Abstract
Primary liver cancer is the fourth leading cause of cancer-related deaths worldwide, with hepatocellular carcinoma (HCC) comprising the vast majority of primary liver malignancies. Imaging plays a central role in HCC diagnosis and management. As a result, the content and structure of radiology reports are of utmost importance in guiding clinical management. The Liver Imaging Reporting and Data System (LI-RADS) provides guidance for standardized reporting of liver observations in patients who are at risk for HCC. LI-RADS standardized reporting intends to inform patient treatment and facilitate multidisciplinary communication and decisions, taking into consideration individual clinical factors. Depending on the context, observations may be reported individually, in aggregate, or as a combination of both. LI-RADS provides two templates for reporting liver observations: in a single continuous paragraph or in a structured format with keywords and imaging findings. The authors clarify terminology that is pertinent to reporting, highlight the benefits of structured reports, discuss the applicability of LI-RADS for liver CT and MRI, review the elements of a standardized LI-RADS report, provide guidance on the description of LI-RADS observations exemplified with two case-based reporting templates, illustrate relevant imaging findings and components to be included when reporting specific clinical scenarios, and discuss future directions.
An invited commentary by Yano is available online.
Online supplemental material is available for this article.
Work of the U.S. Government published under an exclusive license with the RSNA.
SA-CME Learning Objectives
After completing this journal-based SA-CME activity, participants will be able to:
■ Describe the contents of the LI-RADS reporting templates.
■ Discuss when to report multiple observations individually, in aggregate, or by using a combination of the two approaches, depending on the number of observations and their LI-RADS categories.
■ Identify specific clinical scenarios to issue radiology reports that are tailored to address patients’ needs and adequately inform treatment decisions.
Introduction
Hepatocellular carcinoma (HCC) comprises the majority of primary liver cancers, which are the fourth leading cause of cancer-related death worldwide (1). HCC is unique in that the diagnosis may be made primarily with imaging, without the need for histopathologic confirmation (2,3). As radiology reports often support management decisions, they must communicate findings clearly and consistently and include all relevant imaging information.
Radiology reports come in many forms, including free text, standardized, and structured. Free-text reports may lack clarity and consistency, potentially leading to errors in translation to management and longitudinal reporting (4–6). Conversely, standardized or structured reports streamline medical content by using precise terminology and are scripted to cover all clinically relevant data, promoting consistent and complete reporting of all data elements (5–8). They improve clinical care and workflow by providing systematic image analysis and reduced dictation times when compared with free-text reports (9). Furthermore, the adoption of structured reports facilitates data extraction for indexing, teaching, and research purposes (10,11).
Different systems have been developed for the standardization of liver imaging (12). The Liver Imaging Reporting and Data System (LI-RADS) is a comprehensive approach to all HCC imaging components, from image acquisition to interpretation. LI-RADS provides a precise lexicon of imaging features, unambiguous diagnostic categories, and reporting templates for applying the system in practice. A central tenet of LI-RADS is standardization, which is intended to reduce interpretation variability, improve communication between health care providers, and ultimately improve patient care. A standardized LI-RADS report is intended to clearly and concisely reflect the radiologist’s diagnostic impression and provides suggested management considerations (13).
LI-RADS provides standardization in screening and surveillance with US; in diagnostic imaging with CT, MRI, and contrast-enhanced US; and for assessment of treatment response at CT and MRI. In this article, we clarify terminology that is pertinent to reporting and highlight the benefits of structured reports. We discuss the applicability of LI-RADS and focus on the elements of a standardized LI-RADS report for liver CT and MRI. We provide guidance for describing LI-RADS observations, exemplified with two case-based reporting templates, and illustrate relevant imaging findings and components to be included when reporting specific clinical scenarios. We briefly discuss future directions. While we provide standardized terminology and templates for structured reporting, the level of detail should be adapted according to the preferences and needs of individual radiologists, referrers, practices, institutions, regional preferences, and liver transplantation requirements when applicable. A structured report template for liver CT and MRI using LI-RADS is available at https://www.radreport.org/home/RPT50860.
Reporting Terminology
Six terms that are pertinent to reporting in the radiology literature require clarification: narrative reporting, unstructured reporting, free-text reports, standardized reporting, structured reports, and contextual reports (10,14–18). In this manuscript, we prefer the term narrative reporting to designate the use of narrative unstructured text to report imaging information, as this is the most commonly used term in the radiology literature. Similarly, because of the widespread use in the radiology literature, we prefer the broader term structured reports, although some examples in this article can be considered contextual reports, as these focus on a specific clinical indication. Structured reports arrange the medical content into predefined formats that are consistently organized in ordered sections and use standardized language. Reporting terminology is summarized in Table 1.
Table 1:
Types of Radiology Reporting
Chronologically, terminology must be standardized before structured reports can be created. In this manuscript, we use the term standardized reporting to refer to use of the LI-RADS lexicon and algorithms for describing liver observations. In specific sections, we use the term structured reports to refer to reporting templates that facilitate the communication of imaging findings and the extraction of information for indexing, research, and teaching purposes (Table 2).
Table 2:
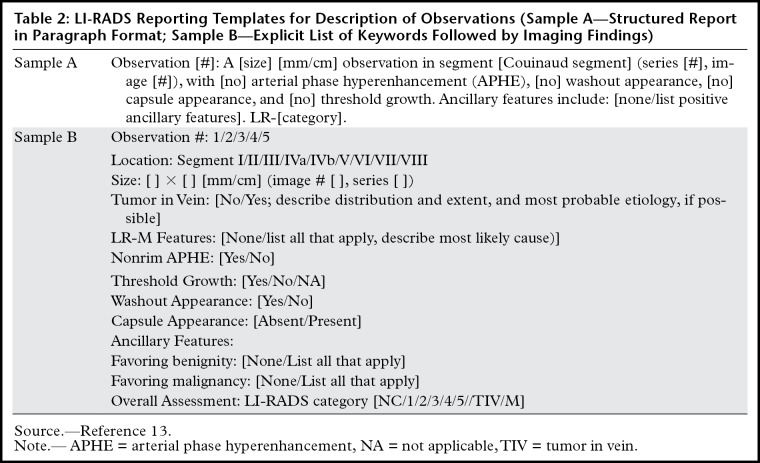
LI-RADS Reporting Templates for Description of Observations (Sample A—Structured Report in Paragraph Format; Sample B—Explicit List of Keywords Followed by Imaging Findings)
Benefits of Structured Reports
In addition to standardization, structured reports provide several benefits. In a clinical setting, a structured report improves clarity of communication, reduces variability in key feature description, and links individual observations to staging and treatment recommendations (19,20). Structured reports promote adherence to guidelines and diagnostics systems, while appropriately documenting technique (21). Finally, they encourage a consistent pattern for image analysis that may reduce diagnostic errors and potentially improve workflow (8,9). Flusberg et al (7) reported significant differences in the frequencies of reporting important elements of HCC imaging, such as major features (Fig 1), observation size, and location, when comparing free-text reports to LI-RADS templates. Structured reports are essential for data mining, facilitating calculation of diagnostic performance, and pooling of data from different sites for multicenter studies (11).
