exercising group (n=12) versus those in the non-exercising group (n=10) of F(1,19) = 6.67, p<.019 with a Cohen’s d=1.18. Thus, with a sample of 48, in our within subjects study, we would have power of 91% with a sample of only N=10 to find an effect on sleep onset of similar magnitude. However, the authors did not find a significant difference in difficulty staying asleep, a variable in which we were interested. They did not report the difference between groups on that variable during the first week of nicotine withdrawal, but noted that it was not significant across the entire study. Lack of group difference may have been due to the small sample size and between subjects comparison. Thus, given our interest in multiple sleep variables, we conservatively estimated having at least 80% power to detect changes with a sample size of n=48.
Results
Participants flow is shown in Figure 1. Sample characteristics are featured in Table 1 [insert Table 1 here]. Those individuals who, at any point in the study, expressed the desire to permanently quit smoking were given educational material on smoking cessation, referred to specialty counseling, and were excluded from the study (N=1). Five participants were excluded for failure to maintain abstinence. Preliminary analyses examined sociodemographic predictors of sleep variables in mixed model regression, while controlling for baseline sleep of the respective dependent variable. Ordering of experimental condition (e.g., magazine control-AM exercise-PM exercise) was unrelated to all sleep dependent variables, p’s> .1. Age, female gender, ethnicity, education level and Fagerstrom Nicotine Dependence score were all significantly related to the dependent variables and were hence entered as covariates alongside baseline values and experimental treatment order in all subsequent analyses for the respective dependent variable.
Table 1.
Descriptive Statistics of Sample Characteristics
| Variables | Freq (%) | M (SD) |
|---|---|---|
| Age in Years | 27.5 (6.5) | |
| Length of Time Smoking in Years | 11.7 (6.2) | |
| Number Cigarettes Smoked Per Day | 14.3 (4.03) | |
| Fagerström Test of Nicotine Dependence | 4.8 (1.7) | |
| Gender | ||
| Male | 26 (53%) | |
| Female | 23 (47%) | |
| Ethnicity | ||
| African American | 13 (27%) | |
| Asian | 3 (6%) | |
| White | 30 (61%) | |
| Native American | 1 (2%) | |
| Unspecified | 2 (4%) | |
| Education | ||
| Some High School | 2 (4%) | |
| High School Diploma | 11 (22%) | |
| Some College | 18 (37%) | |
| Baccalaureate Degree | 9 (18%) | |
| Some Graduate School | 4 (8%) | |
| Graduate Degree | 5 (10%) |
Changes in sleep parameters as a function of amount of time abstinent and withdrawal severity.
Several changes in sleep parameters were observed as a function of amount of time abstinent including: Significant increase in number of arousals during the sleeping period (B = 4.2, SE = .78; t(1273) = 5.4, p < .001), Percentage of delta sleep (B = .6, SE = .16; t(1273) = 3.9, p < .001), and Decrease percentage of REM sleep (B = .71, SE = .14; t(1273) = 5.2, p < .001) in the third night of abstinence compared to the first and second night. Results remained significant after Bonferroni post hoc adjustment. Thus, third night of abstinence from smoking was linked to significantly more arousals and higher percentage of delta sleep, when compared to both first and second nights, p’s< .01. Regarding REM sleep, the first night exhibited significantly lower percentage of REM sleep when compared to the second and third nights of sleep, p’s< .001.
Significant reductions were found as a function of time since abstinence for the following dependent variables: time spent asleep (B = −11.3. SE = 1.4; t(1273) = −8.0, p< .001), number of REMs (B = −.08, SE = .02; t(1273) = −3.2, p = .001), sleep maintenance (B = −9.3, SE = .17; t(1272) = −5.5, p< .001), sleep efficiency (B = −1.2, SE = .21; t(1273) = −5.6, p< .001); and percentage stage 2 sleep (B = −1.3, SE = .17; t(1273) = −7.9, p< .001), revealing that the longer participants were abstinent, the greater the reduction in these sleep parameters. Specifically, Bonferroni post hoc analyses revealed the third night of sleep to demonstrate significantly lower values than the first and second nights of sleep, p’s< .01.