Figure 1.
Axial MR images obtained before and after contrast material injection in late arterial, portal venous, and delayed phases in a 64-year-old man with hepatitis C cirrhosis show LI-RADS major features for HCC diagnosis: observation size, arterial phase hyperenhancement (arrowheads), capsule (white arrows), and washout (black arrow).
To our knowledge, no studies have shown detrimental effects of structured reports. However, criticisms include the risk for errors if prepopulated information is not adequately removed, a potential loss of the big picture due to overly structured content, and limitations for describing more complex cases due to rigid and limited descriptors (22). Implementation of structured reports also requires an adaptation period during clinical adoption, which may momentarily affect workflow and productivity (5).
Current Initiatives
Initiatives to promote standardization of radiologic terms and reports have been proposed in recent years. The RSNA RadLex initiative aims to standardize the language to communicate imaging diagnostic results (23). It is a computer-based application that provides a comprehensive set of standardized radiology terms for use in radiology reports, decision support, data mining and data registries, education, and research (24). RadLex has already incorporated the LI-RADS lexicon.
Another RSNA initiative, in collaboration with the American College of Radiology (ACR), is the Common Data Elements (CDEs) for Radiology (25). This initiative defines the attributes and allowable values of a unit of information (eg, radiology report) so that information can be assembled, exchanged, and stored uniformly across different applications and institutions. In essence, a CDE is a predefined question and a set of allowable answers to that question (14). Radiology CDEs are indexed and cataloged in a dictionary and can be grouped in lists that are pertinent to particular applications. The RSNA and ACR have already incorporated LI-RADS–specific data elements.
LI-RADS CT and MRI Standardized Reporting
The LI-RADS CT and MRI diagnostic algorithm was developed to standardize the imaging diagnosis of HCC. It comprises a stepwise approach to assigning a probability of HCC (LR-1 through LR-5), malignancy (LR-M), or vascular-invasive disease or tumor in vein (LR-TIV) to liver observations at imaging. To maintain a high positive predictive value for the diagnosis of HCC, the diagnostic algorithm is restricted for application in patients who are at high risk for HCC. A companion lexicon standardizes the vocabulary of all aspects of liver imaging. While the use of this lexicon is advocated for the description of liver observations in patients who are at risk for HCC, it is also encouraged for the description of liver observations in all patients.
When to Use LI-RADS Algorithms
The first step in LI-RADS standardized reporting is to identify the appropriate algorithm. To use LI-RADS, radiologists must first establish that the patient meets the LI-RADS population criteria, namely: cirrhosis, chronic hepatitis B infection even in the absence of cirrhosis, or a personal history of HCC. LI-RADS should not be applied to patients without the previously mentioned risk factors, patients younger than 18 years, or patients with congenital hepatic fibrosis or cirrhosis due to vascular disorders (26). Depending on the clinical scenario, users may apply the CT and MRI LI-RADS diagnostic algorithm for untreated observations, the LI-RADS Treatment Response (LR-TR) algorithm for assessing response to local-regional treatment of pathologically proven or imaging-presumed malignancies, or both if untreated and treated observations are present (13).
Conditional Application of LI-RADS Algorithms
The LI-RADS algorithms should only be applied in patients who are at high risk for HCC. However, it is not always known at the time of imaging whether a patient meets high-risk criteria. Conditional application may occur when an observation is encountered in a patient with presumed, but unproven, high-risk status. The following conditional statement may be included in the report: “In the clinical setting of cirrhosis or chronic hepatitis B, this observation meets the criteria for LR-5 (definitely HCC)” (13). Figure 2 illustrates conditional LI-RADS application. In these circumstances, the algorithms are followed as usual, but the final category is provisional until applicability can be confirmed (ie, risk status established or treatment history acquired).
Figure 2.
Axial contrast-enhanced CT images obtained in late arterial, portal venous, and delayed phases in a 60-year-old woman with an unknown medical history. If risk factors are likely present but not established, LI-RADS may be applied conditionally. Imaging findings can be described as follows: There is a 25-mm observation (arrows) with nonrim arterial phase hyperenhancement (APHE) and nonperipheral washout appearance in segment VII. An enhancing capsule is also noted. In the clinical setting of cirrhosis or chronic hepatitis B, this meets the criteria for LR-5 (definitely HCC).
When to Use the LI-RADS Lexicon
The LI-RADS lexicon is available on the ACR website, and it is currently undergoing further review and refinements (27). The lexicon standardizes the language of all aspects of liver imaging, including when the LI-RADS algorithm is not applicable. LI-RADS lexicon terms are labeled as “LI-RADS specific” or “broad use.” LI-RADS–specific terms are used solely in the context of LI-RADS, such as “threshold growth,” and may not be useful in a broader context. Terms identified for broad use may be applied to any liver imaging study performed for any indication. These lexicon terms can be adopted to enable precise and consistent reporting of liver phases, anatomy, and observations.
LI-RADS Templated Report
The elements comprising a LI-RADS templated report are clinical indication and history, comparison examinations, technique, findings, and impression sections (Table E1). The details suggested by LI-RADS for each of the reporting elements are described here.
Clinical History and Indication
In addition to a standard history, it is important to report the basis by which the patient meets the inclusion criteria for the LI-RADS population (13). Treatment history and pathologic results should also be included, if applicable.
Comparison Examinations
Prior examination modality and date are essential elements to include for prior studies. Patients with HCC frequently undergo many prior examinations with different modalities and, in some instances, have a complex treatment history.
The longitudinal review of imaging studies is important for assessing the initial category and T stage, as well as for assigning individual diagnostic or treatment response categories. For instance, threshold growth, defined as an increase in the size of the mass by 50% or more within 6 months, is a major feature of HCC. Subthreshold growth is an ancillary feature favoring malignancy, and spontaneous resolution or size stability after 2 years or longer is an ancillary feature favoring benignity. We recommend congruency of measurement methodology (plane, orientation, image phase, or sequence) between examinations to avoid the risk of misinterpretation of size change due to technical differences. It is also recommended that the pretreatment images for each observation be evaluated to establish the pretreatment characteristics such as size and enhancement pattern, which are essential factors for accurately assigning the LR-TR category.
Technique
The description of type (eg, iodinated CT contrast material, extracellular or hepatobiliary MRI gadolinium-based contrast agent) and amount of contrast material is recommended and might be required for insurance reimbursement purposes. When subtraction images are used to assess arterial phase hyperenhancement (APHE) or washout, this may be described in the technique section.