Regarding perceived withdrawal severity, higher self-report of withdrawal severity was associated with prolonged sleep latency (B = .29, SE = .11; t(1271) = 2.5, p = .01) and percentage of stage 1 sleep (B = .02, SE = .01; t(1271) = 2.04, p = .04),reduced time spent asleep (B = −.97, SE = .23; t(1271) = −4.3, p< .001), number of REMs (B = −.01, SE = .004; t(1271) = −3.8, p< .001), and sleep efficiency (B = −.1, SE = .03; t(1271) = −3.1, p = .003). Changes in perceived nicotine withdrawal severity as a function of time since abstinence had significant effects on time spent asleep (B = −.44, SE = .4; t(1269) = 2.2, p = .03) and percentage stage 1 sleep (B = −.03, SE = .01; t(1269) = −2.5, p = .01), indicating that the larger the increase in perceived nicotine withdrawal severity over time, the greater the reductions in time spent asleep for the last two nights (p’s < .01) [insert Figures 2 and 3 here]
Figure 2.
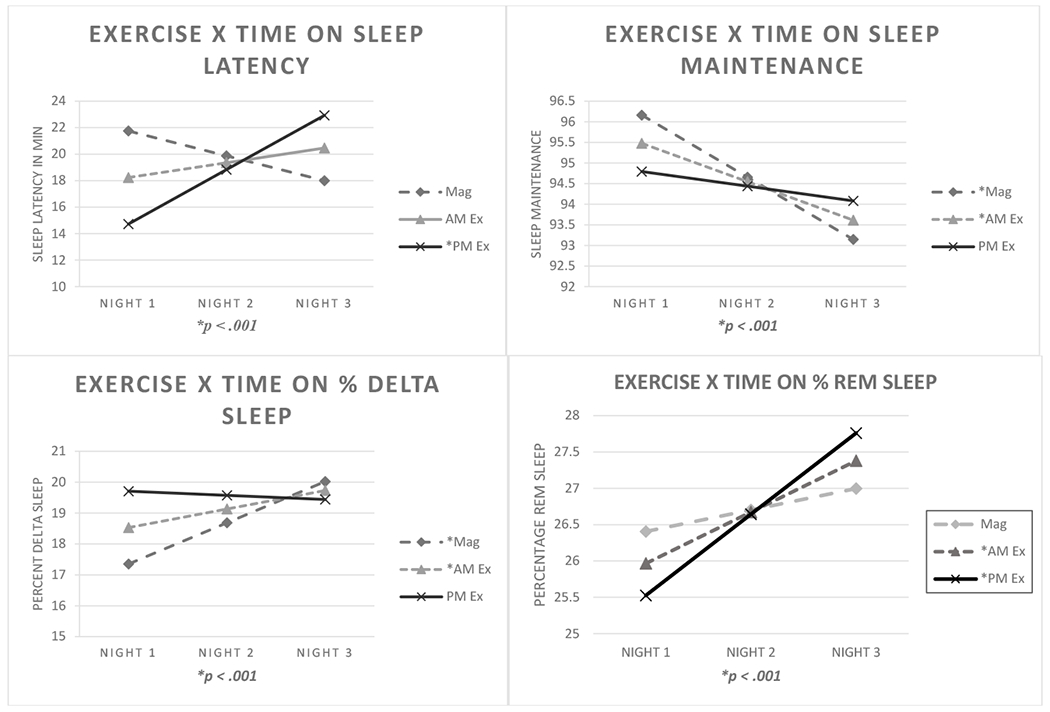
Mixed model regression interactions between exercise condition X time on sleep latency, sleep maintenance, percentage of delta sleep, and percentage REM sleep. Simple slopes analyses indicated that PM exercise was unrelated to changes in sleep maintenance and percentage of delta sleep, whereas the other conditions were linked to reductions in sleep maintenance over time, in addition to increases in percentage of delta sleep. PM exercise condition predicted significant increases in sleep latency over time. Both exercise conditions elevated percentage REM sleep. Note. Mag = Magazine Control Condition; AM Ex = AM Exercise; PM Ex = PM Exercise.
Figure 3.