While not needed for diagnostic interpretation, reporting parameters like field strength and injection rate may be useful for data mining for future research studies. As a memory aid, reporting the protocol and imaging phases can also be a good opportunity to verify if the examination is technically adequate and meets minimum LI-RADS requirements and recommendations (28). If the LI-RADS technical requirements are not met, clarifying the reason for inadequacy and how it affected the analysis in the report is recommended. Examples include omission of contrast enhancement phases, inadequate coverage, or suboptimal protocol.
Findings
Examination Limitations Related to Patient Factors.— While limitations in acquisition are described in the technique section of the report, limitations related to patient factors are best described in the findings section of the report. Examples include substantial image degradation due to motion or artifacts related to implanted metal, extensive liver iron or fat, or large-volume ascites. While these issues may limit diagnostic confidence, it may still be possible to provide a final diagnostic category (eg, if targetoid appearance is demonstrated on any of the obtained images) or to narrow the range of potential categories. If technical factors or image omissions prevent narrowing of the range of potential diagnostic categories (ie, determination between LR-1 and LR-2 or between LR-4 and LR-5 is not possible), an LR-NC (not categorizable) category should be assigned, individually or in aggregate. In that case, short-term (ie, ≤3 months) repeat or alternative diagnostic imaging or contrast-enhanced imaging may be recommended.
Background Liver Findings.— Background liver findings potentially inform the severity of liver disease and may impact treatment options. When available, quantitative techniques such as liver stiffness as a biomarker of liver fibrosis or proton density fat fraction as a biomarker of liver steatosis can help further describe background liver alterations. Background liver alterations may also impact application of LI-RADS major and ancillary features. For example, reporting the presence of background steatosis or iron overload may be relevant, especially when category adjustments are made by relying on ancillary features. If a patient has undergone a hepatectomy or liver transplant, a description of postsurgical changes should be included.
Vascular and Biliary Anatomy.— Description of the vascular and biliary anatomy is important in several clinical settings. In pretransplant evaluation, anatomic variants may influence the choice of surgical technique and determine surgical eligibility. In patients who have undergone transplant, an evaluation of anastomotic patency is required to help detect complications.
The description of venous patency is important in patients with chronic liver disease and those at risk for HCC because of the high risk of vessel occlusions. If vessels are occluded, the report should include a description of the involved vessels, type of occlusion (bland thrombosis or tumor in vein [TIV]), and presence of cavernous transformation. The patency of arterial and venous anastomoses is an important element of the report in patients who have undergone transplant. Hepatic arterial anatomic variants should also be described, particularly in patients who are candidates for liver transplant or transarterial therapies.
Biliary findings such as focal or diffuse ductal dilatation, causes of ductal dilatation (if discernible), and gallbladder abnormalities should be reported. Similar to the vascular anatomy, biliary anatomic variants in transplant candidates and the patency of biliary anastomoses in the posttransplant setting are important reporting elements.
Observations.— In LI-RADS, the term observation refers to an area with an imaging appearance that is distinctive from the background liver. It may consist of a pseudolesion or a true lesion, with the latter either malignant or benign and of hepatocellular or nonhepatocellular origin (26). When multiple observations are detected, a decision must be made to report them individually, in aggregate, or as a combination of the two, depending on clinical context and observation category. Descriptors of individual observations are listed below. Recommendations on how to report observations in aggregate are discussed later in the text.
The recommended descriptors for LI-RADS untreated observations include a unique identifier (ie, observation 1); location; major features, including size and growth; ancillary features (if applied); comparison with prior imaging, if available; and final LI-RADS category.
Figure 3 provides an example of recommended descriptors with two sample LI-RADS report templates. The inclusion of unique lesion identifiers and series and image numbers and saving of key images help to facilitate longitudinal follow-up and multidisciplinary discussions (Fig 4). To facilitate longitudinal follow-up, the unique lesion identifier should remain unchanged over time (eg, an observation assigned identifier 1 should remain observation 1 on all subsequent studies, even if treated or resolved). Reporting the Couinaud segment and additional anatomic information such as the subcapsular location or proximity to vessels informs percutaneous or surgical approaches.
Figure 3a.
Sample structured reports with key images. (a) Axial precontrast and contrast-enhanced MR images in a 55-year-old man depict hepatitis C cirrhosis. (b) Two samples of structured reports for a focal observation are provided: structured text in a single continuous paragraph (left), and a structured report with keywords and findings (right). HBP = hepatobiliary phase, N/A = not applicable.
Figure 4.
Axial contrast-enhanced CT images obtained in the hepatobiliary phase as a baseline image, at 6 months, and at 12 months in a 66-year-old man with hepatitis C cirrhosis. Two observations (arrows #1, #2) are seen in segment 8 in the baseline image and in the 6-month image. A new observation (arrow #3) is seen in segment IVa in the 12-month follow-up image. The use of consistent identification numbers facilitates longitudinal monitoring.
Figure 3b.
Sample structured reports with key images. (a) Axial precontrast and contrast-enhanced MR images in a 55-year-old man depict hepatitis C cirrhosis. (b) Two samples of structured reports for a focal observation are provided: structured text in a single continuous paragraph (left), and a structured report with keywords and findings (right). HBP = hepatobiliary phase, N/A = not applicable.
Size measurement should be performed with the imaging sequence or in the phase where the observation is most conspicuous, preferably in the axial plane. Measurements on diffusion-weighted images should be avoided because of image distortion (26). The measurements should be made from the outer edge to the outer edge, including the capsule, when present. On the basis of expert consensus, LI-RADS recommends against measuring in the arterial phase because of concerns related to the variability of measurements in this vascular phase, although one recent publication suggests that the variability in this phase is no different than on other postcontrast images (29). The other major and ancillary features considered for categorization are briefly described, followed by the individual observation LI-RADS category.
Reporting LR-M and LR-TIV merits special consideration and is discussed further later.
When pathologically proven observations are present, radiologists are instructed to report the observation and imaging features, but with the histologic diagnosis rather than a LI-RADS category. However, if the observation is deemed to be a precursor to a hepatocellular lesion (eg, dysplastic nodule) at histopathologic assessment, then both histologic diagnosis and LI-RADS category should be reported, as the change in category may signal progression.
The recommended descriptors for LI-RADS treated observations include a unique identifier (ie, observation 1); location; LR-TR features of viability; size of equivocal or viable tumor; final LR-TR category; and reference to treatment and comparison with prior imaging, if available.