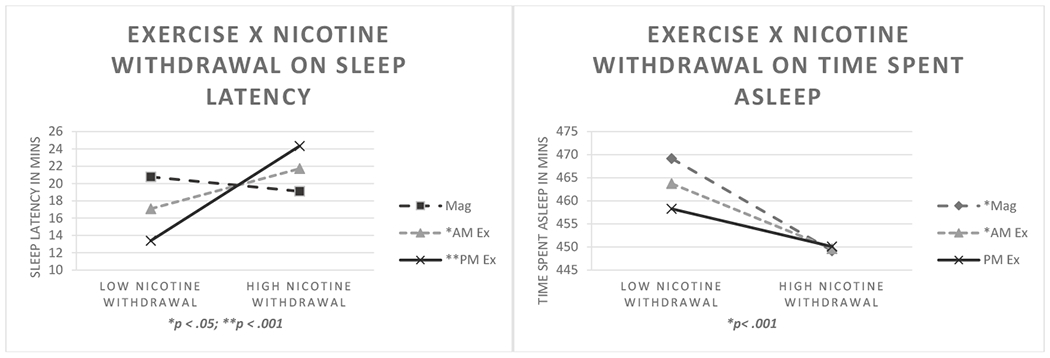
Mixed model regression interactions between exercise condition X perceived nicotine withdrawal severity on sleep latency and time spent asleep (TSA). Points were plotted at 1 SD below and above the centered mean of perceived nicotine withdrawal severity to represent low and high values, respectively. Simple slopes analyses indicated that elevated nicotine withdrawal predicted significant reductions in TSA for all conditions except PM exercise. Both exercise conditions predicted significant reductions in sleep latencies in the context of low perceived nicotine withdrawal, whereas elevated nicotine withdrawal predicted increases in latencies for both exercise conditions. Note. Mag = Magazine Control Condition; AM Ex = AM Exercise; PM Ex = PM Exercise.
Changes in sleep parameters as a function of Exercise Condition and perceived withdrawal severity.
Compared to magazine control, exercise led to a significant decrease in percentage of stage 1 sleep (B = −.17, SE = .07; t(1273) = −2.3, p =.02) and increase in percentage of delta sleep (B = .44, SE = .16; t(1273) = 2.85, p< .01). Bonferroni post hoc analysis indicated that AM exercise was associated with lower percentage of stage 1 sleep when compared to the magazine control conditions, p = .01, while the increase in percentage of delta sleep was driven by the PM exercise condition. Mixed models and descriptive statistics pertaining to Bonferroni post hoc comparisons for both time and exercise condition are displayed in Table 2. These include: sleep latency (B = 3.0, SE = .89; t(1271) = 3.4, p< .001); number of arousals (B = −2.8, SE = .95; t(1271) = −3.0, p = .003); time spent asleep (B = 4.5, SE = 1.7; t(1271) = 2.6, p = .008); sleep maintenance (B = .58, SE = .21; t(1270) = 2.8, p = .005); percentage of delta sleep (B = −.7, SE = .19; t(1271) = −3.9, p< .001; and percentage of REM sleep (B = .41, SE = .17; t(1271) = 2.5, p = .01), which were all differentially affected by time of day of exercise (Figure 2). Further, simple slopes analyses indicated that abstinence-induced changes for sleep maintenance and percentage of delta sleep were reduced in the PM exercise condition, but not in the AM exercise and the non-exercising conditions. Simple slopes analyses also indicated that PM exercise was associated with worsening sleep latency as a function of time since abstinence, compared to AM exercise and magazine control. Percentage of REM sleep showed greater increases as a function of time into abstinence in the PM and AM exercise condition compared to magazine condition. Thus, PM exercise disrupted sleep latency, with longer latency to sleep over time since abstinence. Additionally, both exercise conditions increased percentage of REM, whereas there was no such effect for magazine control.
Table 2.
Main Effect Mixed Model Regressions and Bonferroni Post Hoc Analyses for Time and Exercise Condition
| Variable | First Night | Second Night | Third Night | ||||
|---|---|---|---|---|---|---|---|
| B | SE | t | M (SE) | M (SE) | M (SE) | ||
|
| |||||||
| Arousals | 4.2 | .78 | 5.4** | 30 (2.6) | 33.4 (2.7) | 38.4 (2.7)b | |
| TSA | −11.3 | 1.4 | −8.0** | 435 (5.7) | 431 (5.7) | 412 (5.7)a | |
| Number REMs | −.08 | .02 | −3.2** | 4.4 (.09) | 4.4 (.09) | 4.2 (.09)a | |
| Sleep Maintenance | −9.3 | .17 | −5.5** | 93.6 (.5) | 92.8 (.5) | 91.8 (.5)a | |
| Sleep Efficiency | −1.2 | .21 | −5.6** | 90 (.77) | 89.2 (.77) | 87.6 (.77)a | |
| % Stage 2 Sleep | −1.3 | .17 | −7.9** | 54.2 (.87) | 53.3 (.87) | 51.6 (.87)a | |
| % Delta Sleep | .6 | .16 | 3.9** | 14.5 (.7) | 14.2 (.7) | 15.8 (.7)b | |
| % REM Sleep | .7 | .14 | 5.2** | 25.8 (.42)c | 27 (.42) | 27.2 (.42) | |
| Mag | AM Ex | PM Ex | |||||
| B | SE | t | M (SE) | M (SE) | M (SE) | ||
|
|
|||||||
| % Stage 1 Sleep | −.17 | .07 | −2.3* | 5.7 (.3) | 5.3 (.3)d | 5.4 (.3) | |
| % Delta Sleep | .44 | .16 | 2.9* | 14.64 (.7) | 14.42 (.7) | 15.5 (.7)e | |
Notes. n = 49. TSA = Time Spent Asleep. Mag = Magazine. Ex = Exercise.
p < .05;
p ≤ .001.
Third night is significantly lower than the first and second nights, p’s < .01.
Third night is significantly greater than first and second nights, p’s < .01.
First night is significantly lower than second and third nights, p’s < .001.
AM Exercise condition is significantly lower than sedentary magazine conditions, p = .01.
PM Exercise condition is significantly greater than Magazine and AM Exercise conditions, p’s ≤ .01
Regarding sleep arousals, abstinence-induced increases in number of arousals was significantly reduced by the PM exercise condition, compared to AM exercise and sedentary conditions. Both PM and AM exercise were associated with shorter time spent asleep on the first night of abstinence, compared to sedentary condition an effect that became non-significant by the second and third night into abstinence. Collectively, these findings suggest that evening exercise may help mitigate effects on sleep linked to acute cessation, such as frequency of arousals and reductions in sleep maintenance, but may lengthen the onset of sleep as evidenced by overall longer latency to sleep following PM exercise. Self-report of perceived nicotine withdrawal severity was also an important modifier on the effects of exercise on sleep, particularly on time spent asleep, and sleep latency sleep latency (B = .43, SE = .1; t(1268) = 4.4, p< .001). Mixed model regressions showed that non-exercising condition and AM exercise, but not PM exercise, were associated with decreased time spent asleep as a function of increasing perceived nicotine withdrawal, (B = .40, SE = .19; t(1269) = 2.1, p = .04) . Figure 3 illustrates these interaction effects, with simple slopes analyses indicating the direction of changes as a function of condition and perceived nicotine withdrawal. In both exercise conditions, low perceived withdrawal was associated with significantly shorter sleep latency, whereas high perceived nicotine withdrawal was associated with increased sleep latency. These findings suggest that PM exercise may help to mitigate the changes in time spent asleep associated with nicotine withdrawal, and that exercise engagement may be maximally beneficial to decrease sleep latency only under conditions of lower perceived nicotine withdrawal severity (Figure 3).
Discussion
Our study confirmed prominent sleep changes during the acute phase of nicotine withdrawal, showing progressive worsening of sleep maintenance, sleep efficiency, total time spent asleep, as well as changes in percentage of REM and NREM sleep. Overall, the changes ensued rapidly and showed a progressive trend, increasing from the first to the third night of nicotine withdrawal. A novel finding from this study is the moderating effect of perceived nicotine withdrawal severity on sleep changes, with high perceived severity being associated with worse sleep latency and efficiency, shorter time spent asleep, as well as changes in sleep architecture. That is, smokers who report greater withdrawal severity during early abstinence experience more sleep disruptions. While it is known that these sleep changes are problematic and often associated with relapse (Jaehne et al., 2015), available pharmacological treatments, such as nicotine replacement, bupropion or varenicline, do not improve sleep and may in fact worsen sleep disturbances (Aubin, 2002; Thomas et al., 2015). Thus, the current finding, that moderate exercise can attenuate specific symptoms of sleep disruption, suggest its promise as a means of aiding some smokers when they attempt to quit.