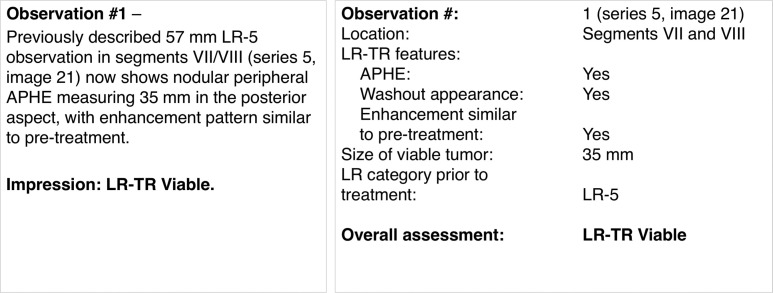
Three LR-TR categories are assigned: LR-TR nonviable, LR-TR equivocal, or LR-TR viable. Each treated observation should be reported individually with the response category and the size of the viable or equivocal component, when applicable. The size measurement should be across the largest enhancing area and not traversing nonviable tumor (30). Figure 5 shows the LI-RADS treatment response algorithm with an example, illustrating how an LR-TR viable or LR-TR equivocal observation should be measured. Reporting of the posttreatment cavity size of nonviable observations is not mandatory but might be recommended per some institutions. When referencing prior images, the inclusion of the treatment modality, pretreatment LI-RADS category, and pretreatment size provides a more comprehensive context for assessment of response.
Figure 5a.
LI-RADS treatment response algorithm. (a) LI-RADS treatment response algorithm shows the categorization of treated observations. (Reprinted, with permission, from reference 13.) (b) Axial MR image demonstrates how the measurement (double-headed arrow) across the enhancing component (LR-TR viable) should not traverse nonviable tumor. (c) Two samples of standardized reports show how a treated observation is described, either as structured text in a single continuous paragraph (left) or as a structured report with keywords and findings (right).
Figure 5b.

LI-RADS treatment response algorithm. (a) LI-RADS treatment response algorithm shows the categorization of treated observations. (Reprinted, with permission, from reference 13.) (b) Axial MR image demonstrates how the measurement (double-headed arrow) across the enhancing component (LR-TR viable) should not traverse nonviable tumor. (c) Two samples of standardized reports show how a treated observation is described, either as structured text in a single continuous paragraph (left) or as a structured report with keywords and findings (right).
Figure 5c.
LI-RADS treatment response algorithm. (a) LI-RADS treatment response algorithm shows the categorization of treated observations. (Reprinted, with permission, from reference 13.) (b) Axial MR image demonstrates how the measurement (double-headed arrow) across the enhancing component (LR-TR viable) should not traverse nonviable tumor. (c) Two samples of standardized reports show how a treated observation is described, either as structured text in a single continuous paragraph (left) or as a structured report with keywords and findings (right).
LI-RADS Reporting Templates for Observations.—Table 2 shows two recommended LI-RADS reporting templates for the description of individual observations (13). The paragraph format example consists of structured text in a single continuous paragraph with a description of observation size, units of measurements, Couinaud segment, series number, image number, explicit description of presence or absence of major imaging features (APHE, washout appearance, capsule appearance, and threshold growth), list of ancillary features present, and LR category.
The structured report example presents the same information but uses an explicit list of keywords followed by imaging findings. Conceptually, keywords can be thought of as predefined questions and findings as a set of allowable answers to these questions. This template was designed to adopt the common data elements framework described by Rubin and Kahn (14). For example, the location keyword corresponds to text and has a set of allowable answers from segments I to VIII. The observation size is a number, reported in millimeters or centimeters, and can only take positive values.
Extrahepatic Findings.— Extrahepatic findings relevant to the clinical context include imaging signs of portal hypertension (eg, ascites, portosystemic shunts, spleen size), extrahepatic metastasis, or other primary abdominal neoplasms, which help to stratify risk and define treatment in patients with end-stage liver disease.
Impression
Observation Assessment.— It is preferred to list the observations in order of identifiers, and these should remain the same on all subsequent studies. If multifocal disease is suspected, describing observations in aggregate rather than listing them individually may provide a clearer picture of tumor burden, as discussed later. Likewise, LR categories may not be applied if a better description fits, such as reporting a hemangioma instead of an LR-1 or LR-2 observation.
In some practices, particularly in centers that offer multidisciplinary management or liver transplantation for HCC, listing observations fulfilling Organ Procurement and Transplantation Network (OPTN) criteria for definite HCC and the radiologic T stage is preferred to inform patient treatment and transplant eligibility (31).
T Stage Assessment.— Radiologic T stage is based on the number and size of definite HCCs (LR-5 or OPTN 5). Initial T stage reflects the tumor burden for untreated HCC, whereas downstaged or posttreatment T stage reflects the tumor burden of residual viable tumor after treatment. T2 stage is of particular importance for transplant purposes and is discussed later.
Management Recommendations.— Management recommendations help guide the referring physician, particularly when imaging follow-up or additional diagnostic workup is needed. LI-RADS provides recommendations tailored to each category (2,32). When biopsy is recommended, a rationale should be provided. Examples of sentences that reflect this rationale include “Biopsy may be necessary to distinguish between HCC and [another entity.]” and “...probably HCC. To establish a definite diagnosis, biopsy may be considered” (32).
Reporting LI-RADS M Observations
An important category to ensure the specificity of the LI-RADS algorithm for HCC is the LR-M category. This category has high sensitivity for malignancy in general, including some atypical HCCs (33). Therefore, management recommendations for LR-M include multidisciplinary discussion and additional diagnostic workup. Descriptors for LR-M observations follow the same recommendations as for other categories. Major features of HCC may be present, although the dominant imaging criteria for LR-M include targetoid appearance, marked restricted diffusion, extensive necrosis, or other features that in the radiologist’s judgment suggest a malignancy other than HCC. When reporting an LR-M observation, radiologists are encouraged to include the most likely etiology, if possible (Fig 6). It is estimated that 36% of LR-M observations are HCCs (34). Imaging features that may suggest hepatocellular origin include fat in mass, iron in mass, blood products in mass, nodule-in-nodule architecture, mosaic architecture, intrinsic T1 hyperintensity, and hepatobiliary phase (HBP) hyperintensity (if applicable). Observations with a targetoid appearance in patients who meet the LI-RADS population criteria are likely to represent intrahepatic cholangiocarcinomas, although combined tumors and HCCs can also have this imaging appearance.
Figure 6.
Reporting flow diagram demonstrates how to report the most likely etiology of disease. The diagram is not exhaustive. It addresses only the more common diagnostic considerations encountered in patients at risk of HCC. cHCC-CCA = combined hepatocellular-cholangiocarcinoma, iCCA = intrahepatic cholangiocarcinoma. *Imaging features suggestive of hepatocellular origin include fat in mass, iron in mass, blood products in mass, nodule-in-nodule architecture, mosaic architecture, intrinsic T1 hyperintensity, and HBP hyperintensity (if applicable).