Overall, moderate exercise attenuated several abstinence-induced sleep disturbances in both male and female smokers over 3 continuous days (72 hours) of smoking deprivation. Specifically, exercise engagement reduced some of the changes in sleep architecture associated with acute nicotine deprivation (Colrain et al., 2004)and other effects on sleep related to time since abstinence, such as frequency of arousals and reductions in sleep maintenance(Prosise et al., 1994). Highly novel to this study, time of day of exercise differentially affected sleep changes associated with acute nicotine deprivation, both as a function of time into withdrawal and of perceived severity of withdrawal. Collectively, this suggests that evening exercise may help stave off time-related effects on sleep linked to acute cessation particularly arousals and poor sleep maintenance. Results also point to important moderating effects of time of day and nicotine withdrawal severity, showing that PM exercise may help to mitigate the effect of high perceived nicotine withdrawal on total time spent asleep. Increased attention has been recently placed on timing of activities (including exercise) due to the differential effects exerted on circadian rhythms, of which sleep wake cycle is one of the most evident phenomena (Buxton et al., 2003; Baehr et al., 2003). Further, exercise engagement may be maximally beneficial to reduce sleep latency under conditions of lower perceived nicotine withdrawal severity.
Of note, one possible limitation of the present study was the inclusion of a non-treatment seeking sample of smokers, with the ability to commit to a fairly intensive study. The aim of the study was to glean information about assessing and treating sleep disruption during the first 72 hours of nicotine withdrawal, which necessitated total abstinence for three days on three different occasions. Having treatment-seeking smokers quit and resume smoking every few weeks would not have been ethically sound. Thus, the results may not entirely generalize to a treatment seeking population, who are making a positive decision to quit and who have a goal of prolonged abstinence. Our study exclusively focuses on individuals who smoke cigarettes, hence generalizability to other models of nicotine consumption, such as vaping or chewing, needs to be tested. Desire to quit is a key factor in successful cessation, as even medication can have limited efficacy in reducing smoking among those not interested in cessation (Benowitz et al., 1998). Thus, the generalizability of these findings needs to be addressed in future cessation studies with treatment-seeking smokers to determine if exercise helps maintain abstinence, or even possibly slows the rapid return to pre-cessation levels of smoking that most smokers follow when they relapse (Conklin et al., 2005). The latter of which might afford a longer window for re-intervention.
While acute exercise bouts showed an effect on certain sleep parameters, other sleep features, such as total sleep time, were unaffected. It is possible that certain sleep parameters would change only in response to a longer period of exercise training and an improved aerobic fitness level, thus suggesting that the mechanisms through which exercise exerts its benefits may be multifold. Along these lines, an additional constraint to the interpretation of our results is the lack of measures assessing fitness levels. Further, we did not control for menstrual cycle phase. Research suggests that menstrual phase may impact smoking behavior and cessation (Weinberger et al., 2015) and future studies should account for this aspect in female participants. Regardless of limitations, the present study used a tightly controlled setting, with all subjects in nicotine withdrawal for the same length of time during all phases of testing, particularly during sleep assessment. Abstinence was verified with both CO breathalyzer and urinary cotinine, and exercise was well-monitored for both duration and intensity. All participants were non-exercising and exercised exclusively within the setting of our study protocol, thus reducing the potential confounding effects of lifestyle differences on our results.
Overall, our results suggest a potential role for exercise as an adjunct smoking cessation treatment to specifically target sleep in early acute nicotine withdrawal. The novel finding that time of the day of exercise and intensity of daytime withdrawal symptoms moderate the effects on nicotine deprivation induced sleep disturbances, should be explored by future studies aimed at personalizing treatment for different nicotine withdrawal phenotypes. Characterizing clinical phenotypes of acute nicotine withdrawal, specifically levels of perceived withdrawal severity, cue-provoked craving, and negative affect, might also help determine which smokers are most likely to benefit from a timed exercise intervention and to further test whether this ultimately leads to higher rates of abstinence. Further work on the role of exercise in smokers with pre-existing sleep disturbances is also warranted. While our participants did not meet current clinical diagnostic criteria for sleep disorders, sleep disorders are more prevalent in smokers compared to non-smokers (Wetter & Young, 1994) and pre-existing insomnia symptoms increase the likelihood of relapse after an attempt to quit smoking (Peltier et al., 2017).
Smoking cessation trials incorporating exercise adjuncts clearly warrant further research and should consider investigating for whom these treatments work best, as well as determining if exercise leading up to a smoking cessation attempt increases abstinence success. Nair and colleagues, for example, have shown that increasing levels of physical activities in sedentary individuals prior to an attempt to quit smoking, may improve cessations rates (Nair et al., 2017). Our study further expands on this, suggesting that specific phenotypes (i.e. individuals with high perceived nicotine withdrawal and increased sleep disturbances), may benefit from an adjunct targeted bout of evening exercise. Further work should explore the effects of different intensities and duration of exercise bouts, as well as whether the efficacy of exercise as a cessation aid increases as a function of smokers’ improved fitness, as past research has shown that smoking inhibits physical fitness and exercise capacity(Conway & Cronan, 1992; Mesquita et al., 2015).
Public significance statement:
This study suggests that moderate exercise can attenuated several abstinence-induced sleep disturbances in both male and female smokers over initial 72 hours of smoking deprivation. While it is known that these sleep changes are problematic and often associated with relapse, available pharmacological treatments not only do not improve sleep, but may in fact worsen sleep disturbances. Thus, the current finding, that moderate exercise can attenuate specific symptoms of sleep disruption, suggest its promise as a means of aiding some smokers when they attempt to quit.
Disclosures and Acknowledgments:
This study was funded by National Institute for Drug Abuse (NIDA) R01DA027508 - 01 (IS and CAC).
The Authors have no conflicts of interest to declare.
The Authors would like to tank Ms. Jodilyn Roberts for manuscript editing.
References:
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Sage Publications, Inc. [Google Scholar]
- Ashare RL, Lerman C, Tyndale RF, Hawk LW, George TP, Cinciripini P, & Schnoll RA (2017). Sleep Disturbance During Smoking Cessation: Withdrawal or Side Effect of Treatment? Journal of Smoking Cessation, 12(2), 63–70. 10.1017/jsc.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin H-J (2002). Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs, 62 Suppl 2, 45–52. 10.2165/00003495-200262002-00005 [DOI] [PubMed] [Google Scholar]
- Baehr EK, Eastman CI, Revelle W, Olson SHL, Wolfe LF, & Zee PC (2003). Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 284(6), R1542–1550. 10.1152/ajpregu.00761.2002 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zevin S, & Jacob P (1998). Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. The Journal of Pharmacology and Experimental Therapeutics, 287(3), 958–962. [PubMed] [Google Scholar]
- Biochemical verification of tobacco use and cessation. (2002). Nicotine & Tobacco Research, 4(2), 149–159. 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, & Roberts MR (1999). Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors, 24(3), 399–410. 10.1016/s0306-4603(98)00088-4 [DOI] [PubMed] [Google Scholar]
- Boutou AK, Tsiata EA, Pataka A, Kontou PK, Pitsiou GG, & Argyropoulou P (2008). Smoking cessation in clinical practice: Predictors of six-month continuous abstinence in a sample of Greek smokers. Primary Care Respiratory Journal: Journal of the General Practice Airways Group, 17(1) 32–38. 10.3132/pcrj.2008.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP, Phillips BA, Youngstedt SD, Kline CE, & Hirshkowitz M (2014). Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America Poll. Sleep Medicine, 15(7), 755–761. 10.1016/j.sleep.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, & Van Cauter E (2003). Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 284(3), R714–724. 10.1152/ajpregu.00355.2002 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall ML, Moul DE, Nofzinger EA, & Kupfer DJ (2007). Daytime Symptoms in Primary Insomnia: A Prospective Analysis Using Ecological Momentary Assessment. Sleep Medicine, 8(3), 198–208. 10.1016/j.sleep.2006.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, & Tiffany ST (2001). The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology, 9(2), 183–190. [DOI] [PubMed] [Google Scholar]
- CDCTobaccoFree. (2019, March 4). Health Effects of Cigarette Smoking. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm [Google Scholar]