Reporting TIV
Determining vascular invasion is one of the first steps in the LI-RADS diagnostic decision tree. To assign TIV, the unequivocal presence of enhancing soft tissue in a vein, regardless of the presence of a parenchymal mass, is required (13). Imaging features suggestive of vascular invasion (ie, occluded vein with ill-defined walls or restricted diffusion or in contiguity with a malignant parenchymal mass; or heterogeneous vein enhancement not attributable to artifact) may also be reported along with suspicion of possible vascular invasion. Most LR-TIV in patients at risk is associated with HCC. However, intrahepatic cholangiocarcinomas (iCCs), biphenotypic tumors (combined hepatocellular cholangiocarcinoma [cHCC-CCA]), or other malignancies may also manifest with TIV. Radiologists are encouraged to include in the report the most probable etiology (35). CT and MRI LI-RADS version 2018 provides guidance on how to report TIV and the probable etiology on the basis of imaging features of an adjacent mass when present (Figs 6, 7) (13). Finally, the distribution and extent of the TIV should always be included in the report.
Figure 7a.
Reporting multiple observations. (a) Report observations individually, in aggregate, or by using a combination of the two, depending on the number of observations and their LI-RADS categories. (b) In general, widespread disease, TIV, and lower-category observations should be reported in aggregate.
Figure 7b.
Reporting multiple observations. (a) Report observations individually, in aggregate, or by using a combination of the two, depending on the number of observations and their LI-RADS categories. (b) In general, widespread disease, TIV, and lower-category observations should be reported in aggregate.
Reporting Observations in Aggregate
Multiple observations can be described individually, in aggregate, or by using a combination of the two, depending on the number of observations and the LI-RADS category (Fig 7). The choice of describing individual or aggregate observations is left to the radiologist’s judgment, with recommendation for the option that provides the greatest clarity.
LI-RADS recommends that up to five of the highest-categorized observations (LR-4, LR-5, LR-TR, and LR-M) are reported individually (13). Exceptions include more than five treated observations with varying degrees of response, for individual planning of additional therapy and restaging purposes. When more than five observations are present, care should be taken to preserve the big picture. It may be best to report in aggregate (eg, multiple individual LR-4 observations that, in aggregate, are likely to represent multifocal HCC). LR-3 observations can be reported in aggregate or individually. Multiple benign observations should not be reported individually as a general rule, to avoid a lengthy and confusing report. However, reporting of LR-1 and LR-2 observations individually may be appropriate if they correspond to lesions identified at screening US or if there is concern that they may mimic malignancy.
LI-RADS favors the term widespread disease to refer to numerous focal lesions, infiltrative disease with or without TIV, or the coexistence of both (Figs 8, 9) (13). When numerous focal or diffuse observations are interpreted in aggregate as malignant, it should be considered widespread disease and reported accordingly (Fig 10). Reporting them individually may be burdensome to radiologists as well as overwhelming and confusing for referring physicians, with little advantage to patient care. Widespread disease should be reported in aggregate, indicating the presence of widespread disease, and include, if applicable, the size, features, and LI-RADS category of one or two of the largest or index observations, along with a description of the segmental distribution and other findings that are relevant to patient treatment.
Figure 8.
Schematic representation of widespread disease. Widespread disease refers to numerous focal lesions, infiltrative disease, or the coexistence of both. When multiple observations are interpreted in aggregate as malignant, it should be considered widespread disease.
Figure 9.
Axial contrast-enhanced CT images obtained in late arterial, portal venous, and delayed phases in a 64-year-old man with chronic hepatitis C cirrhosis and widespread disease show the coexistence of arterial phase hyperenhancement and subtle washout appearance of infiltrative disease (*) and multiple focal observations (arrows).
Figure 10a.
Reporting numerous focal observations. (a) Axial contrast-enhanced MR images obtained in late arterial, portal venous, delayed, and diffusion-weighted (b = 800 sec/mm2) phases in a 58-year-old man with chronic hepatitis B cirrhosis depict numerous focal observations throughout the liver parenchyma. (b) Two samples of structured reports for widespread disease demonstrate reporting as structured text in a single continuous paragraph (left) and as a structured report with keywords and findings (right). N/A =not applicable.
Figure 10b.
Reporting numerous focal observations. (a) Axial contrast-enhanced MR images obtained in late arterial, portal venous, delayed, and diffusion-weighted (b = 800 sec/mm2) phases in a 58-year-old man with chronic hepatitis B cirrhosis depict numerous focal observations throughout the liver parenchyma. (b) Two samples of structured reports for widespread disease demonstrate reporting as structured text in a single continuous paragraph (left) and as a structured report with keywords and findings (right). N/A =not applicable.
Reporting in Specific Clinical Scenarios
Patients Considered for Liver Transplant in the United States
In Western societies, liver cirrhosis is the main risk factor for HCC development, and because of associated poor liver function, liver transplant is often the only curative option (36). In the United States, the OPTN is the national organization that regulates and assures the fair distribution of organs across the country (36).
Patients with HCC may receive priority on the transplant waitlist if their disease is within the Milan criteria (ie, T2 radiologic stage), with exception points added to their Model for End-stage Liver Disease (MELD) score (37). Therefore, it is important to convert LI-RADS categories to the corresponding OPTN classes in reports for a transplant candidate, in addition to explicitly describing the observations contributing to T staging (Figs 11, 12). Table 3 describes the radiologic (United Network for Organ Sharing [UNOS] and OPTN) T-staging system (31,36,38). Currently, OPTN allows exception point allocation for patients with T2- stage disease or greater either before therapy or after therapy, as long as they were initially within University of California, San Francisco (UCSF) criteria (39,40). It is important to note that for OPTN, only definitely HCC (OPTN-5) can be considered for transplant after downstaging. Reporting pre- and posttreatment measurements allows an estimation of the percentage of response and is of utmost importance in patients undergoing local-regional therapies as a bridge for transplant for restaging purposes.
Figure 11.
LI-RADS categories that count toward T staging for OPTN are pathologically proven HCCs, most LR-5 observations, LR-TR viable observations, and LR-TIV. LI-RADS categories that do not count toward T staging for OPTN are LR-3, LR-4, LR-M, LR-5 (if there are 10–19-mm observations with APHE and nonperipheral washout but without an enhancing capsule, or threshold growth [TG] ), and LR-TIV if due to other malignancies.
Figure 12.
Reporting flow diagram shows how to report observations in patients who are candidates for transplant in the United States.