- Colrain IM, Trinder J, & Swan GE (2004). The impact of smoking cessation on objective and subjective markers of sleep: Review, synthesis, and recommendations. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 6(6), 913–925. 10.1080/14622200412331324938 [DOI] [PubMed] [Google Scholar]
- Committee, P. A. G. A., & others. (2008). Physical activity guidelines advisory committee report, 2008. Washington, DC: US Department of Health and Human Services, 2008, A1–H14. [Google Scholar]
- Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, & Marcus MD (2005). The return to smoking: 1-year relapse trajectories among female smokers. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 7(4), 533–540. 10.1080/14622200500185371 [DOI] [PubMed] [Google Scholar]
- Conklin CA, Soreca I, Kupfer DJ, Cheng Y, Salkeld RP, Mumma JM, Jakicic JM, & Joyce CJ (2017). Exercise attenuates negative effects of abstinence during 72 hours of smoking deprivation. Experimental and Clinical Psychopharmacology, 25(4), 265–272. 10.1037/pha0000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway TL, & Cronan TA (1992). Smoking, exercise, and physical fitness. Preventive Medicine, 21(6), 723–734. 10.1016/0091-7435(92)90079-w [DOI] [PubMed] [Google Scholar]
- Diener E, & Emmons RA (1984). The independence of positive and negative affect. Journal of Personality and Social Psychology, 47(5), 1105–1117. [DOI] [PubMed] [Google Scholar]
- First MB, & Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment (pp. 134–143). John Wiley & Sons Inc. [Google Scholar]
- Fucito LM, Redeker NS, Ball SA, Toll BA, Ikomi JT, & Carroll KM (2014). Integrating a Behavioural Sleep Intervention into Smoking Cessation Treatment for Smokers with Insomnia: A Randomised Pilot Study. Journal of Smoking Cessation, 9(1), 31–38. 10.1017/jsc.2013.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, & McNeil JJ (1999). Predictors and timing of adverse experiences during trandsdermal nicotine therapy. Drug Safety, 20(6), 545–555. 10.2165/00002018-199920060-00007 [DOI] [PubMed] [Google Scholar]
- Grove JR, Wilkinson A, Dawson B, Eastwood P, & Heard P (2006). Effects of exercise on subjective aspects of sleep during tobacco withdrawal. Australian Psychologist, 41(1), 69–76. 10.1080/00050060500395127 [DOI] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294. [DOI] [PubMed] [Google Scholar]
- Jaehne A, Unbehaun T, Feige B, Cohrs S, Rodenbeck A, Schütz A-L, Uhl V, Zober A, & Riemann D (2015). Sleep changes in smokers before, during and 3 months after nicotine withdrawal. Addiction Biology, 20(4), 747–755. 10.1111/adb.12151 [DOI] [PubMed] [Google Scholar]
- Janse Van Rensburg K, Taylor A, Hodgson T, & Benattayallah A (2009). Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: An fMRI study. Psychopharmacology, 203(3), 589–598. 10.1007/s00213-008-1405-3 [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, & Baker TB (1999). A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England Journal of Medicine, 340(9), 685–691. 10.1056/NEJM199903043400903 [DOI] [PubMed] [Google Scholar]
- Klinsophon T, Thaveeratitham P, Sitthipornvorakul E, & Janwantanakul P (2017). Effect of exercise type on smoking cessation: A meta-analysis of randomized controlled trials. BMC Research Notes, 10(1), 442. 10.1186/s13104-017-2762-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic A, Mavros Y, Heisz JJ, & Fiatarone Singh MA (2018). The effect of resistance exercise on sleep: A systematic review of randomized controlled trials. Sleep Medicine Reviews, 39, 52–68. 10.1016/j.smrv.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, & Otto MW (2015). The effects of physical activity on sleep: A meta-analytic review. Journal of Behavioral Medicine, 38(3), 427–449. 10.1007/s10865-015-9617-6 [DOI] [PubMed] [Google Scholar]
- Lowe H, Haddock G, Mulligan LD, Gregg L, Fuzellier-Hart A, Carter L-A, & Kyle SD (2019). Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clinical Psychology Review, 68, 1–12. 10.1016/j.cpr.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Mesquita R, Gonçalves CG, Hayashi D, Costa V. de S. P., Teixeira D. de C., de Freitas ERFS, Felcar JM, Pitta F, Molari M, & Probst VS (2015). Smoking status and its relationship with exercise capacity, physical activity in daily life and quality of life in physically independent, elderly individuals. Physiotherapy, 101(1), 55–61. 10.1016/j.physio.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Moreno-Coutiño A, Calderón-Ezquerro C, & Drucker-Colín R (2007). Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 9(3), 389–396. 10.1080/14622200701188901 [DOI] [PubMed] [Google Scholar]