Table 3:
Radiologic T Staging System for HCC
OPTN designates treated OPTN-5 lesions as OPTN-5T, with the letter T indicating that treatment has been performed, but not assessing viability. Thus, there are no OPTN classes analogous to LR-TR categories. Although assessing tumor viability might be more informative for staging purposes, it remains unclear if TR-equivocal observations are routinely counted toward T staging for listing (38).
Currently, OPTN and LI-RADS diagnostic criteria for definite HCC are nearly identical, except for observations measuring 10–19 mm with APHE and nonperipheral washout but without an enhancing capsule. According to LI-RADS, these are assigned an LR-5 category (definitely HCC). For OPTN, these observations do not fulfill criteria for definite HCC and hence do not contribute to OPTN T staging. OPTN uses the qualifier g to indicate HCC diagnosis on the basis of growth (ie, ≥10 mm with APHE and threshold growth equals OPTN class 5-g) and X for HCCs that are larger than 5 cm in diameter (ie, OPTN class 5X). LIRADS does not use qualifiers. LR-4 and LR-M observations do not contribute to OPTN staging or MELD exception points. Nevertheless, they should be included in the report because of the high likelihood of representing HCC and malignancy. LR-TIV observations and extrahepatic disease contribute to overall T stage and transplant eligibility and must be reported clearly.
Patients Considered for Hepatic Resection
In patients considered for tumor resection, additional information should be included. Description of the biliary and vascular anatomy is critical. A description of the tumor location relative to major hepatic veins and biliary structures, distance to the liver capsule, and contact with adjacent organs is helpful for adequate surgical planning (41). A description of the background liver and presence of portal hypertension may help to assess the future liver remnant.
In some institutions, liver volumetry is performed preoperatively, with schematics of the resection line and segmental or sectorial volumes inserted in the report (42). Radiologists working in centers where liver resections are performed are encouraged to become familiar with and include in their report descriptions of procedures aiming to increase the function and size of the future liver remnant such as portal vein embolization, portal vein ligation, and associated liver partition and portal vein ligation for staged hepatectomy.
Patients with Observations Treated with Radiation-based Therapies
Radiation therapies (eg, radioembolization and stereotactic body radiation therapy [SBRT]) may result in periobservation signal alterations or perfusional abnormalities that challenge the ability to evaluate tumor viability. These radiation-induced injuries may resemble or obscure tumor enhancement and may evolve for months after treatment, with some risk for false-positive or false-negative assessment of viability (43,44). This pitfall is particularly relevant when treated areas demonstrate APHE lasting several months after treatment, even in successfully treated HCCs (44). Although the current LR-TR algorithm does not specifically address these changes, when unsure, radiologists should choose the category reflecting lower certainty; that is, LR-TR equivocal (13). Future refinements in the LR-TR algorithm should consider the challenges associated with radiation-based therapies in the assessment of response to treatment.
Patients with Observations Treated with Systemic Therapies
LI-RADS does not currently provide an algorithm for assessing response to systemic therapies. When reporting response to systemic therapy, radiologists may adopt different criteria (eg, modified Response Evaluation Criteria in Solid Tumors [RECIST]) following individual or institutional preferences. Caution should be exercised when applying LR-TR algorithms in patients who have undergone local-regional therapy in conjunction with systemic therapies, since concomitant systemic therapies may result in an unusual posttreatment appearance.
Future Directions
For structured reports to be incorporated in daily practice, the means to deploy structured reports need to be available and fully integrated into dictation or reporting tools. Natural language processing (NLP) applications offer the possibility of converting unstructured text or dictations into structured outputs and, therefore, facilitating the adoption of structured reports in clinical practice. These tools can also assess quality and perform diagnostic surveillance, as well as perform semantic analysis on the outputs by identifying ontology relations to assure that these are populated with standardized language (ie, lexicon) (45).
Other information technology–based reporting tools are also under development to facilitate and explore the benefits of structured radiology reports. ACR Assist, a collection of CDE modules, is under development, and the LI-RADS Assist module, finalized in 2019, is being currently tested in a clinical environment. The LI-RADS Assist module has been developed in collaboration with the ACR programmers and the members of the LI-RADS steering committee and has been rigorously tested and validated to faithfully capture the LI-RADS CT and MRI diagnostic and treatment response algorithms. This schema is to be integrated by the commercial vendors into their dictation software, allowing radiologists to input the predefined data elements (eg, observation location, size, major features, and LR-M features) for the schema to assign the correct LI-RADS category while generating a comprehensive standardized report (46).
Further, the input of the prescribed data elements may be simplified by incorporation of NLP software for extraction of all required data elements from free dictation, with the radiologist being prompted by the software for missing data elements, if needed. The implementation of LI-RADS Assist across multiple institutions and diverse clinical practice settings will likely result in the collection of comprehensive geographically and practice-diverse data, which can be used for LI-RADS validation and refinement, quality assurance, registry development, and research.
We also expect that wide implementation of the ACR Assist module will aid in utilization of LI-RADS in busy clinical practices, particularly in nonacademic centers, while improving adherence to management guidelines (47). To this day, a massive amount of clinical data are still underutilized, as most are contained in unstructured free-text reports. The implementation of the CDE schema and structured reports in multiple institutions and in various clinical practice settings will allow collection of uniformly curated data over time, which can be used for quality assurance, data collection, and development of large registries. These large labeled clinical datasets will certainly increase the capability of training and fine-tune artificial intelligence–based algorithms to aid radiologists during image analysis, reporting, and overall patient care.
Conclusion
As HCC diagnosis and treatment are to a large extent determined by imaging, it is essential that radiology reports clearly and consistently inform stakeholders involved in the care of patients who are at risk for HCC. Standardized LI-RADS reports are designed to provide all necessary information to guide treatment decisions that are tailored to individual patients’ needs. This article provides general guidance on the usage of LI-RADS standardized reporting to describe imaging findings in patients who are at risk, highlighting relevant imaging findings and components to be included when reporting specific clinical scenarios. Radiologists should leverage or adapt the sections and elements described in this article to suit the requirements of their referrers, practices, and institutions, as well as regional or societal preferences.
Author Affiliations.–Department of Radiology, University of California San Diego, Liver Imaging Group, La Jolla, Calif (G.M.C., K.J.F., C.B.S.); Department of Radiology, Mount Sinai West, New York, NY (A.R.); Department of Diagnostic, Molecular and Interventional Radiology, BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY (B.T.); Department of Radiology, Oregon Health & Science University, Portland, Ore (A.W.F.); Department of Abdominal Imaging, The University of Texas MD Anderson Cancer Center, Houston, Tex (K.M.E.); Department of Radiology, Naval Medical Center San Diego, San Diego, Calif (R.M.M.); Department of Radiology, Uniformed Services University of the Health Sciences, Bethesda, Md (R.M.M.); Inland Imaging, Spokane, Wash (I.C.); Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY (N.H.); Department of Radiology, University of São Paulo, São Paulo, Brazil (N.H.); Department of Radiology, Montefiore Medical Center, Bronx, NY (V.C.); Department of Radiology, Centre Hospitalier de l'Université de Montréal, Montréal, Quebec, Canada (A.T.); and Centre de Recherche du Centre Hospitalier de l'Université de Montréal, Montréal, Quebec, Canada (A.T.).
Current address: Department of Radiology, Beth Israel Deaconess Medical Center, Boston, Mass.
For this journal-based SA-CME activity, the authors G.M.C., K.J.F., K.M.E., V.C., C.B.S., and A.T. have provided disclosures (see end of article); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The author(s) are military service members. This work was prepared as part of official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.”
A.T supported by the Fonds de Recherche du Québec—Santé (Career Award 34939).
Disclosures of Conflicts of Interest.— : G.M.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other activities: member of the LI-RADS steering committee, international working group, and writing group. K.J.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant to Epigenomics; institution received grants from GE and Bayer; travel expenses paid by Bayer. Other activities: disclosed no relevant relationships. K.M.E. Activities related to the present article: Editorial board member of RadioGraphics (not involved in the handling of this article). Activities not related to the present article: disclosed no relevant relationships. Other activities: disclosed no relevant relationships. V.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant to Bayer. Other activities: disclosed no relevant relationships. C.B.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant to Epigenomics, Blade, and Boehringer; institution received grants from GE, Siemens, Bayer, Foundation for the National Institutes of Health (NIH), and Gilead Sciences; received royalties from Wolters Kluwer; received payment for development of educational presentations from Medscape; travel prepaid by Sun Yat-sen University; institution received payment for consulting for GE Digital, IBM Watson, AMRA Medical, Bristol Myers Squibb, and Exact Sciences; institution has laboratory service agreements with Enanta Pharmaceuticals, Gilead Sciences, ICON, Intercept Pharmaceuticals, NuSirt Biopharma, Shire, Synageva, and Takeda. Other activities: disclosed no relevant relationships. A.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: speaking honoraria from Siemens Healthineers and Eli Lilly. Other activities: disclosed no relevant relationships.
Abbreviations:
- ACR
- American College of Radiology
- APHE
- arterial phase hyperenhancement
- CDE
- common data element
- HBP
- hepatobiliary phase
- HCC
- hepatocellular carcinoma
- LI-RADS
- Liver Imaging Reporting and Data System
- OPTN
- Organ Procurement and Transplantation Network
- TIV
- tumor in vein
References
- 1. Sayiner M , Golabi P , Younossi ZM . Disease burden of hepatocellular carcinoma: a global perspective . Dig Dis Sci 2019. ; 64 ( 4 ): 910 – 917 . [DOI] [PubMed] [Google Scholar]
- 2. Marrero JA , Kulik LM , Sirlin CB , et al . Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases . Hepatology 2018. ; 68 ( 2 ): 723 – 750 . [DOI] [PubMed] [Google Scholar]
- 3. Heimbach JK , Kulik LM , Finn RS , et al . AASLD guidelines for the treatment of hepatocellular carcinoma . Hepatology 2018. ; 67 ( 1 ): 358 – 380 . [DOI] [PubMed] [Google Scholar]
- 4. Corwin MT , Lee AY , Fananapazir G , Loehfelm TW , Sarkar S , Sirlin CB . Nonstandardized terminology to describe focal liver lesions in patients at risk for hepatocellular carcinoma: implications regarding clinical communication . AJR Am J Roentgenol 2018. ; 210 ( 1 ): 85 – 90 . [DOI] [PubMed] [Google Scholar]
- 5. Goldberg-Stein S , Chernyak V . Adding value in radiology reporting . J Am Coll Radiol 2019. ; 16 ( 9 Pt B ): 1292 – 1298 . [DOI] [PubMed] [Google Scholar]
- 6. Bosmans JM , Neri E , Ratib O , Kahn CE Jr . Structured reporting: a fusion reactor hungry for fuel . Insights Imaging 2015. ; 6 ( 1 ): 129 – 132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flusberg M , Ganeles J , Ekinci T , et al . Impact of a structured report template on the quality of CT and MRI reports for hepatocellular carcinoma diagnosis . J Am Coll Radiol 2017. ; 14 ( 9 ): 1206 – 1211 . [DOI] [PubMed] [Google Scholar]
- 8. Sahni VA , Silveira PC , Sainani NI , Khorasani R . Impact of a structured report template on the quality of MRI reports for rectal cancer staging . AJR Am J Roentgenol 2015. ; 205 ( 3 ): 584 – 588 . [DOI] [PubMed] [Google Scholar]
- 9. Hanna TN , Shekhani H , Maddu K , Zhang C , Chen Z , Johnson JO . Structured report compliance: effect on audio dictation time, report length, and total radiologist study time . Emerg Radiol 2016. ; 23 ( 5 ): 449 – 453 . [DOI] [PubMed] [Google Scholar]
- 10. Kahn CE Jr , Langlotz CP , Burnside ES , et al . Toward best practices in radiology reporting . Radiology 2009. ; 252 ( 3 ): 852 – 856 . [DOI] [PubMed] [Google Scholar]
- 11. European Society of Radiology (ESR) . ESR paper on structured reporting in radiology . Insights Imaging 2018. ; 9 ( 1 ): 1 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim TH , Kim SY , Tang A , Lee JM . Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update . Clin Mol Hepatol 2019. ; 25 ( 3 ): 245 – 263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CT/MRI LI-RADS® v2018 . American College of Radiology; . https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed August 10, 2020 . [Google Scholar]
- 14. Rubin DL , Kahn CE Jr . Common data elements in radiology . Radiology 2017. ; 283 ( 3 ): 837 – 844 . [DOI] [PubMed] [Google Scholar]
- 15. Chen MC , Ball RL , Yang L , et al . Deep learning to classify radiology free-text reports . Radiology 2018. ; 286 ( 3 ): 845 – 852 . [DOI] [PubMed] [Google Scholar]
- 16. Hong Y , Kahn CE Jr . Content analysis of reporting templates and free-text radiology reports . J Digit Imaging 2013. ; 26 ( 5 ): 843 – 849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nobel JM , Kok EM , Robben SGF . Redefining the structure of structured reporting in radiology . Insights Imaging 2020. ; 11 ( 1 ): 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mamlouk MD , Chang PC , Saket RR . Contextual radiology reporting: a new approach to neuroradiology structured templates . AJNR Am J Neuroradiol 2018. ; 39 ( 8 ): 1406 – 1414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz LH , Panicek DM , Berk AR , Li Y , Hricak H . Improving communication of diagnostic radiology findings through structured reporting . Radiology 2011. ; 260 ( 1 ): 174 – 181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brook OR , Brook A , Vollmer CM , Kent TS , Sanchez N , Pedrosa I . Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning . Radiology 2015. ; 274 ( 2 ): 464 – 472 . [DOI] [PubMed] [Google Scholar]
- 21. Kahn CE Jr , Heilbrun ME , Applegate KE . From guidelines to practice: how reporting templates promote the use of radiology practice guidelines . J Am Coll Radiol 2013. ; 10 ( 4 ): 268 – 273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sistrom CL , Honeyman-Buck J . Free text versus structured format: information transfer efficiency of radiology reports . AJR Am J Roentgenol 2005. ; 185 ( 3 ): 804 – 812 . [DOI] [PubMed] [Google Scholar]
- 23. RadLex radiology lexicon . Radiological Society of North America; . https://www.rsna.org/en/practice-tools/data-tools-and-standards/radlex-radiology-lexicon. Accessed November 30, 2020 . [Google Scholar]
- 24. RadLex Web site . http://radlex.org/. Accessed November 30, 2020 .