- Myllymäki T, Kyröläinen H, Savolainen K, Hokka L, Jakonen R, Juuti T, Martinmäki K, Kaartinen J, KINNUNEN M-L, & Rusko H (2011). Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. Journal of Sleep Research, 20(1pt2), 146–153. [DOI] [PubMed] [Google Scholar]
- Myllymäki T, Rusko H, Syväoja H, Juuti T, Kinnunen M-L, & Kyröläinen H (2012). Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. European Journal of Applied Physiology, 112(3), 801–809. 10.1007/s00421-011-2034-9 [DOI] [PubMed] [Google Scholar]
- Nair US, Patterson F, Rodriguez D, & Collins BN (2017). A telephone-based intervention to promote physical activity during smoking cessation: A randomized controlled proof-of-concept study. Translational Behavioral Medicine, 7(2), 138–147. 10.1007/s13142-016-0449-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Levine MD, Houck P, Perkins KA, & Marcus MD (2011). Subjective sleep disturbance during a smoking cessation program: Associations with relapse. Addictive Behaviors, 36(8), 861–864. 10.1016/j.addbeh.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Grandner MA, Malone SK, Rizzo A, Davey A, & Edwards DG (2019). Sleep as a Target for Optimized Response to Smoking Cessation Treatment. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 21(2), 139–148. 10.1093/ntr/ntx236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Lee J, Ma P, Businelle MS, & Kendzor DE (2017). The influence of sleep quality on smoking cessation in socioeconomically disadvantaged adults. Addictive Behaviors, 66, 7–12. 10.1016/j.addbeh.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Peters EN, Fucito LM, Novosad C, Toll BA, & O’Malley SS (2011). Effect of night smoking, sleep disturbance, and their co-occurrence on smoking outcomes. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 25(2), 312–319. 10.1037/a0023128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosise GL, Bonnet MH, Berry RB, & Dickel MJ (1994). Effects of abstinence from smoking on sleep and daytime sleepiness. Chest, 105(4), 1136–1141. 10.1378/chest.105.4.1136 [DOI] [PubMed] [Google Scholar]
- Samet JM (2013). Tobacco Smoking: The Leading Cause of Preventable Disease Worldwide. Thoracic Surgery Clinics, 23(2), 103–112. 10.1016/j.thorsurg.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Taylor A, & Katomeri M (2007). Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 3(11), 1183–1190. 10.1080/14622200701648896 [DOI] [PubMed] [Google Scholar]
- The aasm manual for the scoring of sleep and associated events rules terminology and technical. (n.d.). Retrieved September 19, 2019, from https://sway.office.com/KWDgmSSdDdNNfTTH
- Thomas C, Jones H, Whitworth-Turner C, & Louis J (2020). High-intensity exercise in the evening does not disrupt sleep in endurance runners. European Journal of Applied Physiology, 120(2), 359–368. 10.1007/s00421-019-04280-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KH, Martin RM, Knipe DW, Higgins JPT, & Gunnell D (2015). Risk of neuropsychiatric adverse events associated with varenicline: Systematic review and meta-analysis. BMJ (Clinical Research Ed.), 350, h1109. 10.1136/bmj.h1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Shioda K, Morita Y, Kubota C, Ganeko M, & Takeda N (2012). Exercise effects on sleep physiology. Frontiers in Neurology, 3, 48. 10.3389/fneur.2012.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, & Faulkner G (2012). Exercise interventions for smoking cessation. The Cochrane Database of Systematic Reviews, 1, CD002295. 10.1002/14651858.CD002295.pub4 [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, & Faulkner GEJ (2014). Exercise interventions for smoking cessation. The Cochrane Database of Systematic Reviews, 8, CD002295. 10.1002/14651858.CD002295.pub5 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, & McKee SA (2015). Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(4), 407–421. 10.1093/ntr/ntu249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, & Young TB (1994). The relation between cigarette smoking and sleep disturbance. Preventive Medicine, 23(3), 328–334. 10.1006/pmed.1994.1046 [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Elliott JA, & Kripke DF (2019). Human circadian phase-response curves for exercise. The Journal of Physiology, 597(8), 2253–2268. 10.1113/JP276943 [DOI] [PMC free article] [PubMed] [Google Scholar]