- 25. RadElement Web site . https://www.radelement.org/. Accessed November 30, 2020 .
- 26. Chernyak V , Fowler KJ , Kamaya A , et al . Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients . Radiology 2018. ; 289 ( 3 ): 816 – 830 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lexicon Table 2020 . American College of Radiology; . https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LIRADS-Lexicon-Table.pdf. Accessed October 3, 2020 . [Google Scholar]
- 28. Kambadakone AR , Fung A , Gupta RT , et al . LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 56 – 74 [Published correction appears in Abdom Radiol (NY) 2018;43(1):240.] . [DOI] [PubMed] [Google Scholar]
- 29. Cunha GM , Kwon H , Wolfson T , et al . Examining LI-RADS recommendations: should observation size only be measured on non-arterial phases? Abdom Radiol (NY) 2020. ; 45 ( 10 ): 3144 – 3154 . [DOI] [PubMed] [Google Scholar]
- 30. Kielar A , Fowler KJ , Lewis S , et al . Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 218 – 230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang A , Fowler KJ , Chernyak V , Chapman WC , Sirlin CB . LI-RADS and transplantation for hepatocellular carcinoma . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 193 – 202 . [DOI] [PubMed] [Google Scholar]
- 32. Mitchell DG , Bruix J , Sherman M , Sirlin CB . LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions . Hepatology 2015. ; 61 ( 3 ): 1056 – 1065 . [DOI] [PubMed] [Google Scholar]
- 33. Kim YY , Kim MJ , Kim EH , Roh YH , An C . Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS version 2018 . Radiology 2019. ; 291 ( 1 ): 72 – 80 . [DOI] [PubMed] [Google Scholar]
- 34. van der Pol CB , Lim CS , Sirlin CB , et al . Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy—a systematic review . Gastroenterology 2019. ; 156 ( 4 ): 976 – 986 . [DOI] [PubMed] [Google Scholar]
- 35. Fowler KJ , Potretzke TA , Hope TA , Costa EA , Wilson SR . LI-RADS M (LR-M): definite or probable malignancy, not specific for hepatocellular carcinoma . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 149 – 157 . [DOI] [PubMed] [Google Scholar]
- 36. Wald C , Russo MW , Heimbach JK , Hussain HK , Pomfret EA , Bruix J . New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma . Radiology 2013. ; 266 ( 2 ): 376 – 382 . [DOI] [PubMed] [Google Scholar]
- 37. Organ Procurement and Transplantation Network: Policies . U.S. Department of Health & Human Services; . https://optn.transplant.hrsa.gov/governance/policies/. Accessed July 18, 2020 . [Google Scholar]
- 38. Cunha GM , Tamayo-Murillo DE , Fowler KJ . LI-RADS and transplantation: challenges and controversies . Abdom Radiol (NY) 2019. . 10.1007/s00261-019-02311-w. Published online November 6, 2019 . [DOI] [PubMed]
- 39. Yao FY , Ferrell L , Bass NM , et al . Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival . Hepatology 2001. ; 33 ( 6 ): 1394 – 1403 . [DOI] [PubMed] [Google Scholar]
- 40. Yao FY . Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria . Am J Transplant 2008. ; 8 ( 10 ): 1982 – 1989 . [DOI] [PubMed] [Google Scholar]
- 41. Sahani D , Mehta A , Blake M , Prasad S , Harris G , Saini S . Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery . RadioGraphics 2004. ; 24 ( 5 ): 1367 – 1380 . [DOI] [PubMed] [Google Scholar]
- 42. Orcutt ST , Anaya DA . Liver resection and surgical strategies for management of primary liver cancer . Cancer Contr 2018. ; 25 ( 1 ): 1073274817744621 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. King MJ , Tong A , Dane B , Huang C , Zhan C , Shanbhogue K . Response assessment of hepatocellular carcinoma treated with yttrium-90 radioembolization: inter-reader variability, comparison with 3D quantitative approach, and role in the prediction of clinical outcomes . Eur J Radiol 2020. ; 133 109351 . [DOI] [PubMed] [Google Scholar]
- 44. Mendiratta-Lala M , Gu E , Owen D , et al . Imaging findings within the first 12 months of hepatocellular carcinoma treated with stereotactic body radiation therapy . Int J Radiat Oncol Biol Phys 2018. ; 102 ( 4 ): 1063 – 1069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pons E , Braun LM , Hunink MG , Kors JA . Natural language processing in radiology: a systematic review . Radiology 2016. ; 279 ( 2 ): 329 – 343 . [DOI] [PubMed] [Google Scholar]
- 46. Alkasab TK , Bizzo BC , Berland LL , Nair S , Pandharipande PV , Harvey HB . Creation of an open framework for point-of-care computer-assisted reporting and decision support tools for radiologists . J Am Coll Radiol 2017. ; 14 ( 9 ): 1184 – 1189 . [DOI] [PubMed] [Google Scholar]
- 47. Lu MT , Rosman DA , Wu CC , et al . Radiologist point-of-care clinical decision support and adherence to guidelines for incidental lung nodules . J Am Coll Radiol 2016. ; 13 ( 2 ): 156 – 162 . [DOI] [PubMed] [Google Scholar]


















![LI-RADS categories that count toward T staging for OPTN are pathologically proven HCCs, most LR-5 observations, LR-TR viable observations, and LR-TIV. LI-RADS categories that do not count toward T staging for OPTN are LR-3, LR-4, LR-M, LR-5 (if there are 10–19-mm observations with APHE and nonperipheral washout but without an enhancing capsule, or threshold growth [TG] ), and LR-TIV if due to other malignancies.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c6b3/8415040/045fd3625a81/rg.2021200205.fig11.jpg)

